Abstract
Synaptojanin 1 is a polyphosphoinositide phosphatase implicated in synaptic vesicle recycling. We used FM1-43 imaging and electron microscopy in cultured cortical neurons from control and synaptojanin 1 knockout mice to study how the absence of this protein affects specific steps of the synaptic vesicle cycle. Exo/endocytosis after a moderate stimulus was unchanged. However, during prolonged stimulation, the regeneration of fusion-competent synaptic vesicles was severely impaired. In stimulated nerve terminals, there was a persistent accumulation of clathrin-coated vesicles and a backup of newly reformed vesicles in the cytomatrix-rich area around the synaptic vesicle cluster. These findings demonstrate that synaptojanin 1 function is needed for the progression of recycling vesicles to the functional synaptic vesicle pool.
Neurotransmission and its reliability depend on the efficient and precise recycling of synaptic vesicles in the presynaptic nerve terminal (1, 2). Vesicle fusion and neurotransmitter release are followed by rapid retrieval and repackaging of synaptic vesicle membrane components into new functional vesicles. Morphologic, biochemical, genetic, and functional evidence points to an important role of the clathrin-mediated recycling pathway in this process (1, 3–5). Alternative and parallel pathways may also participate in recycling (1, 2, 6–9).
Growing evidence suggests that phosphoinositides play an important role in the synaptic vesicle cycle via their interaction with endocytic and actin regulatory proteins (10–13). Phosphoinositides, PI(4,5)P2 in particular, bind clathrin adaptors such as AP-2 (14–16), AP180 (17, 18), and epsin (19, 20), as well as the GTPase dynamin (21, 22), which is critically required for the fission of endocytic vesicles (23, 24). They also regulate a variety of proteins that control actin nucleation (13, 25, 26). Actin, in turn, is thought to play an important role in clathrin-mediated recycling by generating and organizing cytoskeletal scaffolds at endocytic zones (27–29) and, possibly, by contributing to the translocation of newly formed endocytic vesicles to the synaptic vesicle cluster (30–34).
Synaptojanin 1 is a polyphosphoinositide phosphatase highly enriched in presynaptic nerve terminals and localized to recycling intermediates in synaptosomes (35, 36). Its COOH-terminal proline-rich domain interacts with several accessory factors implicated in synaptic vesicle recycling, including amphiphysin (35), endophilin (37), (38), intersectin (34, 39), and syndapin/pacsin (40, 41). Its NH2-terminal Sac1-like module hydrolyzes the phosphates of PI(3)P, PI(4)P, and PI(3,5)P2 (42), whereas its central phosphatase module hydrolyzes the 5′ phosphate of PI(4,5)P2 and PI(3,4,5)P3 (43, 44). By means of these enzymatic activities, synaptojanin can dephosphorylate polyphosphoinositides to phosphatidylinositol and thereby function as a negative regulator of the interactions between membranes, coat proteins, and regulatory factors for actin.
In mice knockout studies, the absence of synaptojanin 1 leads to a failure to thrive and 100% mortality within 2 weeks of birth, elevated steady-state levels of PI(4,5)P2, an increased number of clathrin-coated vesicles in presynaptic terminals, and increased synaptic depression in hippocampal slices (43). Paired cell recordings of inhibitory neurons in cultures further demonstrate the importance of synaptojanin 1 for the slow component of synaptic depression during prolonged stimulation (45). In lamprey reticulospinal axons, microinjection of anti-synaptojanin antibodies or of peptides that inhibit synaptojanin recruitment induces a stimulus-dependent depletion of synaptic vesicles, an increase in clathrin-coated vesicles, an expansion of the plasma membrane, and an accumulation of actin at endocytic zones (46). In Caenorhabditis elegans, the synaptojanin null mutant unc-26 exhibits uncoordinated behavior and pleiotropic morphologic effects, including depletion of synaptic vesicles, cytoskeletal defects, and an accumulation of endocytic pits, coated vesicles, and endosome-like compartments (47). In yeast, inactivation of synaptojanin-like proteins results in an accumulation and mislocalization of PI(4,5)P2, as well as defects in actin and endocytosis (48–50).
Collectively, these studies suggest that synaptojanin 1 plays some essential role in synaptic vesicle recycling, particularly during prolonged stimulation. However, a precise correlation between the physiology of synaptic transmission, the synaptic vesicle cycle, and the cytology of the synapse has not been established. In this study, we have combined quantitative imaging of FM1-43 turnover (51, 52) with electron microscopy on cultured cortical neurons from synaptojanin 1 knockout mice to determine the role that synaptojanin 1 plays in specific steps of the synaptic vesicle cycle under physiological conditions.
Methods
Cell Culture.
Synaptojanin 1-deficient mice were obtained by targeted disruption as described (43). Newborn littermates of control and homozygous synaptojanin 1 mutant mice from four different litters were used in the analysis. Primary cultures of cortical neurons were prepared as described (53, 54). Cultures were maintained in Neurobasal/B27 medium (GIBCO) at 37°C in a 95% air/5% CO2 humidified incubator for 9–18 days before use.
Quantitative FM1-43 Imaging and Analysis.
Neurons grown on coverslips were mounted in a rapid-switching, laminar flow perfusion and electrical field stimulation chamber (53). The chamber was mounted on an inverted Zeiss Axiovert 35 microscope equipped with a Princeton Instruments Pentamax 12-bit cooled CCD camera (Roper Scientific, Trenton, NJ) driven by the PC-based metamorph image acquisition and analysis program (Universal Imaging, Brandywine, PA). Action potentials (APs) were evoked by passing 1-ms bipolar current pulses through Pt electrodes, yielding fields of ≈10 V/cm through the chamber. Cells were continuously superfused at room temperature (≈24°C) in a saline solution containing 130 mM NaCl, 3 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 25 mM Hepes (pH 7.35), 30 mM glucose, 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and 50 μM d-2-amino-5-phosphonovaleric acid (APV) (Research Biochemicals, Natick, MA). FM1-43 (Molecular Probes) was used at 10 μM. Fluorescence images were acquired by averaging two frames captured through a 40×, 1.3-N.A. Zeiss objective onto the CCD camera. Quantitative measurements of fluorescence intensity at individual synapses were obtained by averaging a 4 × 4 area of pixel intensities centered about the optical center of mass of a given fluorescent punctum (52).
Electron Microscopy and Morphometry.
Neurons grown on coverslips were mounted in the same electrical field stimulation chamber used for optical studies, and superfused with the physiologic saline solution. Horseradish peroxidase (HRP; 10 mg/ml) or HRP-conjugated wheat germ agglutinin (HRP-WGA; 1 mg/ml) (both from Sigma) were applied to neurons in the extracellular bath and used as endocytic tracers for ultrastructural analysis. At the appropriate times, the saline was rapidly exchange with 2% glutaraldehyde/2% sucrose in 0.1 M sodium phosphate buffer (pH 7.4) and fixed for 1 h at room temperature. Samples were processed for electron microscopy as described (54). Morphometric analysis was done on electron micrographs at a final magnification of ×21,000. Compartments labeled by HRP were identified by the presence of DAB/peroxidase reaction product. Counts of synaptic vesicles and clathrin-coated vesicles were performed and values of clathrin-coated vesicles expressed as a percentage of total small vesicles. Synaptic vesicles were defined as small, homogeneously sized, round or slightly ovoid compartments forming large clusters in presynaptic regions. Clathrin-coated vesicles were defined as round or slightly ovoid compartments that had a complete or partial clathrin coat and were located in close proximity to the synaptic vesicle cluster. From four different neuron cultures, a total of 349 control and 196 knockout nerve terminals were analyzed.
Results
The Functional Recycling Pool Is Smaller in Synaptojanin 1-Deficient Neurons.
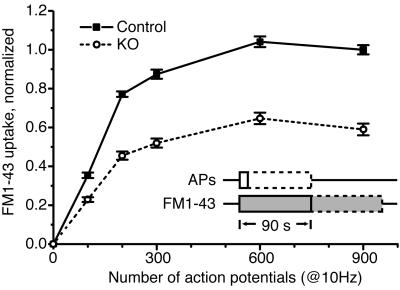
To directly assess whether the exo/endocytotic turnover of synaptic vesicles is altered in synaptojanin 1-deficient neurons, the uptake of FM1-43 into a functional pool of synaptic vesicles in response to increasing numbers of APs was measured in primary cultures of cortical neurons. For a loading sequence (Fig. 1), normal saline was replaced with saline containing FM1-43. Then, the neurons were electrically stimulated to fire a defined AP train at 10 Hz and kept in the presence of dye for an additional 90 s after the end of the stimulus. After a thorough washout period of 10 min in dye-free solution, the remaining fluorescent puncta corresponded to FM1-43 trapped within presynaptic boutons. These nerve terminals, loaded with dye-filled synaptic vesicles, were then maximally unloaded with a long train of APs (900 APs at 10 Hz) in dye-free saline. The difference in fluorescence intensity ΔF before and after complete unloading reflected the amount of fluorescence trapped in the recycling synaptic vesicle pool during the loading sequence. With increasing numbers of exo/endocytic events, the boutons became more fluorescent until they reached a steady-state maximum where additional stimuli did not further increase the fluorescence.
Fig 1.
Overall synaptic vesicle turnover is intact in synaptojanin 1-deficient mice, but the functional recycling pool is 40% smaller. A plot of FM1-43 uptake ΔF (see text) as a function of AP number shows that knockout and control neurons have similarly shaped loading curves, both reaching maximal FM1-43 uptake with 600 APs. However, the absolute amount of trapped fluorescence in knockout synapses is 40% less than in control synapses.
The overall pattern of synaptic vesicle turnover was the same in knockout and control neurons, and agreed with previous findings (52, 53). Analysis of FM1-43 uptake ΔF as a function of AP number revealed that both knockout and control neurons reached maximal dye loading by 600 APs (Fig. 1). The similar shape of the loading curves indicated that there was no difference in the relationship between evoked APs and the relative amounts of synaptic vesicle turnover. However, although the overall pattern of synaptic vesicle turnover was the same, the absolute amount of trapped fluorescence in knockout synapses was ≈40% less than in control synapses. Thus, although the synaptic vesicle cycle was grossly intact, the difference in maximal dye uptake suggests that the functional synaptic vesicle pool in knockout neurons was on average 40% smaller than in control neurons.
Endocytosis Kinetics After a Short Stimulatory Burst Are Unchanged in Synaptojanin 1-Deficient Neurons.
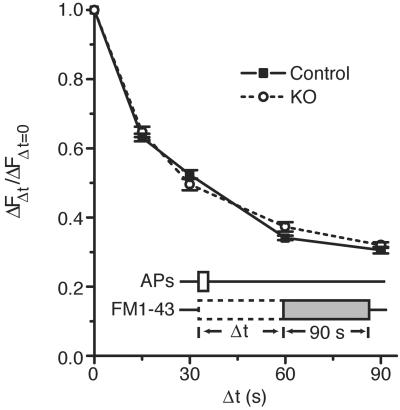
To determine whether synaptojanin 1 is involved in the initial retrieval of synaptic membrane from the cell surface, the time course of dye internalization after a short train of APs was measured in synaptojanin 1-deficient neurons. Neurons were stimulated with a train of 100 APs at 10 Hz that triggered the turnover of, on average, one-third of the synaptic vesicle pool. A 90-s FM1-43 pulse was begun after a variable time delay Δt ranging from 0 to 90 s after the start of the stimulus train (Fig. 2, schematic). Synaptic membrane that underwent endocytosis during the delay period escaped labeling, whereas endocytic vesicles retrieved during the dye pulse were labeled. The amount of FM1-43 taken up into the releasable pool was revealed by comparing the fluorescence of boutons before and after a complete 900-AP unloading. Analysis of FM1-43 uptake ΔF as a function of the delay Δt revealed that the kinetics of endocytosis after the 100-AP train were indistinguishable between knockout and control neurons (Fig. 2). In both cases, the time t1/2 for half the fused synaptic vesicle membrane to undergo endocytosis was ≈15 s. Moreover, the overall shapes of the curves show no difference in the time course of endocytosis over a period of 180 s (up to a 90-s delay and including a 90-s dye pulse). These results agreed with previous findings (52) and suggested that the basic properties and machinery of endocytosis were intact. Therefore, the function of synaptojanin 1 did not appear to be essential in these early steps of synaptic vesicle recycling.
Fig 2.
Endocytosis kinetics of synaptic vesicle membrane are unchanged after moderate stimulation (100 APs at 10 Hz). A plot of FM1-43 uptake as a function of dye pulse delay shows that the half time (t1/2) for endocytosis is 15 s in both knockout and control neurons, and the overall shape of the curves is identical.
The Reformation of Fusion-Competent Synaptic Vesicles After a Round of Exo/Endocytosis Is Delayed in Synaptojanin 1-Deficient Neurons.
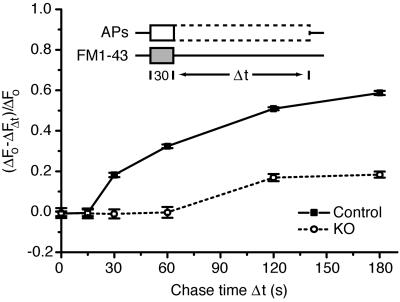
The observations described above left open the question of whether synaptojanin 1 functions in the synaptic vesicle cycle downstream of membrane internalization. After endocytosis, newly retrieved synaptic membrane progresses through a multistep process to be repackaged into fusion-competent (or primed) synaptic vesicles ready for the next round of exocytosis. To determine whether synaptojanin 1 plays such a role, the kinetics of synaptic vesicle “repriming” was measured in synaptojanin 1-deficient neurons. The loading phase consisted of a 30-s pulse of FM1-43 where the start of the pulse was coincident with the start of a 10-Hz AP train (Fig. 3, schematic). The stimulus continued beyond the dye pulse for a variable amount of chase time Δt ranging from 0 to 180 s while the neurons were bathed in dye-free saline. During this chase time, internalized fluorescent dye traveled through the recycling pathway. With longer AP trains, newly formed vesicles containing dye reentered the functional pool and released their trapped fluorescence in a second round of exocytosis. The releasable net fluorescence ΔFΔt remaining after a given chase time Δt was unloaded and measured after a 10-min recovery period at rest. This fluorescence value ΔFΔt corresponded to synaptic membrane that was trapped “en route” from endocytosis to exocytosis during the prolonged stimulus. This residual fraction of fluorescence was compared with the total releasable fluorescence ΔFo, or the maximal amount of fluorescence trapped during a 300-AP, 30-s dye pulse loading phase measured when there was no chase period (Δt = 0 s). The progress of dye through the synaptic vesicle cycle was plotted by calculating the fraction (ΔFo − ΔFΔt)/ΔFo of the total releasable internalized dye that was released at various intervals Δt of the chase period.
Fig 3.
Repriming kinetics reveal a delay and impairment of synaptic vesicle reformation during prolonged stimulation. A plot of the ratio (ΔFo − ΔFΔt)/ΔFo (see text) gives the fraction of fluorescence depleted by the APs fired during the chase period Δt. During continuous stimulation, knockout neurons exhibit a minimum repriming interval of 60 s compared with 15 s in control. In addition, only 20% of the retrieved membrane is releasable during the sustained stimulus in the knockout compared with 60% in the control.
Analysis of repriming kinetics revealed that the regeneration of functional synaptic vesicles during prolonged stimulation was delayed in synaptojanin 1-deficient neurons (Fig. 3). In control neurons, the minimum repriming time was ≈15 s, and 60% of the internalized and releasable dye was secreted during the continuous stimulus. The remaining fluorescence could be released after a 10-min recovery period at rest, similar to previous findings (52, 53). In contrast, in synaptojanin 1-deficient neurons, the minimum repriming time was 60 s, indicating a substantial kinetic delay in synaptic vesicle reformation. Furthermore, knockout synapses released only 20% of their total releasable fluorescence during the prolonged high-frequency stimulus. These results demonstrate the existence of two defects in synaptic vesicle recycling under these conditions: a kinetic delay in synaptic vesicle recycling and a severe backup of synaptic vesicle membrane that persists as long as stimulation is maintained. They suggest that synaptojanin 1 plays a critical role after vesicle membrane internalization in one or multiple steps of a recycling pathway leading from an endocytic vesicle to a fusion-competent vesicle.
A Stimulus-Dependent Increase in Clathrin-Coated Vesicles Is Persistent in Synaptojanin 1-Deficient Neurons.
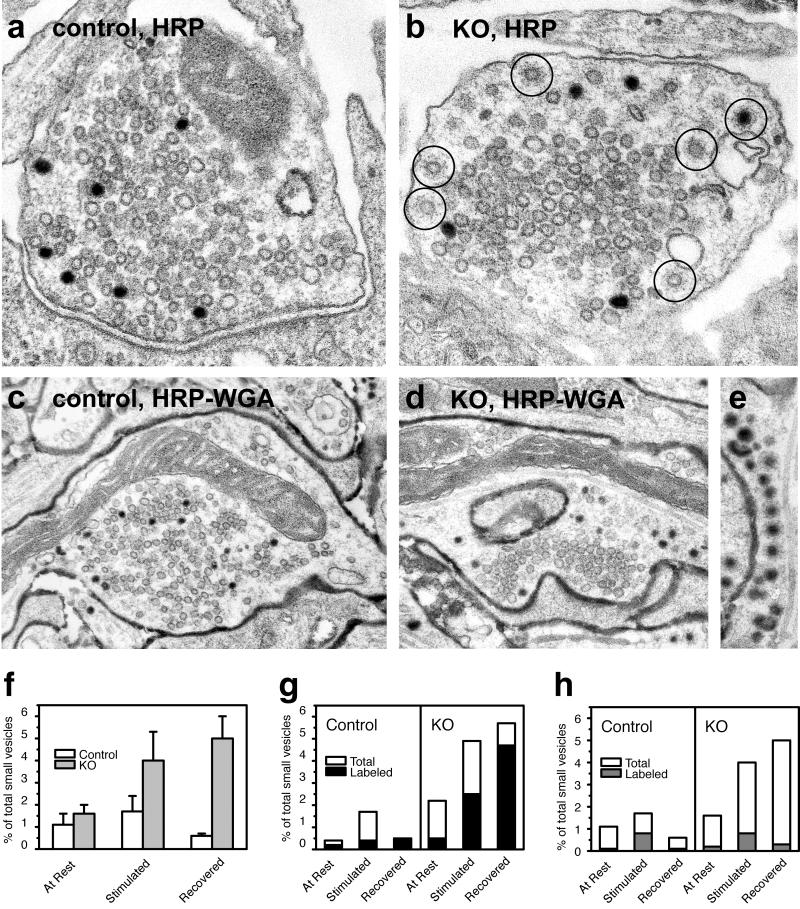
To gain insight into the cellular mechanisms underlying this functional delay in synaptic vesicle reformation, we performed correlative ultrastructural studies involving pulse–chase experiments with endocytic tracers on cortical neuronal cultures. Knockout and control neurons were kept at rest for 30 min then stimulated with 900 APs (90 s at 10 Hz) in the presence of either the fluid-phase marker HRP or HRP-WGA. HRP-WGA binds to synaptic membranes and labels clathrin-coated vesicles and synaptic vesicles more efficiently than does fluid-phase HRP (55). After the stimulus, neurons were washed and allowed to recover for 10 min in the absence of extracellular tracer. Cultures were fixed before the beginning of the stimulus (At Rest), at the end of the 900-AP train (Stimulated), or after the 10-min recovery period (Recovered; Fig. 4a). The “stimulated” condition corresponded to the 900-AP load in the synaptic vesicle turnover assay and to the Δt = 60 s time point in the repriming kinetics assay. Samples were then processed for electron microscopy, and counts of clathrin-coated vesicles and synaptic vesicles in nerve terminals were performed. Numbers of clathrin-coated vesicles as a percentage of total small vesicles (clathrin-coated and noncoated) were calculated for each condition.
Fig 4.
Electron microscopy of synaptojanin 1-deficient synapses shows a persistent, stimulus-dependent increase in clathrin-coated vesicles. (a–e) Electron micrographs show tracer-labeled (HRP and HRP-WGA as indicated) recycling vesicles in control (a and c) and knockout (b, d, and e) nerve terminals after a 900-AP train (10 Hz) and 10-min recovery period. In control nerve terminals, labeled vesicles are randomly intermixed in the cluster of synaptic vesicles. In knockout nerve terminals, there is an accumulation of labeled and unlabeled clathrin-coated vesicles (circles in b) that are often segregated at the periphery of the synaptic vesicle cluster. Note the row of clathrin-coated vesicles in e. (f) Morphometry of total clathrin-coated vesicles (labeled plus unlabeled) showing the persistent accumulation of clathrin-coated vesicles after stimulation. (g) HRP-WGA-labeled vesicles make up a large fraction of the accumulated clathrin-coated vesicles in knockout neurons, confirming that they are truly endocytic. (h) Fluid-phase HRP-labeled clathrin-coated vesicles decrease after a 10-min recovery period, showing that at least some of the vesicles progress to downstream recycling steps.
Previous findings in cortical cultures from the synaptojanin 1 knockout mouse revealed an accumulation of clathrin-coated vesicles in presynaptic terminals not subjected to any exogenous stimulation (43). In agreement with these observations, pooled counts from all experiments here revealed a general increase in clathrin-coated vesicles in synaptojanin 1-deficient synapses. The 900-AP train induced an increase in clathrin-coated vesicles over resting levels in both control and knockout synapses (Fig. 4f). A striking difference, however, was observed between control and knockout synapses 10 min after the completion of the stimulus. Whereas the number of clathrin-coated vesicles returned to resting levels in control synapses, it remained elevated in knockout synapses (compare Fig. 4 a and b with f). HRP-WGA, which binds the membrane, was not efficiently removed by the washout at the beginning of the recovery and continued to label endocytic vesicles that reformed during this period. The fact that a large fraction of clathrin-coated vesicles were positive for HRP-WGA in “recovered” knockout nerve terminals confirmed their endocytic origin (Fig. 4g). Given that the absence of synaptojanin 1 did not significantly affect endocytosis kinetics, the accumulation of labeled clathrin-coated vesicles suggests a delay in clathrin uncoating. On the other hand, the fact that the number of clathrin-coated vesicles labeled by fluid-phase HRP (a probe efficiently removed by the washout) decreased after the recovery period indicated that, even in knockout synapses, the clathrin-coated vesicles do eventually uncoat (Fig. 4h).
The increase of clathrin-coated vesicles in recovered knockout synapses correlated with a spatial segregation of tracer-labeled, newly reformed vesicles from the synaptic vesicle cluster. In normal presynaptic terminals, transient recycling intermediates such as clathrin-coated pits and endosome-like cisternae were generally localized in the periphery of the synapse, but newly reformed vesicles rapidly and randomly intermixed with other vesicles in the synaptic vesicle cluster (Fig. 4 a and c). In many knockout synapses, however, tracer-labeled uncoated vesicles were more concentrated in the cytomatrix surrounding the synaptic vesicle cluster (Fig. 4 b and d). This segregation of labeled vesicles suggests a delayed incorporation of newly reformed vesicles into the functional synaptic vesicle pool.
Discussion
In synaptojanin 1-deficient neurons, the exo/endocytic turnover of synaptic vesicles after moderate stimulation is unaffected. Specifically, there is no difference in the relationship between the number of evoked APs and the fraction of the functional pool of synaptic vesicles mobilized. However, the functional synaptic vesicle pool size is 40% smaller in synaptojanin 1 knockout mice, even though levels of synaptic vesicle proteins observed biochemically are unchanged (43). Together with the recycling changes described here, the smaller vesicle pool may have contributed to the enhanced synaptic depression observed in previous studies (43, 45).
The kinetics of endocytosis after a moderate stimulus are unchanged in synaptojanin 1-deficient neurons. For the population of synaptic vesicles that recycle after 100 APs at 10 Hz, the machinery of exo/endocytosis seems to be functioning normally, suggesting that synaptojanin 1 does not play a major role in the endocytic reaction during the first rounds of recycling. A delay in the time course of endocytosis is only observed after longer AP trains (900 APs at 10 Hz; data not shown). Under those conditions where PI(4,5)P2 is persistently elevated, some endocytic proteins may fail to shed from internalized vesicles and remain sequestered on recycling intermediates, unavailable for reuse. Moreover, during prolonged stimulation, the endocytosis kinetics assay does not depend solely on the one-time internalization of a discrete cohort of synaptic vesicles. Instead, it captures multiple rounds of exo/endocytosis for a significant population of vesicles. Therefore, during prolonged stimulation, the observed time course depends not only on the rate of endocytosis but also on all of the subsequent steps in the synaptic vesicle cycle.
The most striking finding in this study is that, during prolonged high-frequency stimulation, the reformation of fusion-competent synaptic vesicles is severely impaired in synaptojanin 1-deficient neurons. In control neurons, the majority of retrieved synaptic membranes is rapidly returned to the functional pool of synaptic vesicles and proceeds to the next round of exocytosis. At the same time, a fraction of these membranes gets backed up and only reenters the functional pool after a recovery period at rest, as previously observed (52, 53). In contrast, when exposed to the same prolonged stimulus, synaptojanin 1-deficient neurons show a marked delay in the acquisition of fusion competence by the fastest recycling membranes. Thus, a much smaller fraction of retrieved membranes manages to reenter the functional synaptic vesicle pool as long as the stimulation is maintained. Previous electrophysiological studies of synaptojanin 1-deficient neurons have shown that neurotransmission continues under these conditions, albeit at a depressed level (43, 45). Therefore, it appears that a small fraction of the total synaptic vesicle pool recycled adequately to maintain some neurotransmission in knockout synapses, but that an abnormally large fraction of the recycling membrane became trapped temporarily in a functionally inert compartment in synaptojanin 1-deficient neurons. It remains to be seen whether a clathrin-independent pathway may account, at least in part, for the persistence of synaptic transmission under these stimulatory conditions.
The use of endocytic tracers and ultrastructural analysis of neurons in different functional states allowed us to correlate functional and structural changes and to gain insight into the cellular mechanisms underlying the recycling defects in synaptojanin 1-deficient nerve terminals. The persistent increase in clathrin-coated vesicles after an exocytic burst demonstrates an impairment of recovery of stimulated nerve terminals. The vast majority of these vesicles were labeled by HRP-WGA, confirming their endocytic origin and arguing against the possibility that synaptic vesicles became coated without first undergoing exocytosis. Because the endocytic reaction does not seem to be a major target of synaptojanin 1 action, these changes likely reflect delayed uncoating of clathrin-coated vesicles, as suggested by indirect evidence in our preliminary study of synaptojanin 1-deficient neurons (43).
In knockout nerve terminals, an abnormally prominent zone of dense cytomatrix was observed around synaptic vesicle clusters that was occupied by tracer-labeled recycling intermediates: clathrin-coated vesicles, deep invaginations of plasma membrane, and endosome-like cisternae. Similar morphologic changes were observed at synapses of the giant reticulospinal axon of the lamprey after microinjection of antibodies and peptides that disrupted synaptojanin recruitment (46). Studies of lamprey and mammalian synapses have demonstrated that synaptic vesicle clusters are surrounded by actin, suggesting that actin is the major component of the matrix present at these endocytic zones (28, 29, 56).
The disruption of the normal balance between PI(4,5)P2 synthesis and catabolism after synaptic activation may lie at the heart of the functional and morphologic changes observed in synaptojanin 1-deficient neurons. Based on our present results, we propose the following hypothesis: The absence of synaptojanin 1 phosphatase function in knockout nerve terminals leads to an imbalance in the phosphorylation/dephosphorylation cycle of PI(4,5)P2. This imbalance, in turn, leads to the disruption of other cyclic reactions that are regulated by PI(4,5)P2, such as the coating/uncoating of endocytic vesicles and the polymerization/depolymerization of actin. The loss of synaptojanin 1 activity may have no effect on endocytosis kinetics because PI(4,5)P2 levels are not rate-limiting for endocytosis. However, synaptojanin 1 function appears to be critical during membrane trafficking steps downstream of endocytosis. Indeed, synaptojanin 1 is localized, at least in part, on coated recycling intermediates in nerve terminals (35, 36). The persistent presence of high PI(4,5)P2 levels on endocytic membranes may delay uncoating. Furthermore, elevated PI(4,5)P2 levels may enhance nucleation of actin at endocytic zones and lead to a hypertrophy of the cytomatrix in synaptojanin 1-deficient neurons. The severe delay in repriming observed in this study may reflect trapping of recycling membranes in this matrix and their impaired reentry into the functional pool of synaptic vesicles.
In conclusion, the findings of this study indicate that the rapid degradation of PI(4,5)P2 by synaptojanin 1 is of critical importance for efficient synaptic vesicle regeneration and for the recovery of normal presynaptic function after an exocytic burst. In the absence of synaptojanin 1, sustained activity leads to a kinetic delay in synaptic vesicle reformation and to an increased, transient backup of synaptic membrane. This study provides direct evidence for the hypothesis that synaptojanin 1 plays a key physiologic role in the transition from early endocytic compartments to newly reformed synaptic vesicles fully incorporated into the functional pool. In a larger context, these results provide new evidence for a critical role of phosphoinositides in synaptic physiology and for their importance in regulating membrane traffic in the presynaptic terminal.
Acknowledgments
We thank Stephen J. Smith, Alberto Bacci, Scott Floyd, and Vladimir Slepnev for their generous advice and assistance at various stages in the study. This work was supported in part by National Institutes of Health Grants NS 36251 and CA46128 (to P.D.C.). S.C. was a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation.
Abbreviations
AP, action potential
HRP, horseradish peroxidase
HRP-WGA, HRP-conjugated wheat germ agglutinin
References
- 1.Heuser J. E. & Reese, T. S. (1973) J. Cell Biol. 57, 315-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceccarelli B., Hurlbut, W. P. & Mauro, A. (1973) J. Cell Biol. 57, 499-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cremona O. & De Camilli, P. (1997) Curr. Opin. Neurobiol. 7, 323-330. [DOI] [PubMed] [Google Scholar]
- 4.Marsh M. & McMahon, H. T. (1999) Science 285, 215-220. [DOI] [PubMed] [Google Scholar]
- 5.Brodin L., Low, P. & Shupliakov, O. (2000) Curr. Opin. Neurobiol. 10, 312-320. [DOI] [PubMed] [Google Scholar]
- 6.Fesce R., Grohovaz, F., Valtorta, F. & Meldolesi, J. (1994) Trends Cell Biol. 4, 1-4. [DOI] [PubMed] [Google Scholar]
- 7.Klingauf J., Kavalali, E. T. & Tsien, R. W. (1998) Nature 394, 581-585. [DOI] [PubMed] [Google Scholar]
- 8.Stevens C. F. & Williams, J. H. (2000) Proc. Natl. Acad. Sci. USA 97, 12828-12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakharenko S., Zablow, L. & Siegelbaum, S. (2002) Neuron 35, 1099. [DOI] [PubMed] [Google Scholar]
- 10.Cremona O. & De Camilli, P. (2001) J. Cell Sci. 114, 1041-1052. [DOI] [PubMed] [Google Scholar]
- 11.Martin T. F. (1998) Annu. Rev. Cell Dev. Biol. 14, 231-264. [DOI] [PubMed] [Google Scholar]
- 12.Osborne S. L., Meunier, F. A. & Schiavo, G. (2001) Neuron 32, 9-12. [DOI] [PubMed] [Google Scholar]
- 13.Sechi A. S. & Wehland, J. (2000) J. Cell Sci. 113, 3685-3695. [DOI] [PubMed] [Google Scholar]
- 14.Gaidarov I. & Keen, J. H. (1999) J. Cell Biol. 146, 755-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins B. M., McCoy, A. J., Kent, H. M., Evans, P. R. & Owen, D. J. (2002) Cell 109, 523-535. [DOI] [PubMed] [Google Scholar]
- 16.Rohde G., Wenzel, D. & Haucke, V. (2002) J. Cell Biol. 158, 209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford M. G., Pearse, B. M., Higgins, M. K., Vallis, Y., Owen, D. J., Gibson, A., Hopkins, C. R., Evans, P. R. & McMahon, H. T. (2001) Science 291, 1051-1055. [DOI] [PubMed] [Google Scholar]
- 18.Mao Y., Chen, J., Maynard, J. A., Zhang, B. & Quiocho, F. A. (2001) Cell 104, 433-440. [DOI] [PubMed] [Google Scholar]
- 19.Itoh T., Koshiba, S., Kigawa, T., Kikuchi, A., Yokoyama, S. & Takenawa, T. (2001) Science 291, 1047-1051. [DOI] [PubMed] [Google Scholar]
- 20.Ford M. G., Mills, I. G., Peter, B. J., Vallis, Y., Praefcke, G. J., Evans, P. R. & McMahon, H. T. (2002) Nature 419, 361-366. [DOI] [PubMed] [Google Scholar]
- 21.Zheng J., Cahill, S. M., Lemmon, M. A., Fushman, D., Schlessinger, J. & Cowburn, D. (1996) J. Mol. Biol. 255, 14-21. [DOI] [PubMed] [Google Scholar]
- 22.Barylko B., Binns, D., Lin, K. M., Atkinson, M. A., Jameson, D. M., Yin, H. L. & Albanesi, J. P. (1998) J. Biol. Chem. 273, 3791-3797. [DOI] [PubMed] [Google Scholar]
- 23.Thompson H. M. & McNiven, M. A. (2001) Curr. Biol. 11, R850. [DOI] [PubMed] [Google Scholar]
- 24.Sever S., Damke, H. & Schmid, S. L. (2000) Traffic 1, 385-392. [DOI] [PubMed] [Google Scholar]
- 25.Rohatgi R., Ma, L., Miki, H., Lopez, M., Kirchhausen, T., Takenawa, T. & Kirschner, M. W. (1999) Cell 97, 221-231. [DOI] [PubMed] [Google Scholar]
- 26.Takenawa T. & Itoh, T. (2001) Biochim. Biophys. Acta 1533, 190-206. [DOI] [PubMed] [Google Scholar]
- 27.Morales M., Colicos, M. A. & Goda, Y. (2000) Neuron 27, 539-550. [DOI] [PubMed] [Google Scholar]
- 28.Dunaevsky A. & Connor, E. A. (2000) J. Neurosci. 20, 6007-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shupliakov O., Bloom, O., Gustafsson, J. S., Kjaerulff, O., Löw, P., Tomilin, N., Pieribone, V. A., Greengard, P. & Brodin, L. (2002) Proc. Natl. Acad. Sci. USA 99, 14476-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qualmann B. & Kelly, R. B. (2000) J. Cell Biol. 148, 1047-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee E. & De Camilli, P. (2002) Proc. Natl. Acad. Sci. USA 99, 161-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orth J. D., Krueger, E. W., Cao, H. & McNiven, M. A. (2002) Proc. Natl. Acad. Sci. USA 99, 167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merrifield C. J., Feldman, M. E., Wan, L. & Almers, W. (2002) Nat. Cell Biol. 4, 691-698. [DOI] [PubMed] [Google Scholar]
- 34.Hussain N. K., Jenna, S., Glogauer, M., Quinn, C. C., Wasiak, S., Guipponi, M., Antonarakis, S. E., Kay, B. K., Stossel, T. P., Lamarche-Vane, N. & McPherson, P. S. (2001) Nat. Cell Biol. 3, 927-932. [DOI] [PubMed] [Google Scholar]
- 35.McPherson P. S., Takei, K., Schmid, S. L. & De Camilli, P. (1994) J. Biol. Chem. 269, 30132-30139. [PubMed] [Google Scholar]
- 36.Haffner C., Takei, K., Chen, H., Ringstad, N., Hudson, A., Butler, M. H., Salcini, A. E., Di Fiore, P. P. & De Camilli, P. (1997) FEBS Lett. 419, 175-180. [DOI] [PubMed] [Google Scholar]
- 37.de Heuvel E., Bell, A. W., Ramjaun, A. R., Wong, K., Sossin, W. S. & McPherson, P. S. (1997) J. Biol. Chem. 272, 8710-8716. [DOI] [PubMed] [Google Scholar]
- 38.Ringstad N., Nemoto, Y. & De Camilli, P. (1997) Proc. Natl. Acad. Sci. USA 94, 8569-8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos J. & Kelly, R. B. (1998) J. Biol. Chem. 273, 19108-19119. [DOI] [PubMed] [Google Scholar]
- 40.Qualmann B., Roos, J., DiGregorio, P. J. & Kelly, R. B. (1999) Mol. Biol. Cell 10, 501-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modregger J., Ritter, B., Witter, B., Paulsson, M. & Plomann, M. (2000) J. Cell Sci. 113, 4511-4521. [DOI] [PubMed] [Google Scholar]
- 42.Guo S., Stolz, L. E., Lemrow, S. M. & York, J. D. (1999) J. Biol. Chem. 274, 12990-12995. [DOI] [PubMed] [Google Scholar]
- 43.Cremona O., Di Paolo, G., Wenk, M. R., Luthi, A., Kim, W. T., Takei, K., Daniell, L., Nemoto, Y., Shears, S. B., Flavell, R. A., et al. (1999) Cell 99, 179-188. [DOI] [PubMed] [Google Scholar]
- 44.Woscholski R., Finan, P. M., Radley, E., Totty, N. F., Sterling, A. E., Hsuan, J. J., Waterfield, M. D. & Parker, P. J. (1997) J. Biol. Chem. 272, 9625-9628. [DOI] [PubMed] [Google Scholar]
- 45.Luthi A., Di Paolo, G., Cremona, O., Daniell, L., De Camilli, P. & McCormick, D. A. (2001) J. Neurosci. 21, 9101-9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gad H., Ringstad, N., Low, P., Kjaerulff, O., Gustafsson, J., Wenk, M., Di Paolo, G., Nemoto, Y., Crun, J., Ellisman, M. H., et al. (2000) Neuron 27, 301-312. [DOI] [PubMed] [Google Scholar]
- 47.Harris T. W., Hartwieg, E., Horvitz, H. R. & Jorgensen, E. M. (2000) J. Cell Biol. 150, 589-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer-Kruger B., Nemoto, Y., Daniell, L., Ferro-Novick, S. & De Camilli, P. (1998) J. Cell Sci. 111, 3347-3356. [DOI] [PubMed] [Google Scholar]
- 49.Stefan C. J., Audhya, A. & Emr, S. D. (2002) Mol. Biol. Cell 13, 542-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srinivasan S., Seaman, M., Nemoto, Y., Daniell, L., Suchy, S. F., Emr, S., De Camilli, P. & Nussbaum, R. (1997) Eur. J. Cell Biol. 74, 350-360. [PubMed] [Google Scholar]
- 51.Betz W. J. & Bewick, G. S. (1992) Science 255, 200-203. [DOI] [PubMed] [Google Scholar]
- 52.Ryan T. A. & Smith, S. J. (1995) Neuron 14, 983-989. [DOI] [PubMed] [Google Scholar]
- 53.Ryan T. A., Smith, S. J. & Reuter, H. (1996) Proc. Natl. Acad. Sci. USA 93, 5567-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mundigl O., Matteoli, M., Daniell, L., Thomas-Reetz, A., Metcalf, A., Jahn, R. & De Camilli, P. (1993) J. Cell Biol. 122, 1207-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stieber A., Erulkar, S. D. & Gonatas, N. K. (1989) Brain Res. 495, 131-139. [DOI] [PubMed] [Google Scholar]
- 56.Roos J. & Kelly, R. B. (1999) Curr. Biol. 9, 1411-1414. [DOI] [PubMed] [Google Scholar]