Abstract
The hypothalamic arcuate nucleus is involved in the control of energy intake and expenditure and may participate in the pathogenesis of eating disorders such as anorexia nervosa (AN) and bulimia nervosa (BN). Two systems are of particular interest in this respect, synthesizing α-melanocyte-stimulating hormone (α-MSH) and synthesizing neuropeptide Y, respectively. We report here that 42 of 57 (74%) AN and/or BN patients studied had in their plasma Abs that bind to melanotropes and/or corticotropes in the rat pituitary. Among these sera, 8 were found to bind selectively to α-MSH-positive neurons and their hypothalamic and extrahypothalamic projections as revealed with immunostaining on rat brain sections. Adsorption of these sera with α-MSH peptide abolished this immunostaining. In the pituitary, the immunostaining was blocked by adsorption with α-MSH or adrenocorticotropic hormone. Additionally, 3 AN/BN sera bound to luteinizing hormone-releasing hormone (LHRH)-positive terminals in the rat median eminence, but only 2 of them were adsorbed with LHRH. In the control subjects, 2 of 13 sera (16%) displayed similar to AN/BN staining. These data provide evidence that a significant subpopulation of AN/BN patients have autoantibodies that bind to α-MSH or adrenocorticotropic hormone, a finding pointing also to involvement of the stress axis. It remains to be established whether these Abs interfere with normal signal transduction in the brain melanocortin circuitry/LHRH system and/or in other central and peripheral sites relevant to food intake regulation, to what extent such effects are related to and/or could be involved in the pathophysiology or clinical presentation of AN/BN, and to what extent increased stress is an important factor for production of these autoantibodies.
Anorexia nervosa (AN) and bulimia nervosa (BN) are two officially recognized eating disorders that affect ≈3% of women during their lifetime (1). Both illnesses usually make their debut at young age and are characterized by hyperactivity and exaggerated concern about body shape and weight, and they often occur in the same patients (2). AN is manifested by an aversion to food, often resulting in life-threatening weight loss and amenorrhea, whereas BN includes large uncontrolled eating episodes followed by compensatory vomiting without significant change in body weight. Even if the cause(s) of AN and BN is still unclear, a body of data exists suggesting a primary neurobiological origin (3), and neuropeptides also have been implicated in these disorders (4). These assumptions are paralleled by growing evidence for a role of hypothalamic peptidergic neurons in conditions associated with energy deprivation or energy excess, providing a concept for central mechanisms controlling food intake and body weight (5–7). In a search of possible mechanisms implicating hypothalamic peptidergic neurons in the etiology and pathogenesis of AN/BN, we hypothesized that hypothalamic systems responsible for the regulation of food intake could be targeted by autoantibodies in AN/BN patients as shown for several other neurological diseases (8). To test our hypothesis, we used immunohistochemistry to explore the possibility that sera from AN/BN patients contain Abs that bind to epitopes present in the rat hypothalamus and pituitary.
Materials and Methods
Human Sera.
Sera from 57 female patients (ages 17–42) with eating disorders, diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Ed. (DSM-IV; ref. 40), were used in this study. Among them 28 AN patients (average body weight ± SD, 39.4 ± 6.2 kg), 22 BN patients (66.1 ± 25 kg), and seven patients with combination of both AN and BN (47.1 ± 1.4 kg) were diagnosed. Sera from 13 healthy female volunteers (age 20–41, 64.7 ± 5.6 kg) served as control.
Immunohistochemistry.
Sprague–Dawley male rats (body weight 200–250 g; B & K Universal, Sollentuna, Sweden) were housed under controlled environmental conditions with a constant light-dark cycle (light on between 6 a.m. and 6 p.m.), a temperature of 21–22°C, and a relative humidity of 40–50%; food and water were given ad libitum. To detect peptides/proteins more readily in neuronal cell bodies, we blocked centrifugal axonal transport in some rats by injection of colchicine (120 μg in 20 μl of 0.9% NaCl) into the brain lateral ventricle under anesthesia with a mixture of 1 ml of Midasolam (5 mg/ml) and 1 ml of Hypnorm (2.7 ml/kg), both given i.p. After colchicine injection (24 h), rats were anaesthetized with sodium pentobarbital (0.15 mg/100 g body weight, i.p.) and perfused via the ascending aorta with Tyrode's Ca2+-free solution at 37°C, followed by a mixture of 4% paraformaldehyde and 0.4% picric acid in 0.16 M phosphate buffer (pH 6.9, 37°C)∥ and then by the same, but ice-cold, mixture. The brains and pituitaries were rapidly dissected out, immersed in the same fixative for 90 min, and rinsed with 10% sucrose in 0.1 M phosphate buffer (pH 7.4) overnight. Tissue was snap-frozen by using solid CO2. Coronal brain and pituitary sections (14 μm thick) were cut on a cryostat (Microm, Heidelberg, Germany) and thaw-mounted on chrome alum-gelatin-coated glass slides.
The tyramide signal amplification immunohistochemical technique (9) was used for single labeling. Incubation with AN/BN or control sera (1:200–1:5,000) overnight at 4°C was followed by horseradish peroxidase-conjugated, donkey anti-human IgG (1:500, Dako) using the Tyramide signal amplification-plus fluorescein system (DuPont/NEN). For double-labeling, the tyramide signal amplification technique was followed by conventional immunohistochemistry (10) with primary rabbit antisera against α-melanocyte-stimulating hormone (αMSH, 1:400; Chemicon) and cocaine- and amphetamine-regulated transcript (CART) peptide (1:400; Phoenix Pharmaceuticals, St. Joseph, MO) or with mouse Abs against adrenocorticotropic hormone (ACTH) (1:500, Peninsula Laboratories) and luteinizing hormone-releasing hormone (LHRH) (1:50, Biogenesis, Kingston, NH). Secondary Abs conjugated with rhodamine red were used at 1:50 dilution (Jackson ImmunoResearch). The specificity of the binding was tested by preadsorption of human sera (1:1,000–1:5,000) with α-MSH, ACTH, β-endorphin, or LHRH (10−6 and 10−5 M) purchased from Bachem. Sections were mounted in a mixture of glycerol and 0.1 M PBS (3:1), pH 7.4, containing 0.1% para-phenylenediamine (Sigma) as antifading agent (11). After processing, the sections were examined in a Bio-Rad Radiance Plus confocal laser scanning system, installed on a Nikon Eclipse E600 fluorescence microscope. Digital images resulting from the confocal scanning microscopy were optimized for image resolution by using Adobe photoshop 6.0 (Adobe Systems, Mountain View, CA).
Results
We found that 42 of 57 (74%) AN and/or BN patients studied had in their plasma Abs that bind to melanotropes and/or corticotropes in the rat pituitary. Among these sera, 8 of 42 (20%) were found to bind selectively to α-MSH-positive neurons and their projections in the rat brain.
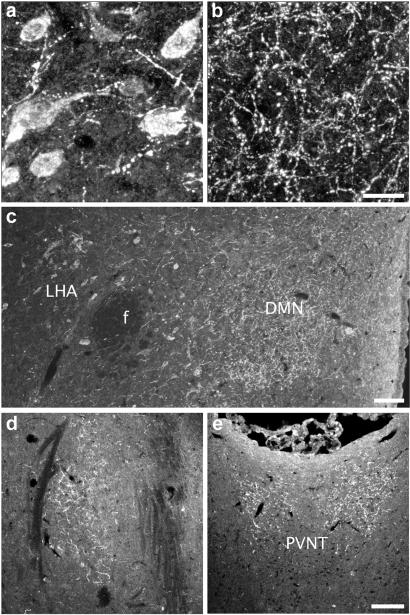
After incubation of sections of colchicine-treated rat brain with patient sera, a selective staining was found with the following eight sera: AN, five sera; AN/BN, two sera; and BN, one serum. Thus, a strong immunoreactivity was detected in neuronal somata (Fig. 1a) in the lateral part of the arcuate nucleus and in neuronal processes (Fig. 1b) distributed over hypothalamic and extrahypothalamic sites. This pattern was similar to the previously described distribution of the arcuate pro-opiomelanocortin (POMC) neurons and their projections (see ref. 12). Using serum from the BN patient with the most distinct staining, immunopositive varicose fibers were found in the hypothalamic periventricular region and dorsomedial nucleus (Fig. 1c) as well as in the amygdala (Fig. 1d) and the paraventricular thalamic nucleus (Fig. 1e). Additionally, this serum resulted in weak immunostaining of cell bodies in the lateral hypothalamic area (Fig. 1c). We could not see any distinct differences between AN and BN sera immunoreactivity. In 17 sera a distinct staining of tanycytes in the median eminence was observed. Among the sera from control subjects, two sera displayed peptide-like immunostaining similar to the one described above.
Fig. 1.
Rat brain sections show binding of serum from a BN patient to neuronal cell bodies in the arcuate nucleus (a) and to varicose fibers in the dorsomedial nucleus (DMN) (b and c). Immunopositive cell bodies are also observed in the lateral hypothalamic area (LHA) (c). Immunopositive fibers are found in extrahypothalamic areas, such as the amygdala (d) and paraventricular thalamic nucleus (PVNT) (e). f, fornix. [Scale bars = 20 μm (a and b) and 100 μm (c–e).]
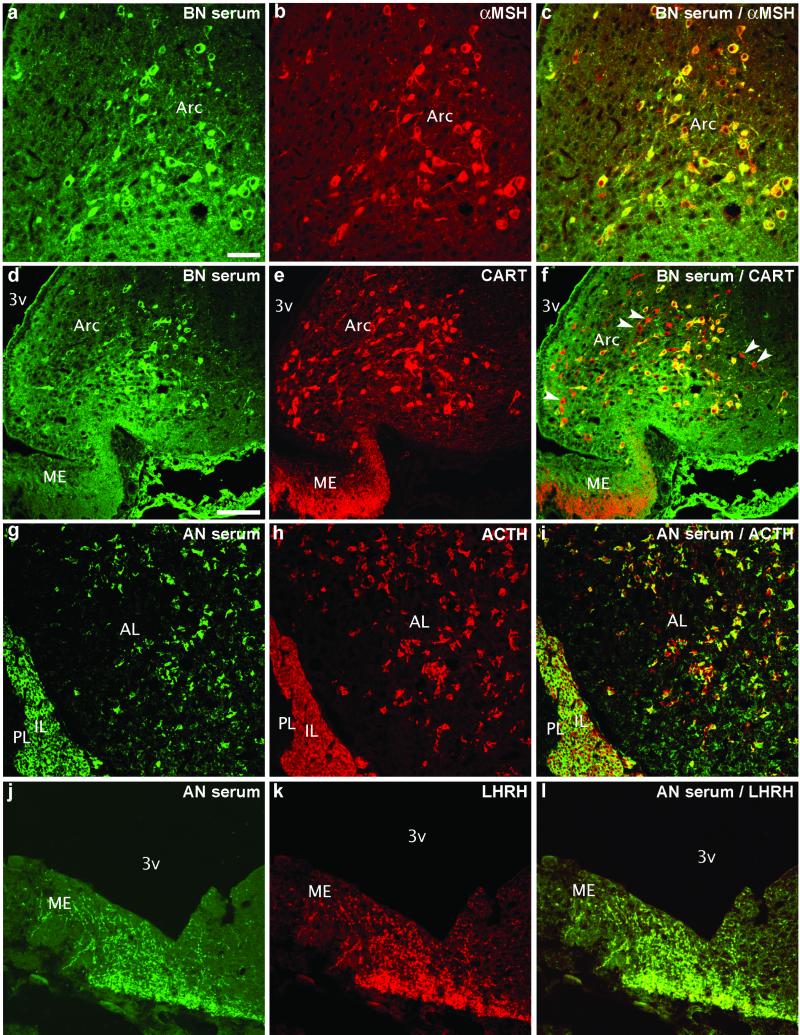
To determine the neurochemical phenotype of neurons that were labeled with AN/BN sera, we used double-immunohistochemistry for α-MSH or CART, which are normally colocalized in arcuate nucleus neurons (13). The results showed a total overlap of AN/BN sera-immunoreactive cells and terminals with α-MSH-positive neurons (Fig. 2 a–c). CART immunostaining showed that most of the arcuate CART-positive neurons were also positive for AN/BN sera. However, several CART-positive neurons and a dense network of CART-positive terminals in the external layer of the median eminence were AN/BN sera-negative (Fig. 2 d–f), thereby excluding CART as a possible antigen targeted by the human sera.
Fig. 2.
Double-staining with a BN serum shows complete colocalization with α-MSH (a–c) and partial colocalization with CART peptide (d–f) in the arcuate nucleus (Arc). However, some CART-positive/BN-negative cells (arrowheads) are seen in this nucleus and in terminals in the median eminence (ME) (d–f). In the pituitary, AN serum stains melanotropes in the intermediate lobe (IL) and corticotropes in the anterior lobe (AL) as revealed with ACTH double-staining (g–i). Colocalization of AN serum immunostaining with LHRH in fibers in the ME (j–l). PL, posterior pituitary lobe; 3v, third ventricle. [Scale bars = 50 μm (a–c and g–l) and 100 μm (d–f).]
Incubation of rat pituitary sections with sera from AN/BN patients yielded a strong immunostaining of melanotropes in the intermediate lobe and corticotropes in the anterior lobe (Fig. 2 g–i). This was seen in 74% of the sera. However, only eight of these sera resulted in the neuronal immunostaining in the brain described above. Three AN sera bound to terminals in the lateral median eminence, which by double staining with mAbs were identified as LHRH terminals (Fig. 2 j–k).
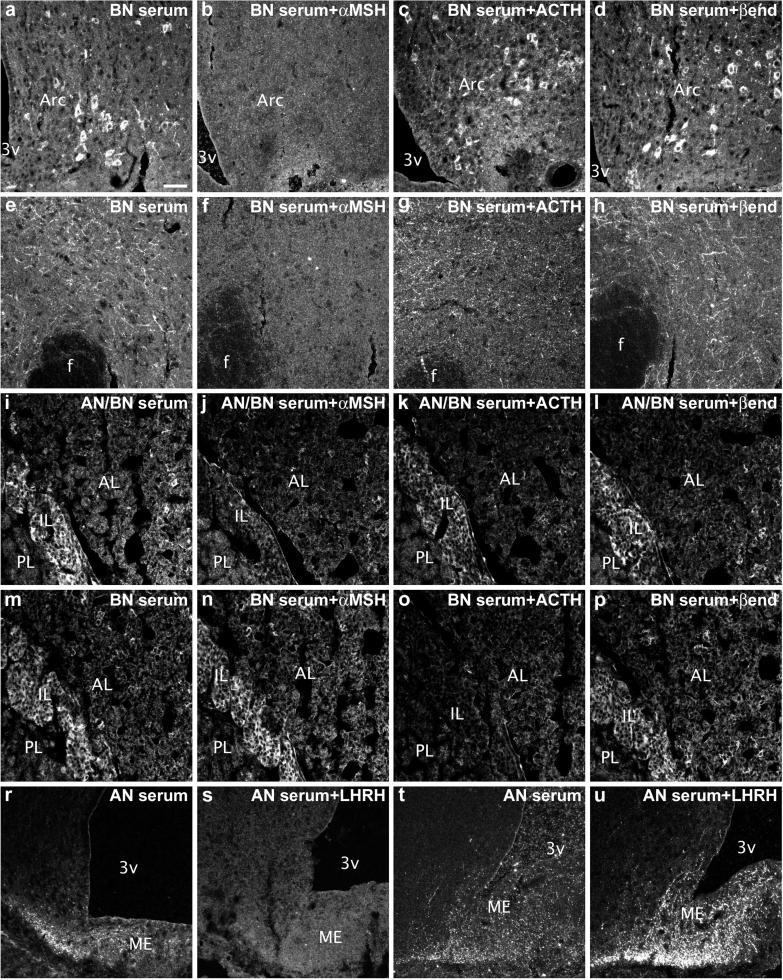
To determine which antigen is targeted by AN/BN sera in ACTH-positive neurons as well as in melanotropes and corticotropes, we used adsorption test with different peptides present in the POMC precursor, including α-MSH, ACTH, or β-endorphin. α-MSH at both 10−6 and 10−5 M completely abolished AN/BN sera-positive staining in the cell bodies and nerve terminals in the brain (compare Fig. 3 b and f to a and e). Adsorption test with β-endorphin did not result in a detectable change in intensity of the staining (Fig. 3 d and h), whereas staining after ACTH adsorption at most appeared somewhat weaker (Fig. 3c). In the pituitary intermediate lobe, immunostaining was reduced by adsorption with 10−5 M α-MSH (compare Fig. 3 j to i, k, and l). However, with some sera, immunostaining in both melanotropes and corticotropes was reduced only by adsorption with 10−5 M ACTH (compare Fig. 3 o to m, n, and p). Adsorption of sera that stained LHRH terminals with LHRH resulted in reduction of immunostaining for two sera (compare Fig. 3 s to r), whereas one serum displayed no reduction but rather an increase of immunostaining after adsorption with the peptide (compare Fig. 3 u to t).
Fig. 3.
Adsorption tests of a BN serum with POMC-derived peptides (α-MSH, b and f; ACTH, c and g; β-endorphin, d and h) show a complete blockade of immunostaining in both cell bodies (compare b and a) and terminals (compare f and e) only when α-MSH was used. Adsorption test of AN/BN serum with POMC-derived peptides in the pituitary shows a reduction of immunostaining by use of α-MSH (i–l) and by use of ACTH in a BN serum (m–p). Adsorption with LHRH completely abolishes staining of LHRH-like fibers in the median eminence (ME) in one AN serum (r and s), but not in another one (t and u). Arc, arcuate nucleus; f, fornix; AL, IL, or PL, anterior, intermediate, or posterior pituitary lobe, respectively. 3v, third ventricle. [Scale bar = 50 μm (a–u).]
Discussion
In the present work we found that ≈74% (42 of 57) of sera from AN/BN patients bind to pituitary melanotropes and corticotropes, including ≈20% (8 of 42), which stained hypothalamic α-MSH-expressing neurons and their projections. We also found that staining in the brain was completely blocked by α-MSH peptide, and in pituitary melanotropes and corticotropes it was reduced by either α-MSH or ACTH peptide, suggesting that a significant subset of AN/BN sera contain α-MSH and/or ACTH autoantibodies. Because α-MSH represents the first 13 aa of ACTH, it is not surprising that autoantibodies may recognize both or preferentially one of these two molecules. The fact that some AN/BN sera also weakly stained neurons in the lateral hypothalamus, which normally do not express α-MSH or ACTH, could be explained by the fact that these cells express melanin-concentrating hormone, the precursor of which is known to have epitopes that cross react with α-MSH Abs (14).
Because the melanocortin system seems to play an important role in the regulation of food intake and body weight (refs. 15–18; see refs. 19–23), our data raise the possibility that the disturbance in food intake observed in AN/BN could involve an autoimmune process against α-MSH/ACTH in a subpopulation of patients. The function of the melanin-concentrating hormone precursor (14) is currently unknown; however, it can perhaps not be excluded that the melanin-concentrating hormone system could also be affected by an autoimmune process in patients who have α-MSH-Abs and may therefore contribute to feeding abnormalities.
If and how autoantibodies to α-MSH/ACTH could interfere with signaling in the melanocortinergic pathway in vivo is obscure. However, one can envisage several scenarios. First, because the medial part of the arcuate nucleus is outside the blood–brain barrier, α-MSH/ACTH autoantibodies may access arcuate food intake regulatory neurons such as neuropeptide Y neurons and the dendrites of POMC neurons and disrupt interarcuate melanocortin signaling at the MC3 receptor, which is expressed on both neuropeptide Y and POMC neurons (24). Second, α-MSH/ACTH autoantibodies could interfere with signaling on the MC4 receptor, another melanocortin receptor that is involved in the regulation of body weight (17), by penetration through the blood–brain barrier. This barrier does not always seem to prevent Abs from reaching their targets in the brain, as reflected in a number of neurological autoimmune diseases (see ref. 8). A third possibility is that α-MSH/ACTH autoantibodies may block α-MSH function to suppress production of cytokines such as IL-1 and tumor necrosis factor-α (see ref. 25), which are potent inhibitors of food intake (see ref. 26). Considering that POMC and its products are also expressed in leukocytes (27), both central and peripheral-impaired melanocortin signaling by α-MSH/ACTH autoantibodies may trigger anorectic (28) and hypothalamo-pituitary-adrenal axis stimulatory (29) actions of cytokines. A final possibility could be related to a previously reported common feature of α-MSH, ACTH, as well as LHRH to bind serotonin (30). Occupation of such serotonin-binding sites by autoantibodies could potentially lead to interference with serotonin action and consequently to increased serotonin concentration, which in fact has been found in plasma of AN patients (31). Furthermore, dysregulation of the central serotonergic system is a common feature in AN/BN patients (see ref. 32).
The origin of α-MSH/ACTH autoantibodies in AN/BN patients has not been identified. A host of mechanisms by which tolerance might be broken has been proposed to explain autoimmunity in neurological diseases (see ref. 8). For instance, this could be related to the concept of molecular mimicry, whereby the specific immune response triggered by an invading pathogen results in propagation of T cell clones or Abs that crossreact by happenstance with self tissue (33). This concept also has been postulated to be relevant to pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection, in which antistreptococcal Abs react with basal ganglia proteins (34), and which can be also accompanied by anorexia (35). Conversely, in 27% of AN patients with symptoms of obsessive-compulsive disorder, Abs against human putamen have been identified (36). However, because α-MSH neurons do not project to the corpus striatum, these findings could be relevant to a specific subgroup of AN distinct from the one discussed here. Another possibility for the induction of autoimmunity in AN can be related to genetic polymorphism, and several mutations of the POMC gene in AN have been identified (37). Interestingly, serotonin-binding sites on α-MSH/ACTH and LHRH were also found to bind muramyl dipeptide, a component of Freund's adjuvant (38).
It is also possible that α-MSH/ACTH autoantibodies appear as a result of concomitant activation of the hypothalamo-pituitary-adrenal axis and the immune system. In fact, the activation of the hypothalamo-pituitary-adrenal axis is one characteristic feature of AN (see ref. 40). Of interest, the two control subjects carrying α-MSH Abs both had supranormal plasma cortisol levels. These data also suggest that the presence of α-MSH/ACTH autoantibodies does not necessarily imply the presence of AN or BN. In fact, severe stress may represent a common denominator for production of such antibodies. It will therefore be necessary to analyze sera of a number of other, especially stress-related, disorders with regard to autoantibodies, as well as a group of apparently healthy subjects much larger than studied here.
In conclusion, we have demonstrated that subpopulations of AN/BN patient sera have α-MSH/ACTH or LHRH Abs. To what extent these Abs can target the central melanocortinergic pathway/the neuroendocrine LHRH loop and contribute to the development AN/BN remains to be established, as does the mechanism underlying this immune response.
Acknowledgments
Special thanks are forwarded to the Uppsala Centre for Eating Disorders (Adults), Uppsala University Hospital (Uppsala, Sweden). This study was supported by the Swedish Medical Research Council (04X-2887; 03X-10350), Marianne and Marcus Wallenberg's Foundation, Knut and Alice Wallenberg's Foundation, Torsten and Ragnar Söderberg's Foundations, an Unrestricted Bristol-Myers Squibb Neuroscience Grant, and the European Union (QLG1-CT-2001-01521). S.O.F. was supported by the Wenner-Gren Foundations and Tore Nilsons Stiftelse för Medicinsk Forskning.
Abbreviations
α-MSH, α-melanocyte-stimulating hormone
AN, anorexia nervosa
BN, bulimia nervosa
LHRH, luteinizing hormone-releasing hormone
ACTH, adrenocorticotropic hormone
CART, cocaine- and amphetamine-regulated transcript
POMC, pro-opiomelanocortin
Zamboni, L. & De Martino, C. (1967) J. Cell Biol. 35, 148A (abstr.).
References
- 1.Walsh B. T. & Devlin, M. J. (1998) Science 280, 1387-1390. [DOI] [PubMed] [Google Scholar]
- 2.Kaye W. H., Klump, K. L., Frank, G. K. & Strober, M. (2000) Annu. Rev. Med. 51, 299-313. [DOI] [PubMed] [Google Scholar]
- 3.Bergh C. & Södersten, P. (1998) Lancet 351, 1427-1429. [DOI] [PubMed] [Google Scholar]
- 4.Kaye W. H. (1996) Psychiatry Res. 62, 65-74. [DOI] [PubMed] [Google Scholar]
- 5.Elmquist J. K., Elias, C. F. & Saper, C. B. (1999) Neuron 22, 221-232. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz M. W., Woods, S. C., Porte, D., Jr., Seeley, R. J. & Baskin, D. G. (2000) Nature 404, 661-671. [DOI] [PubMed] [Google Scholar]
- 7.Broberger C. & Hökfelt, T. (2001) Physiol. Behav. 74, 669-682. [DOI] [PubMed] [Google Scholar]
- 8.Whitney K. D. & McNamara, J. O. (1999) Annu. Rev. Neurosci. 22, 175-195. [DOI] [PubMed] [Google Scholar]
- 9.Adams J. C. (1992) J. Histochem. Cytochem. 40, 1457-1463. [DOI] [PubMed] [Google Scholar]
- 10.Coons A. H. (1958) in General Cytochemical Methods, ed. Danielli, J. F. (Academic, New York), pp. 399–422.13574437
- 11.Platt J. L. & Michael, A. F. (1983) J. Histochem. Cytochem. 31, 840-842. [DOI] [PubMed] [Google Scholar]
- 12.Khachaturian H. Y., Lewis, M. E., Tsou, K. & Watson, S. J. (1985) in Handbook of Chemical Neuroanatomy: GABA and Neuropeptides in the CNS, eds. Björklund, A. & Hökfelt, T. (Elsevier, Amsterdam), Vol. 4, pp. 216–272. [Google Scholar]
- 13.Elias C. F., Lee, C., Kelly, J., Aschkenasi, C., Ahima, R. S., Couceyro, P. R., Kuhar, M. J., Saper, C. B. & Elmquist, J. K. (1998) Neuron 21, 1375-1385. [DOI] [PubMed] [Google Scholar]
- 14.Bittencourt J. C., Presse, F., Arias, C., Peto, C., Vaughan, J., Nahon, J. L., Vale, W. & Sawchenko, P. E. (1992) J. Comp. Neurol. 319, 218-245. [DOI] [PubMed] [Google Scholar]
- 15.Poggioli R., Vergoni, A. V. & Bertolini, A. (1986) Peptides 7, 843-848. [DOI] [PubMed] [Google Scholar]
- 16.Fan W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. (1997) Nature 385, 165-168. [DOI] [PubMed] [Google Scholar]
- 17.Huszar D., Lynch, C. A., Fairchild-Huntress, V., Dunmore, J. H., Fang, Q., Berkemeier, L. R., Gu, W., Kesterson, R. A., Boston, B. A., Cone, R. D., et al. (1997) Cell 88, 131-141. [DOI] [PubMed] [Google Scholar]
- 18.Hagan M. M., Rushing, P. A., Schwartz, M. W., Yagaloff, K. A., Brun, P., Woods, S. C. & Seeley, R. J. (1999) J. Neurosci. 19, 2362-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacNeil D., Howard, A., Guan, X., Fong, T., Nargund, R., Bednarek, M., Goulet, M., Weinberg, D., Strack, A., Marsh, D., et al. (2002) Eur. J. Pharmacol. 450, 93-109. [DOI] [PubMed] [Google Scholar]
- 20.Tritos N. A. & Maratos-Flier, E. (1999) Neuropeptides 33, 339-349. [DOI] [PubMed] [Google Scholar]
- 21.Adan R. A. & Gispen, W. H. (2000) Eur. J. Pharmacol. 405, 13-24. [DOI] [PubMed] [Google Scholar]
- 22.Vergoni A. V. & Bertolini, A. (2000) Eur. J. Pharmacol. 405, 25-32. [DOI] [PubMed] [Google Scholar]
- 23.Wikberg J. E., Muceniece, R., Mandrika, I., Prusis, P., Lindblom, J., Post, C. & Skottner, A. (2000) Pharmacol. Res. 42, 393-420. [DOI] [PubMed] [Google Scholar]
- 24.Bagnol D., Lu, X.-Y., Kaelin, C. B., Day, H. E. W., Ollmann, M., Gantz, I., Akil, H., Barsh, G. S. & Watson, S. J. (1999) J. Neurosci. 19, RC26., 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipton J. M. & Catania, A. (1997) Immunol. Today 18, 140-145. [DOI] [PubMed] [Google Scholar]
- 26.Plata-Salaman C. R. (2001) Int. J. Obes. Relat. Metab. Disord. 25, Suppl. 5, S48-S52. [DOI] [PubMed] [Google Scholar]
- 27.Smith E. M. & Blalock, J. E. (1981) Proc. Natl. Acad. Sci. USA 78, 7530-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uehara Y., Shimizu, H., Sato, N., Tanaka, Y., Shimomura, Y. & Mori, M. (1992) Eur. J. Pharmacol. 220, 119-122. [DOI] [PubMed] [Google Scholar]
- 29.Weiss J. M., Sundar, S. K., Cierpial, M. A. & Ritchie, J. C. (1991) Eur. J. Pharmacol. 192, 177-179. [DOI] [PubMed] [Google Scholar]
- 30.Root-Bernstein R. S. & Westall, F. C. (1984) Brain Res. Bull. 12, 425-436. [DOI] [PubMed] [Google Scholar]
- 31.Hassanyeh F. & Marshall, E. F. (1991) Acta Psychiatr. Scand. 84, 561-563. [DOI] [PubMed] [Google Scholar]
- 32.Brewerton T. D. (1995) Psychoneuroendocrinology 20, 561-590. [DOI] [PubMed] [Google Scholar]
- 33.Oldstone M. B. (1998) FASEB J. 12, 1255-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garvey M. A., Giedd, J. & Swedo, S. E. (1998) J. Child Neurol. 13, 413-423. [DOI] [PubMed] [Google Scholar]
- 35.Sokol M. S., Ward, P. E., Tamiya, H., Kondo, D. G., Houston, D. & Zabriskie, J. B. (2002) Am. J. Psychiatry 159, 1430-1432. [DOI] [PubMed] [Google Scholar]
- 36.Harel Z., Hallett, J., Riggs, S., Vaz, R. & Kiessling, L. (2001) Int. J. Eat. Disord. 29, 463-469. [DOI] [PubMed] [Google Scholar]
- 37.Hinney A., Becker, I., Heibult, O., Nottebom, K., Schmidt, A., Ziegler, A., Mayer, H., Siegfried, W., Blum, W. F., Remschmidt, H., et al. (1998) J. Clin. Endocrinol. Metab. 83, 3737-3741. [DOI] [PubMed] [Google Scholar]
- 38.Root-Bernstein R. S. & Westall, F. C. (1990) Brain Res. Bull. 25, 827-841. [DOI] [PubMed] [Google Scholar]
- 39.Stoving R. K., Hangaard, J., Hansen-Nord, M. & Hagen, C. (1999) J. Psychiatr. Res. 33, 139-152. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association, (1994) Diagnostic and Statistical Manual of Mental Disorders (Am. Psychiatric Assoc., Washington, DC).