Abstract
During mammalian spermatogenesis, male germ cells undergo a dramatic transformation, which includes a change of shape, nuclear condensation, and development of specialised structures, such as an acrosome, and a flagellum with a mitochondrial sheath. We have found a previously undescribed pharmacological approach to intervene in these events. After oral administration of the alkylated imino sugar N-butyldeoxynojirimycin (NB-DNJ) to mice, epididymal spermatozoa displayed a spectrum of abnormal head shapes, and acrosomal antigens were mostly absent or displayed irregular patterns. In addition, the mitochondria of these cells often had an aberrant morphology, and were arranged in relatively short and wide mitochondrial sheaths. The motility of the affected spermatozoa was severely impaired. After 3 weeks of administration of NB-DNJ, male mice became sterile, and regained their fertility during the fourth week off drug. The NB-DNJ-induced infertility was not associated with a reduction in the serum testosterone level. Biochemically, the capacity of imino sugars to impair spermatogenesis was associated with their potential to attenuate the biosynthesis of glucosylceramide-based sphingolipids. Our study reveals that male fertility is affected by partial glycosphingolipid depletion, or, alternatively, by a distinct as yet unidentified property that is shared by alkylated imino sugars that inhibit glucosylceramide biosynthesis. These compounds therefore may be new leads in the development of a male contraceptive, especially because NB-DNJ has already been through extensive evaluation in various mammals, including man.
The susceptibility of spermatogenesis to hormonal control has received much attention in the development of a male contraceptive (reviewed in refs. 1–4). However, other nonendocrine approaches for pharmacological intervention in the generation and maturation of male germ cells also can be considered, and would be desirable (5). Possible targets may be found among glycolipids, as these metabolites are involved in mammalian spermatogenesis.
The major glycolipid of mature mammalian spermatozoa is seminolipid, a sulfated galactoglycerolipid; it is almost exclusively present in the testes and epididymides (reviewed in ref. 6). Other glycolipids within the testes and in spermatozoa are glycosphingolipids (7, 8), which comprise the hydrophobic compound ceramide, to which one or more monosaccharides are linked. The significance of glycolipids for spermatogenesis has become evident by creating mice deficient in enzymes involved in their biosynthesis. Spermatogenesis is severely impaired in male mice lacking seminolipid (9, 10), and also in males devoid of complex gangliosides, which are sialylated glucosphingolipids (GSLs) (8); males of these knockout strains are sterile.
The first step in the biosynthetic pathways of a large number of structurally distinct GSLs, including gangliosides, is the transfer of a glucose residue to ceramide; this reaction is catalysed by a ceramide-specific glucosyltransferase, which has its active site on the cytosolic face of an early Golgi compartment. This enzyme is inhibited by the alkylated imino sugar N-butyldeoxynojirimycin (NB-DNJ), in vitro (11) and in vivo (reviewed in ref. 12). NB-DNJ can be orally administered to experimental animals over a wide dose range and is well tolerated even at high doses, effecting extensive and prolonged attenuation of GSL biosynthesis. Importantly, NB-DNJ is well tolerated in humans, and therapeutically effective in mouse models and in humans with impaired GSL degradation (12, 13). NB-DNJ has also been characterized as an inhibitor of α-glucosidases I and II (reviewed in ref. 14), which are localized in the lumen of the endoplasmic reticulum (ER), and involved in the processing of asparagine (N)-linked glycans present on glycoproteins. However, in vivo, NB-DNJ has not been found to affect N-glycan processing of the surface glycoproteins of murine splenocytes (15); it is thought that the difference in subcellular localization between α-glucosidases I/II and ceramide glucosyltransferase is responsible for the lack of effect on the glucosidases in vivo (15). The primary in vivo effect of NB-DNJ is therefore on GSL biosynthesis.
Considering the involvement of glycolipids in mammalian spermatogenesis, it was of interest to study the effects of NB-DNJ on male fertility. We have found that oral administration of this compound causes male mice to become sterile, whereas females remain fertile. The male infertility is fully reversed after drug withdrawal. We have analyzed the effects of NB-DNJ on the reproductive system of male mice, including kinetics, dose-dependence, endocrinology, and sperm morphology and motility, and suggest a putative biochemical basis for the impact of the drug on male fertility.
Materials and Methods
Compounds.
NB-DNJ was a gift from Searle/Monsanto and Oxford GlycoSciences (Abingdon, Oxfordshire, U.K.). NB-DGJ was purchased from Toronto Research Chemicals (Downsview, ON, Canada). DNJ was a gift from Synergy Pharmaceuticals (Somerset, NJ).
Treatment of Mice with Iminosugars.
C57BL/6 mice were housed under standard nonsterile conditions. The mice were provided with water ad libitum and were fed pelleted chow (expanded Rat and Mouse Chow 3, SDS, Witham, Essex, U.K.) before drug administration. To administer imino sugars, the mice were fed a diet of powdered mouse chow (expanded Rat and Mouse Chow 1, ground, SDS) containing either NB-DNJ, NB-DGJ, or DNJ. The diet and compound (both as dry solids) were mixed thoroughly, stored at room temperature, and used within 7 days of mixing.
Mating Assays.
Six-week-old male C57BL/6 mice were administered diet containing compound for 5 weeks (unless otherwise specified), after which each of them was caged with four untreated female C57BL/6 mice, and provided with standard pelleted chow. Females were at least 11 weeks old, and age-matched in each experiment. The male was removed from the females after 7 or 9 days, depending on the experiment; the females were monitored for vaginal mucous plugs, pregnancies, and, if any, litter sizes. To study any effect on female fertility six-week-old female C57BL/6 mice were treated with NB-DNJ for 5 weeks, after which each of them was caged with a nontreated age-matched male for 4 days.
Endocrinology.
Testosterone, luteinising, and follicle-stimulating hormones were measured as described (16–18).
Fluorescence Microscopy.
Freshly exised vasa deferentia were stripped, caudae epididymides were dissociated with forceps in M2 medium, and spermatozoa were allowed to disperse for 15 min. The resultant suspensions were used to prepare smears on glass slides. After drying in air for 1 h, cells were fixed in methanol for 2 min, dried, and stored at room temperature or 4°C for 1–5 days. Before immunostaining, smears were immersed in methanol for 5 min, dried, and wetted in PBS containing 0.5% BSA, and 0.15 M glycine (PBG). Slides were stained with monoclonal antibodies, either Mab18.6 (1:20 in PBG, ref. 19), or anti-sp56 (1:67 in PBG, clone 7C5, QED Bioscience, San Diego; ref. 20). Goat anti-mouse IgG (Sigma) was used as secondary antibody, in the presence of 1 mg/ml RNase A (Sigma). Nuclei were stained with propidium iodide or Hoechst dye 33342. Images were acquired with an Eclipse E800 microscope (Nikon) equipped with a confocal scanning system (Bio-Rad Radiance Plus).
Histology and Electron Microscopy.
Testes and caudae epididymides were obtained from mice perfused with 4% paraformaldehyde, 1% gluteraldehyde, kept in the fixative for 2 days, and transferred to 70% ethanol. Tissues were postfixed with 2% osmium tetroxide and embedded in araldite. Testes were sectioned at 1 μm, and stained with Toluidine Blue. Caudae were sectioned at 70 nm, stained with uranyl acetate/lead citrate, and examined with a JEOL JEM-1010 microscope at 80 kV. For scanning electron microscopy, dried smears of murine spermatozoa were prepared as described above. Cells were fixed for 20 min in 4% paraformaldehyde in PBS, transferred to 70%, 80%, and 95% ethanol, critical-point dried, gold-coated, and examined in a Hitachi S800 field emission gun scanning electron microscope (FEG-SEM) at 3kV.
Sperm Motility Analysis.
Spermatozoa were collected in prewarmed M2 medium (Sigma) by stripping vasa deferentia and by applying mild pressure to caudae epididymides; the cells were incubated at 37°C for 1 h, and videographed by using a Leica DM IRB microscope fitted with a JVC TK-1281 camera. Computer-assisted sperm motility analysis was performed by using a Hobson Sperm Tracker (Hobson Vision, Baslow, Derbyshire, U.K.) at 50 frames per second. Motility parameters of each sperm sample were measured in triplicate, collecting data from 200 sperm tracks per determination.
Statistics.
Quantitative data from multiple experimental groups were analyzed for significance by using multiple comparisons tests. One-way ANOVA with Tukey's post hoc test or nonparametric ANOVA (Kruskal–Wallis test) with Dunn's post hoc test were performed using graphpad instat version 3.0a for Macintosh (GraphPad Software, www.graphpad.com).
Results
Male Mice Become Sterile After Oral Administration of NB-DNJ.
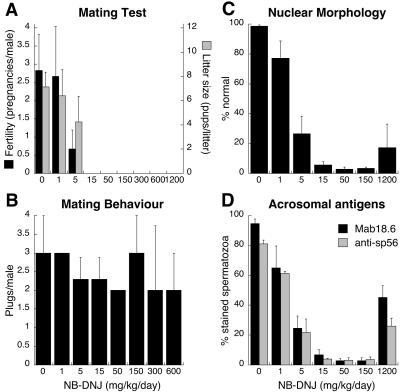
To determine the effects of NB-DNJ on the fertility of male mice, we treated 6-week-old C57BL/6 males with 2,400 mg/kg/day for increasing lengths of time, and then subjected the animals to a mating assay, during which they were caged with untreated female mice for 1 week. At this high dosage level the NB-DNJ concentration in mouse serum is 57 μM (15), because of rapid renal excretion in mice. Fig. 1A shows that untreated (0 week) males were fertile and able to sire litters with normal numbers of pups, and that this capacity was maintained after 1 and 2 weeks of NB-DNJ treatment. The mice were infertile after at least 3 weeks of drug consumption (Fig. 1A). When male mice were taken off the drug after 5 weeks of treatment, they regained fertility after 3 weeks off drug, and sired normal-sized litters that developed normally to adulthood and did not exhibit overt abnormalities (Fig. 1A).
Fig 1.
Mating tests. After drug treatment, fertility of male mice was assessed by caging each of them with four female mice for 7 (A) or 9 (B) days, during which the females were monitored for vaginal mucous plugs (see text), and subsequently for pregnancies and litter sizes. (A) Effects of oral administration and withdrawal of NB-DNJ on fertility of male mice. ON, Animals were given the compound for 1–5 weeks. OFF, Recovery of fertility was studied by using males that had received the compound for 5 weeks. These animals were further assayed for 5 weeks without drug by caging each of them each week with a new set of four female mice. (B) Comparison of effects of imino sugars with different biochemical activities on reproductive capacity of male mice. The data are presented as the mean ± SD for 3 male and 12 female mice per data point.
Biochemistry.
To find out which of the two inhibitory activities of NB-DNJ is responsible for its effects on male fertility, we treated mice with the galactose analogue N-butyldeoxygalactonojirimycin (NB-DGJ) and with the unsubstituted compound DNJ (all at 5 mmol/kg/day, equivalent to 1,200 mg/kg/day of NB-DNJ). NB-DGJ impairs GSL synthesis in a similar fashion as NB-DNJ, but does not inhibit the ER α-glucosidases (21), whereas DNJ inhibits the α-glucosidases in vitro (14) but not GSL biosynthesis (11). We found that DNJ did not affect male fertility, and that NB-DGJ consumption resulted in male sterility (Fig. 1B). Male fertility is thus affected by partial GSL depletion, or, alternatively, by a distinct as yet unidentified property that is shared by alkylated imino sugars that inhibit glucosylceramide biosynthesis.
Dose-Response.
Having established that male mice become infertile when treated with 1,200–2,400 mg/kg/day of NB-DNJ (Fig. 1), we exposed them to reduced doses of the compound, to determine the lowest dose at which it causes infertility. Although 1 mg/kg/day did not affect the males, 5 mg/kg/day significantly reduced their fertility (P < 0.01), and 15 mg/kg/day rendered them completely infertile (Fig. 2A). Thus, the male reproductive system of mice is exceptionally sensitive to NB-DNJ. The drug-induced loss of fertility was not caused by a change in mating behavior, as the frequency of postcoital vaginal mucous plugs was not affected by NB-DNJ treatment (Fig. 2B), nor by NB-DGJ (data not shown). In addition, we found that male mice treated for 6 months with 15 mg/kg/day NB-DNJ regained their fertility after cessation of drug administration (data not shown). The pups derived from these recovered mice developed normally.
Fig 2.
Dose-effect study. Males were given NB-DNJ in a range of concentrations for 5 weeks, and then caged with four female mice each for 9 days (n = 6 males in A, n = 3 males in B). The females were monitored for pregnancies and litter sizes (A) and vaginal mucous plugs (B). Alternatively, after a 5-week treatment, epididymal spermatozoa were obtained and incubated with either monoclonal antibody Mab18.6 or anti-sp56, and the nuclear dye Hoechst 33342. By examining >100 spermatozoa per mouse, we determined the percentage of nuclei with grossly abnormal morphology (n ≥ 4; C) and the percentage of spermatozoa that stain with monoclonal antibodies Mab18.6 (n = 3) or anti-sp56 (n = 2) (D). The data are presented as the mean ± SD.
Fertility of Female Mice.
In contrast to male mice, NB-DNJ treatment at 1,200 mg/kg/day did not cause female mice to become infertile. After a 4-day mating period with untreated males, 100% of treated (n = 5) as well as of nontreated females (n = 4) became pregnant; these mice had 6.2 ± 2.7 and 7.8 ± 1.0 pups per litter, respectively.
Endocrinology.
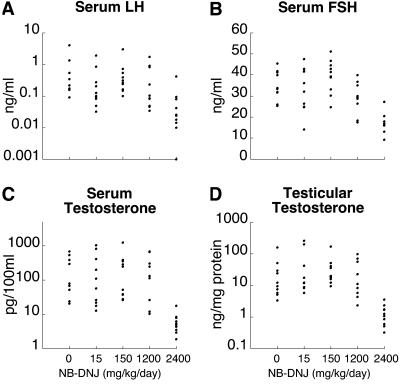
In the mouse strain that is constitutively deficient in complex gangliosides, male sterility is associated with a dramatically reduced serum testosterone level, and an elevated serum concentration of follicle-stimulating hormone (8). Therefore, we determined the serum levels of these hormones, as well as the levels of luteinizing hormone and the testicular testosterone content, in NB-DNJ-treated mice. Fig. 3 shows that 15, 150, or 1,200 mg/kg/day of the compound did not change these endocrinological parameters. In contrast, 2,400 mg/kg/day caused a significant reduction (P < 0.001) in all of these hormone levels (Fig. 3). Thus, the reproductive endocrinology of the imino sugar-treated males is distinct from that of the complex ganglioside-deficient animals. Furthermore, these results indicate that the NB-DNJ-induced infertility (requiring at least 15 mg/kg/day) is not mediated by a depression of reproductive hormones.
Fig 3.
Reproductive endocrinology. Male mice were treated with NB-DNJ at four different doses, and sera and were obtained for determination of luteinizing hormone (A), follicle-stimulating hormone (B), and testosterone (C). (D) Testes were also assayed for testosterone. Data points from individual mice are presented.
Gonad Weights and Sperm Counts.
Previously, it was reported that administration of NB-DNJ at doses of at least 600 mg/kg/day reduces the body weight of mice (15). In this study, we found that 15–150 mg/kg/day did not lower body or gonad weights, whereas 1,200–2,400 mg/kg/day significantly reduced body weight (P < 0.01) (Table 1). At these doses, most organ weights were decreased proportionally to body weight (Table 1). Relative increases in the weights of cauda epididymis/vas deferens (150 mg/kg/day) and testis (1,200 mg/kg/day) were observed, whereas cauda epididymis/vas deferens weight was reduced proportionally more than body weight at 2,400 mg/kg/day (Table 1). Thus, gonadal weight loss is an effect of a high-dose NB-DNJ regimen, and unrelated to induction of male infertility. Accordingly, body and gonad weights were unchanged after 6 months of NB-DNJ administration at 15 mg/kg/day (data not shown). Furthermore, the epididymal sperm count was unaffected at the lower doses of NB-DNJ, and decreased at 1,200 mg/kg/day (Table 1). The testicular homogenization-resistant spermatid count was slightly lower in drug-treated animals, but this was not significant (Table 1).
Table 1.
Tissue weights and sperm counts
|
NB-DNJ, mg/kg/day
|
Weights, % | Counts, 106 | ||||
|---|---|---|---|---|---|---|
| Whole body | Testis | Caput + corpus | Cauda + vas | Sperm/testis | Sperm/cauda | |
| 0 | 100.0 ± 4.6 | 100.0 ± 6.7 | 100.0 ± 6.2 | 100.0 ± 7.3 | 17.8 ± 3.7 | 22.0 ± 3.8 |
| 15 | 95.2 ± 6.2 | 91.5 ± 8.0 | 91.5 ± 23.3 | 101.4 ± 15.1 | 14.1 ± 2.2 | 22.5 ± 6.7 |
| 150 | 95.7 ± 5.3 | 93.8 ± 5.2 | 99.2 ± 6.6 | 104.8 ± 8.2 | 15.1 ± 3.1 | 29.6 ± 10.3 |
| 1,200 | 69.1 ± 6.3 | 81.5 ± 6.9 | 74.8 ± 7.8 | 63.5 ± 13 | 14.5 ± 2.9 | 10.5 ± 3.1 |
| 2,400 | 59.5 ± 4.7 | 67.8 ± 9.3 | 59.2 ± 16 | 42.7 ± 6.7 | ND | ND |
Body and tissue weights are presented as percentages of the mean body and tissue weights from untreated animals, convenient for comparison of change in tissue weights with change in body weight in treated animals. The data are presented as the mean ± SD; for weights n = 10 and for counts n = 4. ND, not determined.
Significantly different from body weight (P < 0.05).
Significantly different from values obtained from untreated mice (P < 0.05).
Testis Histology.
In the seminiferous tubules of NB-DNJ-treated mice, a more extensive vacuolization was observed, at a higher frequency, than in controls (Fig. 4 A and B). In addition, after administration of drug, the nuclei of mature spermatids displayed an aberrant, heterogeneous morphology. Fig. 4B Inset shows an example of such step 13 spermatids: they often had indentations, and were much less elongated than normal step 13 spermatids (Fig. 4A Inset) (for classification of spermatids and testicular staging see ref. 22).
Fig 4.
Histology of the testis and immunocytochemistry of epididymal spermatozoa. Sections from stage I seminiferous tubules from a control mouse (A) and a mouse treated with 50 mg/kg/day NB-DNJ (B). Insets show close-ups of step 13 spermatid heads. Epididymal spermatozoa from normal mice (C), 15 mg/kg/day NB-DNJ-treated mice (D), and mice recovered from a 15 mg/kg/day NB-DNJ regimen (E) were incubated with anti-acrosomal monoclonal Mab18.6 and counterstained with the nuclear dye propidium iodide. (Original magnification of A and B = ×1,000; bar in C–E = 10 μm.)
Morphology of Spermatozoa.
The nucleus of a normal murine epididymal spermatozoon is laterally flattened and has a typically curved, falciform shape. Overlying the nucleus is the acrosome, a relatively large membrane-delineated organelle that contains various hydrolytic enzymes and can be regarded as a specialized regulated secretory vesicle; the acrosome is essential for fertilization (reviewed in ref. 23). In agreement with the histological observations of the testis (see above), cauda epididymal sperm cells from NB-DNJ-treated mice had various abnormally shaped nuclei: they were either near-normal, triangular, oblate, or elongate, the majority being spatulate (Fig. 4D) (morphological categories as described by Hollander et al., ref. 24). Some of these nuclei had a lateral invagination close to the anterior tip (Fig. 4D). Spermatozoa derived from the caput epididymis were similarly affected (data not shown). The percentage of caudal spermatozoa with (near)-normal nuclei decreased dramatically with increasing NB-DNJ dosage, falling below 6% at the minimal dose to achieve complete infertility (Fig. 2C). Curiously, normal nuclear morphology was observed significantly more frequently (P < 0.01) at 1,200 mg/kg/day than at 50–150 mg/kg/day of NB-DNJ (Fig. 2C).
To further characterize these spermatozoa, we used indirect immunofluorescence with monoclonal antibodies Mab18.6 and anti-sp56, which recognize distinct acrosomal antigens (19, 20). Only a small fraction of the spermatozoa from NB-DNJ-treated mice stained with Mab18.6 (Fig. 4D) or anti-sp56 (data not shown), decreasing with drug dose to <10% at 15 mg/kg/day (Fig. 2D). Again, 1,200 mg/kg/day was exceptional: antibody staining at this dose was more frequent than at 15–150 mg/kg/day (Fig. 2D). However, many of the Mab18.6-positive sperm cells from drug-treated mice did not show the normal crescent-shaped acrosomal fluorescence, but displayed an irregular staining pattern (Fig. 4D). In fact, among the Mab18.6-positive cells the incidence of (near)-normal acrosomal staining was equally low from 15 to 1,200 mg/kg/day, on average 31 ± 10% (n = 3). Similar results were obtained with anti-sp56 (data not shown). Sperm samples from mice after administration of 2,400 mg/kg/day had similar characteristics as those from mice treated with 1,200 mg/kg/day (data not shown). Finally, also at the ultrastructural level, the majority of epididymal spermatozoa from drug-treated mice did not show any typical acrosomes (Fig. 5E).
Fig 5.
Morphological features of epididymal spermatozoa from normal mice (A and D), NB-DNJ-treated mice (B and E, 15 mg/kg/day), and mice recovered from a 15 mg/kg/day NB-DNJ regimen (C). (A–C) Scanning electronmicrographs, showing examples of a normal head and midpiece (A), a spatulate sperm head with a disorganized squat mitochondrial sheath (B), and a normal head and midpiece after recovery from drug treatment (C). (D and E) Transmission electronmicrographs, displaying a normal spermatozoon (D) and a spermatozoon with alterations in nuclear shape, tail orientation, and morphology and arrangement of mitochondria (E). (Bar = 2 μm.)
NB-DNJ treatment induced a number of abnormalities in the tails and mitochondria of epididymal spermatozoa. First, in a subset of the spermatozoa from NB-DNJ-treated mice, the mitochondria were found in a much shorter and wider arrangement just behind or adjacent to the sperm head (Fig. 5 B and E). The fibrous tail core structures (outer dense fibers/axoneme) of this type of cell were devoid of a mitochondrial investment over a large part of the midpiece, from the posterior end of the altered mitochondrial sheath to the beginning of the next tail segment, the principal piece. These shortened sheaths were highly disorganized: the mitochondria were irregularly arranged and stacked on top of each other; and they varied widely in size and shape (Fig. 5E).
The tail of a normal murine spermatozoon extends away from the head, and can be mildly curved. However, in a fraction of epididymal spermatozoa from NB-DNJ-treated mice, the proximal part of the tail was wound around the sperm head or nucleus, from a half (Fig. 5B) or single turn to several full loops (data not shown). Coiling of the tail in this fashion positioned the section of the tail carrying the mitochondrial sheath at least in part in the sperm head, adjacent to the nucleus (Fig. 5 B and E). Finally, drug treatment rendered spermatozoal nuclei less condensed, displaying small electronlucent foci (Fig. 5E), which were much less frequent in control cells (Fig. 5D).
Thus, NB-DNJ treatment lead to nuclear dysmorphia of spermatozoa, induced acrosomal abnormalities, and caused disorganization of the tail, including the mitochondrial sheath. A relatively low drug dose induced a very high proportion of abnormal sperm cells. Surprisingly, at the highest doses used, this effect was partially reversed, although the mice remained infertile. The galactose analogue NB-DGJ, used at 1,200 mg/kg/day, induced a similar range of morphological aberrations as seen with NB-DNJ (data not shown).
Finally, we also examined epididymal spermatozoa from mice that had been treated with 15 mg/kg/day NB-DNJ for 5 weeks, and then maintained for 5 weeks without drug administration. The germ cells from the recovered mice had a normal falciform morphology (Figs. 4E and 5C) and displayed a typical acrosomal staining pattern using Mab18.6 (Fig. 4E). Similar results were obtained with animals recovered from 2,400 mg/kg/day NB-DNJ (data not shown), in agreement with the return of fertility to these animals (Fig. 1A).
Sperm Motility.
To assess whether the structural defects of epididymal spermatozoa from NB-DNJ-treated mice had any functional consequences, we performed computer-assisted sperm motility analysis. At 15 mg/kg/day, the motile percentage was significantly below normal (P < 0.05), as were curvilinear velocity and straight line velocity (P < 0.001) (Table 2). Moreover, at 2,400 mg/kg/day, both velocities were significantly lower than at 150 mg/kg/day (P < 0.001) (Table 2). Thus, ingestion of NB-DNJ leads to a severely diminished sperm motility, which is progressive with drug dose.
Table 2.
Sperm motility
| NB-DNJ, mg/kg/day | % Motile | VCL, μm/s | VSL, μm/s |
|---|---|---|---|
| 0 | 74 ± 12 | 182 ± 19 | 52 ± 10 |
| 15 | 26 ± 16 | 74 ± 31 | 27 ± 9 |
| 150 | 15 ± 6 | 89 ± 31 | 27 ± 8 |
| 1,200 | 17 ± 7 | 49 ± 17 | 18 ± 9 |
| 2,400 | 9 ± 2 | 28 ± 21 | 9 ± 5 |
VCL, curvilinear velocity; VSL, straight line velocity. The data are presented as the mean ± SD; n ≥ 3.
, P < 0.05.
Discussion
We have observed that oral administration of an alkylated imino sugar to male mice for 5 weeks results in infertility, and that, on withdrawal of the drug, fertility is regained. On the basis of our data, we conclude that the NB-DNJ-treated males are infertile for two reasons: (i) their spermatozoa have a much reduced capacity to swim to the oviduct, and (ii) the few spermatozoa that migrate successfully are unable to fertilize oocytes. In turn, these functional deficiencies can be attributed to the morphological abnormalities of the spermatozoa; the irregular mitochondrial sheaths, aberrant attachment of tails, and deviant head shapes impair their motility, whereas the lack or malformation of the acrosomes as well as the anomalous head shapes preclude fertilization.
Our results indicate in which phase of male germ cell development the observed abnormalities are induced by NB-DNJ. Sperm counts are unaffected by the imino sugar, suggesting that NB-DNJ-treatment does not interfere with the meiotic divisions. Furthermore, it is known that the latter 15.5 days of murine spermatogenesis are needed for the postmeiotic development (spermiogenesis) (22), and that the epididymal transit time for spermatozoa is 5 days (25); these facts, combined with our data on the kinetics of onset and reversal of NB-DNJ-induced infertility, allow us to speculate that NB-DNJ acts approximately during the Golgi and cap phases (steps 1–8) of murine spermiogenesis.
This study has demonstrated that those imino sugars (NB-DNJ and NB-DGJ) that have been characterized as inhibitors of the ceramide-specific glucosyltransferase can impair spermatogenesis. The central question is whether this property is responsible for the effects of these compounds on male fertility. In a preliminary study using TLC of testicular lipid extracts, we have observed that ganglioside levels in whole mouse testes were reduced ≈60% after administration of 2,400 mg/kg/day (A.C.S., unpublished observation), comparable to 65% decrease in liver GM2 ganglioside (21). This finding demonstrates that NB-DNJ can attenuate testicular GSL metabolism and offers a candidate biochemical mechanism for NB-DNJ-induced male infertility. Alternatively, a nonlysosomal glucosylceramidase activity has been described, hydrolyzing glucosylceramide to glucose and ceramide (26), which is inhibited by both NB-DNJ and NB-DGJ (27). However, this activity has only been measured in human somatic tissues and cells, and has not been characterized at a biochemical or molecular level; its significance for GSL metabolism in male germ cells is thus unclear. Currently, GSL levels are being investigated in testes from animals treated with low doses of NB-DNJ.
Recent studies have shown that complex sialylated GSLs are essential for spermatogenesis. Male mice deficient in ganglioside GM2/GD2 synthase do not produce gangliosides more complex than GM3/GD3; their seminiferous tubules are devoid of elongating spermatids, but contain vacuolated Sertoli cells and giant multinucleate cells composed of round spermatids (8). It is proposed that the complex gangliosides are involved in testosterone transport as these mice have normal testicular testosterone production, but extremely low testosterone levels in serum (8). We have shown that the infertile phenotype of NB-DNJ-treated mice is very different from that of the latter mouse model. This finding suggests, therefore, that GSLs may have an additional role in spermatogenesis.
It is of interest that a number of distinct mouse strains with male infertility share some features with the imino sugar-treated animals. The combination of abnormally shaped sperm heads, and irregularly shaped and stacked mitochondria is seen at low frequency in normal C57BL/6 males (28), as well as in mice lacking the Golgi protein GOPC (29), or the cell surface adhesion protein nectin-2 (30), in ebouriffé (ebo) mutant mice (31), and in males of mouse strains with mutations at the pink-eyed dilution (p) locus (32). The range of aberrant head shapes seen in pink-eyed dilution males (near-normal, triangular, oblate, elongate, or spatulate) is comparable to that of NB-DNJ-treated mice (24, 33). Coiling of the sperm tail has also been observed in ebouriffé mice (31) and in the GOPC-deficient mice (29); in the latter strain the acrosome develops improperly, so that it is lacking from mature spermatozoa. At present, it is unclear whether there is a common biochemical basis underlying these superficial similarities.
Because spermatogenesis is a highly conserved process in mammals, we can speculate that NB-DNJ may also impair spermatogenesis in other mammalian species, including man, though this has yet to be demonstrated. NB-DNJ has been used in two clinical trials in humans. The drug was administered as part of an antiretroviral strategy in HIV-infected patients at 3 g/day (equivalent to ≈43 mg/kg/day), which resulted in a peak serum level of 42.7 ± 6.1 μM (34). NB-DNJ showed efficacy in the treatment of the GSL storage disorder type 1 Gaucher's disease, at 300 mg/day (13). The drug was very recently approved for clinical use for this disease in Europe.
Considering the advanced status of NB-DNJ in clinical application, this compound could be rapidly evaluated for its suitability as a male contraceptive. In man, the major side effect of NB-DNJ, depending on dosage, is osmotic diarrhea caused by inhibition of intestinal sucrase and isomaltases (13, 34). In the clinical study in Gaucher's disease the drug was well tolerated, and gastrointestinal distress was minimal and usually transient (13). This drug does not inhibit proliferation of cells, as indicated by normal bone marrow-derived circulating lymphocyte counts in the treated patients (35). As the dose required to induce male infertility in mice is very low, we can estimate that the dose needed for use as a nonhormonal contraceptive in men is at least an order of magnitude lower than used in the Gaucher's disease trial, assuming that spermatogenesis in humans is equally sensitive to NB-DNJ as in mice. It would be anticipated that side effects of the drug at this level would be negligible.
In summary, NB-DNJ and related compounds are among a small number of nonhormonal agents that can reversibly induce infertility in male mammals. The alkylated imino sugars may have promise as male contraceptives because of their oral availability, low dose action and lack of impact on male endocrinology. In addition, investigation of their effects may shed considerable light on the role of glucosphingolipids in male germ cell development.
Acknowledgments
We thank David Smith, Julia McAvoy, Carol Broadbent, Gabriele Reinkensmeier, Charlotte Walden, Mohan Masih (University of Oxford), and Autumn Ivy (Fogarty Minority International Research Training Program), Nicholas Jenkins (University of Sheffield) for excellent technical assistance; Dr. Margaret Abel, Dr. David Neville, and Dr. Helen White-Cooper (University of Oxford), and Prof. Lynn Fraser (King's College, London) for advice; and Dr. Ilpo Huhtaniemi and Dr. Pirjo Pakarinen (University of Turku, Turku, Finland) for endocrinological assays. We thank Searle/Monsanto and Oxford GlycoSciences for NB-DNJ, Synergy Pharmaceuticals for DNJ, the Medical Research Council Drosophila Cooperative Group (Department of Zoology, University of Oxford) for use of the confocal fluorescence microscope, and the Electron Microscopy and Microanalysis Group (Department of Materials, University of Oxford) for use of and assistance with the scanning electron microscope. F.M.P. is a Lister Institute Research Fellow. R.A.D., T.D.B., and F.M.P. are shareholders in Oxford GlycoSciences, as is the University of Oxford. R.A.D. is currently a nonexecutive director of Oxford GlycoSciences and is on the Board of Directors of Synergy Pharmaceuticals. R.A.D., T.D.B., F.M.P., and A.C.S. are in receipt of grants from Oxford GlycoSciences.
Abbreviations
GSL, glucosphingolipid
NB-DNJ, N-butyldeoxynojirimycin
NB-DGJ, N-butyldeoxygalactonojirimycin
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Anawalt B. D. & Amory, J. K. (2001) Ann. Med. 33, 587-595. [DOI] [PubMed] [Google Scholar]
- 2.Wang C. & Swerdloff, R. S. (2002) Best Pract. Res. Clin. Obstet. Gynaecol. 16, 193-203. [DOI] [PubMed] [Google Scholar]
- 3.McLachlan R. I., O'Donnell, L., Meachem, S. J., Stanton, P. G., de Kretser, D. M., Pratis, K. & Robertson, D. M. (2002) J. Androl. 23, 149-162. [PubMed] [Google Scholar]
- 4.Brady B. M. & Anderson, R. A. (2002) Exp. Opin. Invest. Drugs 11, 333-344. [DOI] [PubMed] [Google Scholar]
- 5.Holden C. (2002) Science 296, 2172-2173. [DOI] [PubMed] [Google Scholar]
- 6.Tanphaichitr N., White, D., Taylor, T., Attar, M., Rattanachaiyanont, M., D'Amours, D. & Kates, M. (1999) in The Male Gamete, ed. Gagnon, C. (Cache River, Vienna, IL), pp. 227–235.
- 7.Ritter G., Krause, W., Geyer, R., Stirm, S. & Wiegandt, H. (1987) Arch. Biochem. Biophys. 257, 370-378. [DOI] [PubMed] [Google Scholar]
- 8.Takamiya K., Yamamoto, A., Furukawa, K., Zhao, J., Fukumoto, S., Yamashiro, S., Okada, M., Haraguchi, M., Shin, M., Kishikawa, M., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 12147-12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto H., Tadano-Aritomi, K., Tokumasu, A., Ito, K., Hikita, T., Suzuki, K. & Ishizuka, I. (2000) J. Biol. Chem. 275, 22623-22626. [DOI] [PubMed] [Google Scholar]
- 10.Honke K., Hirahara, Y., Dupree, J., Suzuki, K., Popko, B., Fukushima, K., Fukushima, J., Nagasawa, T., Yoshida, N., Wada, Y. & Taniguchi, N. (2002) Proc. Natl. Acad. Sci. USA 99, 4227-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platt F. M., Neises, G. R., Dwek, R. A. & Butters, T. D. (1994) J. Biol. Chem. 269, 8362-8365. [PubMed] [Google Scholar]
- 12.Dwek R. A., Butters, T. D., Platt, F. M. & Zitzmann, N. (2002) Nat. Rev. Drug Discovery 1, 65-75. [DOI] [PubMed] [Google Scholar]
- 13.Cox T., Lachmann, R., Hollak, C., Aerts, J., van Weely, S., Hrebicek, M., Platt, F., Butters, T., Dwek, R., Moyses, C., et al. (2000) Lancet 355, 1481-1485. [DOI] [PubMed] [Google Scholar]
- 14.Elbein A. D. (1987) Annu. Rev. Biochem. 56, 497-534. [DOI] [PubMed] [Google Scholar]
- 15.Platt F. M., Reinkensmeier, G., Dwek, R. A. & Butters, T. D. (1997) J. Biol. Chem. 272, 19365-19372. [DOI] [PubMed] [Google Scholar]
- 16.Huhtaniemi I., Nikula, H. & Rannikko, S. (1985) J. Clin. Endocrinol. Metab. 61, 698-704. [DOI] [PubMed] [Google Scholar]
- 17.Haavisto A. M., Pettersson, K., Bergendahl, M., Perheentupa, A., Roser, J. F. & Huhtaniemi, I. (1993) Endocrinology 132, 1687-1691. [DOI] [PubMed] [Google Scholar]
- 18.van Casteren J. I., Schoonen, W. G. & Kloosterboer, H. J. (2000) Biol. Reprod. 62, 886-894. [DOI] [PubMed] [Google Scholar]
- 19.Moore H. D., Hartman, T. D., Brown, A. C., Smith, C. A. & Ellis, D. H. (1985) Exp. Clin. Immunogenet. 2, 84-96. [PubMed] [Google Scholar]
- 20.Cheng A., Le, T., Palacios, M., Bookbinder, L. H., Wassarman, P. M., Suzuki, F. & Bleil, J. D. (1994) J. Cell Biol. 125, 867-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson U., Butters, T. D., Dwek, R. A. & Platt, F. M. (2000) Biochem. Pharmacol. 59, 821-829. [DOI] [PubMed] [Google Scholar]
- 22.Russell L. D., Ettlin, R. A., Sinha Hikim, A. P. & Clegg, E. D., (1990) Histological and Histopathological Evaluation of the Testis (Cache River, Clearwater, FL).
- 23.Yanagimachi R. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. D. (Raven, New York), pp. 189–317.
- 24.Hollander W. F., Bryan, J. H. D. & Gowen, J. W. (1960) Fertil. Steril. 11, 316-324. [DOI] [PubMed] [Google Scholar]
- 25.Dadoune J. P. & Alfonsi, M. F. (1984) Reprod. Nutr. Dev. 24, 927-935. [DOI] [PubMed] [Google Scholar]
- 26.van Weely S., Brandsma, M., Strijland, A., Tager, J. M. & Aerts, J. M. (1993) Biochim. Biophys. Acta 1181, 55-62. [DOI] [PubMed] [Google Scholar]
- 27.Overkleeft H. S., Renkema, G. H., Neele, J., Vianello, P., Hung, I. O., Strijland, A., van der Burg, A. M., Koomen, G. J., Pandit, U. K. & Aerts, J. M. (1998) J. Biol. Chem. 273, 26522-26527. [DOI] [PubMed] [Google Scholar]
- 28.Hillman N. & Nadijcka, M. (1978) J. Embryol. Exp. Morphol. 44, 263-280. [PubMed] [Google Scholar]
- 29.Yao R., Ito, C., Natsume, Y., Sugitani, Y., Yamanaka, H., Kuretake, S., Yanagida, K., Sato, A., Toshimori, K. & Noda, T. (2002) Proc. Natl. Acad. Sci. USA 99, 11211-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchard M. J., Dong, Y., McDermott, B. M., Jr., Lam, D. H., Brown, K. R., Shelanski, M., Bellve, A. R. & Racaniello, V. R. (2000) Mol. Cell. Biol. 20, 2865-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalouette A., Lablack, A., Guenet, J. L., Montagutelli, X. & Segretain, D. (1996) Biol. Reprod. 55, 355-363. [DOI] [PubMed] [Google Scholar]
- 32.Hunt D. M. & Johnson, D. R. (1971) J. Embryol. Exp. Morphol. 26, 111-121. [PubMed] [Google Scholar]
- 33.Wolfe H. G., Erickson, R. P. & Schmidt, L. C. (1977) Genetics 85, 303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischl M. A., Resnick, L., Coombs, R., Kremer, A. B., Pottage, J. C., Jr., Fass, R. J., Fife, K. H., Powderly, W. G., Collier, A. C., Aspinall, R. L., et al. (1994) J. Acquired Immune Defic. Syndr. 7, 139-147. [PubMed] [Google Scholar]
- 35.Lachmann R. H. & Platt, F. M. (2001) Exp. Opin. Invest. Drugs 10, 455-466. [DOI] [PubMed] [Google Scholar]