Abstract
The accumulation of ammonia and associated tissue alkalinization predispose avocado fruit to attack by Colletotrichum gloeosporioides. Secretion of ammonia by C. gloeosporioides in the presence of KNO3 was induced by decreasing the pH from 7.0 to 4.0. When the fungus was grown at pH 4.0 or 6.0 in the absence of a nitrogen source, ammonia did not accumulate, and neither pelB (encoding pectate lyase) transcription nor pectate lyase secretion was detected. Under these nitrogen starvation conditions, only transcriptional activation of areA, which encodes the global nitrogen regulator, was detected. pelB transcription and pectate lyase secretion were both detected when C. gloeosporioides was grown at pH 6.0 in the presence of ammonia accumulated from different nitrogen sources. The early accumulation of ammonia induced early pelB expression and pectate lyase secretion. As the external pH increased from 4.0 to 6.0, transcripts of pac1, the C. gloeosporioides pacC homolog, also could be detected. Nit mutants of C. gloeosporioides, which cannot utilize KNO3 as a nitrogen source, did not secrete ammonia, alkalinize the medium, or secrete pectate lyase. If Nit mutants were grown at pH 6.0 in the presence of glutamate, then pectate lyase secretion was induced. Infiltration of 0.1 M ammonium hydroxide at pH 10 into ripening avocado fruits enhanced the activation of quiescent infection and symptom development by C. gloeosporioides. These results suggest that ambient pH alkalinization resulting from ammonia accumulation and the availability of ammonia as a nitrogen source independently regulate pelB expression, pectate lyase secretion, and virulence of C. gloeosporioides. These data suggest that alkalinization during C. gloeosporioides infection is important for its transformation from the quiescent biotrophic stage to the necrotrophic stage of fungal colonization in the fruit host.
Colletotrichum gloeosporioides is a filamentous ascomycete plant pathogen that attacks the fruits of a variety of pre- and postharvest hosts. C. gloeosporioides germinates on avocado (Persea americana Miller var. drymifolia [Schlechtendal and Chamisso] S. F. Blake) fruit peel and forms appressoria. Infection hyphae from the appressoria penetrate the epidermal cells of the avocado exocarp but remain quiescent until the fruit ripens. The resistance of unripe fruit to fungal attack during quiescence is related to the presence of preformed or inducible antifungal compounds and the lack of secretion of fungal pathogenicity factors. The activation of quiescent infection results from a decline in the concentration of preformed antifungal compounds followed by the activation of fungal pathogenicity factors (27, 28). Pectate lyase (PL) has been implicated as a virulence factor of C. gloeosporioides in avocado fruit (26), and its expression is strongly affected by alkalinization (8). Alkalinization of the tissue occurs naturally during fruit ripening, where the pH of the pericarp increases from 5.2 to 6.1. However, the pathogen also helps increase the amount of ammonia accumulated by the host (16). This increase in accumulated ammonia helps create the alkaline environment necessary for the activation of some extracellular lyase enzymes, including PL (3, 4, 5, 6, 25, 26). The increase in external pH may change the expression of regulatory genes, including pacC, the terminal component of the pH signaling pathway and a transcriptional regulator of pH-dependent genes which is known to regulate the expression of pelB, the gene encoding PL (8). Thus, activation of quiescent infection, which requires alkalinization of the tissue and colonization by C. gloeosporioides, may depend on the availability of exogenous nitrogen sources that can be converted to NH3.
It is not clear, however, whether the environmental conditions that affect PL secretion are determined only by the effect of ammonia on pH or if ammonia also has a direct effect on the regulation of pelB expression by C. gloeosporioides. Our objective in this study was to determine the importance of ammonia accumulation in the secretion of PL. We hypothesized that ammonia and ambient pH are independent signals for the transcriptional regulation of genes required for the disease process of C. gloeosporioides during decay development in avocado fruits. The accumulation of large amounts of ammonia in the decaying tissue ensures transformation from the quiescent biotrophic to the necrotrophic stage of Colletotrichum colonization.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Single-spore cultures of a Cg-14 isolate of C. gloeosporioides, obtained from a decayed Persea americana cv. Fuerte avocado fruit in Israel, were routinely cultured on MS agar containing 250 mg/liter chloramphenicol. The fungus was grown in 40 ml MS medium (23) at pH 5.0 (primary culture) at an inoculation density of 1 × 106 spores/flask. MS medium contains the following reagents (per liter): 2.5 g MgSO4 · 7H2O, 2.7 g KH2PO4, 1.5 g peptone Bios D (Biolife, Milan, Italy), 1.5 g Bacto yeast extract (Difco, Detroit, MI), 15 g sucrose, and 250 mg chloramphenicol. Cultures were incubated at 22 to 24°C in a shaking incubator at 150 rpm for 4 days. Cultures were harvested by filtration through a sterile Büchner funnel fitted with filter paper. The hyphal mat was washed twice by filtering with 40 ml of sterile distilled water each time. The washed mycelia were resuspended in 40 ml of fresh medium (secondary medium) containing the following reagents (per liter): 4 g K2HPO4, 2 g MgSO4 · 7H2O, 5 g KNO3, 0.3 g CaCl2 · 2H2O, 10 mg FeCl3, and 10 g glucose. The medium was buffered with 50 mM phthalate-hydroxide buffer (Sigma, St. Louis, MO) to obtain an initial pH between 4.0 and 7.0. The initial pH for each flask was determined after the medium was autoclaved but prior to inoculation. Experiments were repeated at least three times, with the results of a single representative experiment shown here. Differences between average values for each treatment in each experiment did not differ by more than 2 to 3% between experiments. To create nitrogen starvation conditions, a non-nitrogen-containing medium was used as an intermediate medium between the primary and secondary media. The washed mycelia were resuspended in 40 ml of fresh intermediate medium containing the following reagents (per liter): 4 g K2HPO4, 2 g MgSO4 · 7H2O, 0.3 g CaCl2 · 2H2O, 10 mg FeCl3, and 10 g glucose.
Four nit mutants were obtained from the wild-type isolate Cg-14 as spontaneous mutants growing on potato dextrose agar plates amended with KClO3 (10). When transferred to secondary medium, the nit mutants were grown in the presence of 3 mM glutamate amended with amounts of KNO3 similar to those described above.
Ammonia was detected with an ammonium test kit (Merck KGaA, Darmstadt, Germany) based on a colorimetric reaction. Samples (0.5 ml) from the culture media were diluted with 4.5 ml of double-distilled water, and the concentration of ammonia was determined according to the the manufacturer's instructions. In brief, the sample containing ammonium was adjusted to pH 13 so that ammonium was transformed to ammonia and could be detected as ammonia in a colorimetric reaction. Concentrations are reported as mmol ammonia.
The pH was measured with a microcombination pH electrode (model 9810BN; Orion, Beverly, MA) in 0.5-ml aliquots sampled at various times after fungal inoculation.
Fruits, ripening parameters, and fruit treatments.
Freshly harvested avocado Fuerte fruits from an orchard at Kibbutz Givat Brenner, Israel, were used for fruit treatment and inoculations. The effect of NH4OH on decay development in vivo was determined by infiltration experiments. NH4OH at a concentration of 0.1 M in 0.05 M phthalate buffer (pH 10.0) was infiltrated into freshly harvested avocado fruits ca. 12 h prior to inoculation with C. gloeosporioides conidial suspensions. Infiltration was performed by dipping fruits in the solution, reducing the atmospheric pressure to 20 mm Hg within 60 s, and then releasing the vacuum. Other fruits, harvested at the same time, were similarly infiltrated with H2O as a control (8). Fruits were inoculated by placing 10 μl of a conidial suspension (106 conidia/ml) on six longitudinally spaced inoculation spots, with three on each side, of the 10 different fruits per treatment (60 inoculation replicates per treatment). Following inoculation, fruits were incubated at 22°C in 95% rH for 24 h in covered plastic containers containing wet paper towels. They were then transferred to ambient (70 to 80%) rH at 22°C until fruit ripening and symptom development were observed (16). The average decay diameter for 10 fruits is reported. Inoculation experiments were repeated three times.
The preformed antifungal compound 1-acetoxy-2-hydroxy-4-oxo-heneicosa-12,15 diene (diene) was extracted from the avocado pericarp (2 g of tissue, 1- to 2-mm-thick slices). Pericarp tissue was homogenized in 95% ethanol in a Sorval Omni-Mixer (Kendro, Asheville, NC) at full speed for 3 min as previously described by Yakoby et al. (28). The collected samples were dried, dissolved in 1 ml of running solvent, and analyzed by high-performance liquid chromatography. Unripe fruits were harvested at a firmness of 120 N, and the fruits were considered ripe when the firmness decreased to below 10 N. Firmness, expressed in newtons (N), was measured with a Chatillon digital force gauge (model DFGS50; Ametek Chatillon, Brooklyn, NY).
Detection of PL in liquid medium.
Hyphae grown on secondary medium were separated by filtration (see above), washed twice with sterile water, frozen with liquid N2, lyophilized, and stored at −80°C until used for RNA or protein extraction. The culture medium filtrate was concentrated on a Rotavapor machine (Buchii, Flawil, Switzerland) at 30°C to 5 ml, dialyzed with a SnakeSkin pleated dialysis tube (10,000-molecular-weight cutoff; Pierce, Rockford, IL) for 24 h against 5 liters of 50 mM Tris-HCl, pH 8.5, reconcentrated to 1 ml, lyophilized, and resuspended in 150 μl sterile water. Protein samples were quantified by the Bradford reagent (Bio-Rad Laboratories, Hercules, CA) protein assay, with bovine serum albumin (Sigma) as the standard.
Samples (2.5 μg of secreted proteins) were loaded onto a 12.5% sodium dodecyl sulfate-polyacrylamide gel (Mini-Protean II; Bio-Rad) and run for 1.5 h at a constant 100 V. Western blot analysis was performed with PL antiserum as previously described (2, 27, 28). Blot analyses were repeated three times, and the results from one representative experiment are presented here.
RNA extraction and Northern blot analysis.
For Northern analysis, samples of hyphae were first lyophilized and then homogenized with a Mini Beadbeater (Biospec Products, Bartlesville, OK) in the presence of 1 g zirconia beads for three periods of 20 s each, and total RNA was extracted with 1 ml of Tri-Reagent (Sigma) for every 25 mg of lyophilized hyphae. RNA samples were prepared according to the manufacturer's instructions (Tri-Reagent technical bulletin MB-205, Sigma). RNAs were quantified by GeneQuant (Pharmacia Biotech, Cambridge, United Kingdom).
Northern blot analysis was conducted by running 10 μg of total RNA in a 1.1% formaldehyde denaturing agarose gel (19). The RNA was blotted onto a Hybond+ nylon membrane (Amersham, Buckinghamshire, United Kingdom) by the capillary method (19) with 20× SSC (1× SSC is 17 mM NaCl and 170 mM sodium citrate). RNAs were fixed by baking for 2 h at 80°C and then subjected to hybridization. All hybridizations were carried out at 65°C, and reaction mixtures were washed with 0.1× SSC. Probes were synthesized by using the Prime a Gene labeling system (Promega, Madison, WI) with [32P]dCTP. The hybridization probes were the 1.1-kb full-length pelB clone (GenBank accession no. U32942), the 1.2-kb pac1 coding sequence from C. gloeosporioides (GenBank accession no. AF539700) (8), and the rRNA gene repeat sequence from Neurospora crassa pMF2 (9). The washed blot was autoradiographed and exposed to a Fuji BAS (Bio Analyzing system) sample screen. Images were captured with a Fuji BAS reader (Fujifilm, Tokyo, Japan).
For real-time PCR, RNAs were extracted from 80 mg (dry weight) of mycelium with an RNeasy Plant mini kit (QIAGEN Sciences, Hilden, Germany) and further purified by treatment with RNase-free DNase (QIAGEN). The reverse transcription reaction was performed with a Reverse-it first-strand synthesis kit (ABgene, Surrey, United Kingdom). cDNA samples were diluted 1:10 to the final template concentration for real-time PCR. Real-time detection was performed with ABsolute SYBR green ROX mix in an ABI Prism 7000 machine (Applied Biosystems [ABI], Foster City, CA). The endogenous control was a β-tubulin gene, and the calibrator was a no-nitrogen sample. The primers for pelB were 5′-CACCAAGCCCGACTACAGCT-3′ (forward) and 5′-AGCCTTACCTTGGAGGAGCC-3′ (reverse); the primers for areA (accession no. AY699608) were 5′-ACAGACCACAGGCATTGCAA-3′ (forward) and 5′-TGTGGAGACGAAACCCTGAAG-3′ (reverse); and the primers for β-tubulin were 5′-GCAACAACTGGGCCAAGG-3′ (forward) and 5′-GCGGACAACATCGAGAACCT-3′ (reverse). The primer concentration was 200 nM. Primer efficacy was examined by running different solutions of template and verifying a slope of −3.2 ± 0.2 for a curve of cycle threshold (CT) values versus the log values of template concentrations. Mixtures of all cDNAs used for all the treatments were used as templates for calibration curves. Relative quantification was calculated by the method using ΔΔCT (ABI). The ΔCT value was determined by subtracting the CT results for the target gene from the CT results for the endogenous control gene and then normalized as suggested by ABI (ΔΔCT). The final relative quantification value is 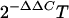 , which represents the level of expression of the gene in relation to the control (no-nitrogen) treatment (relative quantification). Each experiment was repeated three times with similar results, and results for one experiment are presented. The averages presented are the results of two independent cDNA measurements for different RNA samples from the same treatments. The variation between the quantification levels for the same treatment ranged between 10 and 15%.
, which represents the level of expression of the gene in relation to the control (no-nitrogen) treatment (relative quantification). Each experiment was repeated three times with similar results, and results for one experiment are presented. The averages presented are the results of two independent cDNA measurements for different RNA samples from the same treatments. The variation between the quantification levels for the same treatment ranged between 10 and 15%.
RESULTS
Effect of pH on induction of ammonia accumulation.
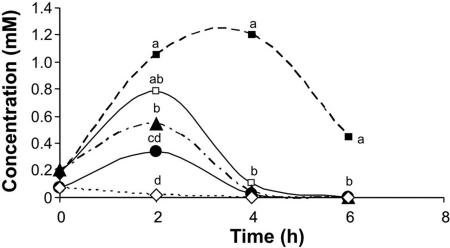
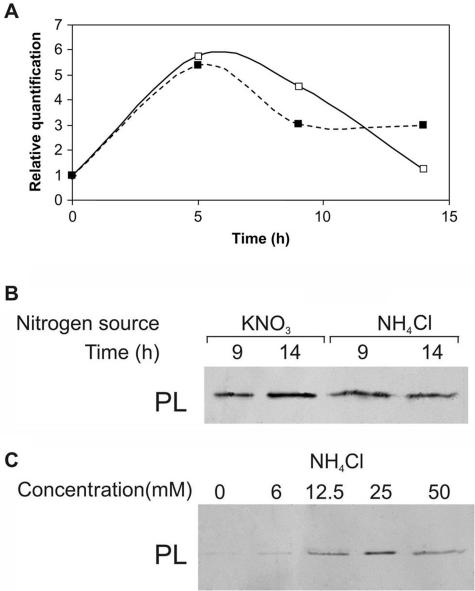
Cultures in primary medium were transferred to secondary medium at a range of pH levels, from 4.0 to 7.0 (Fig. 1). Two hours after transfer of the hyphae to pH 7.0, the amount of accumulated ammonia reached 0.05 mM; at pH 4.0, however, the accumulated amount was 22-fold higher (1.1 mM). Two hours later, hyphae grown at pH 4.0 had secreted more ammonia, reaching up to 1.2 mM, while exposure of the hyphae to higher pH values (5 to 7) resulted in the accumulation of <0.1 mM ammonia. This differential accumulation of ammonia raised the question of whether the amount of ammonia present could differentially activate PL secretion. Ammonia also activated pelB expression and PL secretion (Fig. 2A and B). Growth of the fungus in secondary medium with increasing concentrations of NH4Cl (6 to 25 mM) at pH 6.0 enhanced the increased secretion of PL by C. gloeosporioides (Fig. 2C).
FIG. 1.
Effect of pH on ammonia secretion by C. gloeosporioides. Samples of medium were quantified for ammonia accumulation at different times after transfer to secondary medium containing KNO3, buffered with phthalate buffer to a pH range of 4.0 to 7.0. Symbols: ▪, pH 4; □, pH 5; ▴, pH 6; •, pH 6.5; ⋄, pH 7. Significance was calculated by comparing the concentrations of ammonia at the different initial pHs on each sampling day and was analyzed statistically by analysis of variance of three replications. Values for points on the same day labeled with the same letter were not statistically different (P < 0.05).
FIG. 2.
Effect of NH4Cl as a nitrogen source on pelB expression and PL secretion during growth of C. gloeosporioides in culture medium. At different times after transfer to secondary medium buffered with phthalate buffer (pH 6.0) in the presence of 50 mM NH4Cl (▪), the level of secreted PL was analyzed by Western blot analysis of concentrated, dialyzed culture medium, and pelB expression was analyzed in the mycelia. Growth of the fungus in the presence of 50 mM KNO3 (□) was used as a control. (A) pelB expression by real-time PCR. (B) PL secretion in the presence of NH4Cl. (C) PL secretion in the presence of increasing concentrations of NH4Cl 16 h after transfer to secondary medium. Blot analyses were repeated three times, and results from a representative experiment are presented.
Effect of nitrogen sources on ammonia accumulation, pelB expression, and PL secretion.
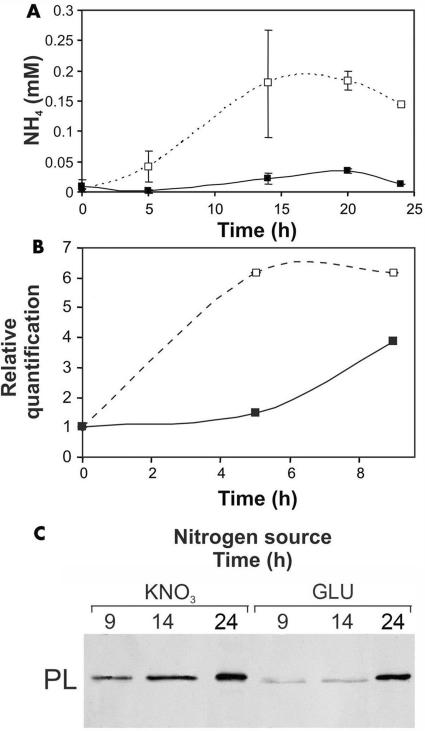
Nitrogen starvation for 15 h before the transfer to secondary medium induced strong expression of areA (3.3-fold higher than that in controls), but this expression level dropped to approximately that of the control 9 h after transfer to secondary medium containing KNO3 or glutamate (results not shown). Under these conditions (secondary medium buffered at pH 6.0 in the presence of KNO3), ammonia accumulation and pelB expression began earlier than if the fungus was grown in culture medium with glutamate as the nitrogen source (Fig. 3).
FIG. 3.
Ammonia secretion, transcript levels of pelB, and PL secretion by C. gloeosporioides at pH 6.0 in the presence of glutamate (GLU) (▪) or KNO3 (□) as the nitrogen source in the culture medium. (A) Ammonia accumulation. (B) pelB transcript levels. (C) PL secretion. Following growth on primary medium, mycelia were transferred for 15 h to a medium lacking nitrogen and then transferred to the secondary medium. At different times after transfer to the secondary medium, the following were analyzed: ammonia accumulation, pelB expression (by real-time PCR), and PL secretion (by Western blot analysis). Western blot analysis of secreted PL was performed on concentrated, dialyzed culture medium. Blot analysis was repeated three times, and the results of one representative experiment are presented.
Effect of nitrogen and pH on pac1 and pelB expression and PL secretion.
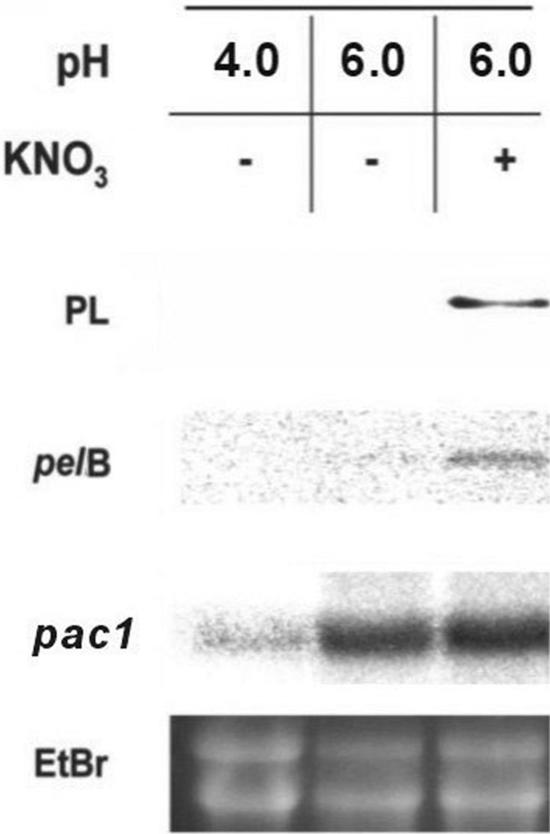
The growth of C. gloeosporioides at pH 6.0 in the presence of KNO3 as the nitrogen source enhanced the transcript levels of pac1 and pelB, as well as PL secretion, 16 h after exposure to the secondary medium. If the nitrogen source was not included in the secondary medium but the pH was kept at 6.0, the pac1 transcript was present at levels similar to those seen when the fungus was grown in medium without nitrate, but no pelB signal or PL secretion was observed. At pH 4.0, the pac1 signal was considerably reduced, and neither the pelB signal nor PL was secreted (Fig. 4).
FIG. 4.
Transcriptional activation of pelB and pac1 and PL secretion by C. gloeosporioides as a function of pH levels and nitrogen amendment. The different factors were analyzed 16 h after transfer to the secondary medium buffered with phthalate buffer at pH 4.0 or 6.0 in the presence or absence of KNO3. Northern blots of total RNA were probed with pelB and then sequentially stripped and reprobed with pac1 and rRNA gene (bottom panel) probes. Secreted PL was analyzed by Western analysis. Ethidium bromide staining was used as a loading control.
Effect of deficiency of ammonia secretion by C. gloeosporioides on alkalinization.
We made four Nit mutants, which are unable to utilize NO3 as a nitrogen source (10). These mutants grew well in the primary medium, which contains peptone and yeast extract as potential nitrogen sources. PL secretion by the wild type and the Nit mutants was similar if they were grown in 50 mM glutamate-amended secondary medium at pH 6.0. Under these conditions, both the Nit mutants and the wild-type strain secreted similar amounts of ammonia into the medium (0.12 μg/ml and 0.11 μg/ml, respectively). Growth of the Nit mutants and the wild type in 3 mM glutamate-amended secondary medium did not affect fungal growth compared to that with higher glutamate concentrations, but alkalinization did not occur. The addition of KNO3 to the 3 mM glutamate-containing, nonbuffered secondary medium at pH 4.0 or 6.0 did not cause the nit mutants to accumulate ammonia, increase the pH of the medium, or secrete PL. The wild-type progenitor strain, Cg-14, increased the amount of ammonia secreted, the pH of the medium (from 4 to 6.25), and the amount of PL secreted.
Effect of ammonia on virulence.
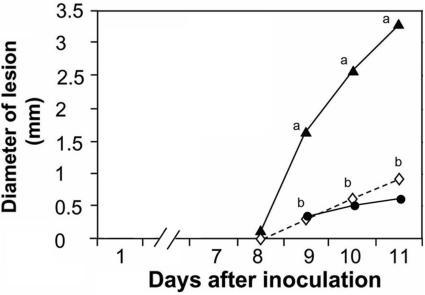
The direct effect of ammonia on C. gloeosporioides virulence was analyzed by measuring the diameter of decay on avocado Fuerte fruit caused by the addition of ammonia to fruits inoculated with the wild-type Cg-14 strain. Infiltration of fruits with a 0.1 M NH4OH solution in 50 mM phthalate buffer, pH 10, following inoculation with Cg-14 significantly enhanced decay development (Fig. 5). The decay development of fruits infiltrated with 50 mM phthalate buffer at pH 10.0 was similar to that of water-treated fruits. NH4OH did not affect fruit ripening, as measured by fruit firmness or ethylene or CO2 evolution on different days after harvesting (results not shown). The levels of the antifungal compound (diene) in NH4OH- and control-treated fruits declined similarly, from 1,320 to 330 μg−1 g fresh weight of fruit peel, suggesting that differential decay development is not affected by a differential decline in the level of the preformed antifungal diene.
FIG. 5.
Effect of NH4OH treatment on decay development by C. gloeosporioides on avocado fruits. Symptoms of decay by C. gloeosporioides wild-type Cg-14 on avocado Fuerte fruits were examined after infiltration with 0.1 M NH4OH in 0.05 M phthalate, pH 10. Fruits were inoculated 12 h after infiltration. Lesion development in NH4OH-treated fruits (▴) was compared to changes occurring on H2O (⋄)- or 0.05 M phthalate (pH 10) (•)-infiltrated fruits. Each experiment contained 10 repetitions (fruits), with a total of 30 replications (10 fruits × 3 replications per fruit). Significance was calculated from comparisons of the diameters of lesion decay by C. gloeosporioides on NH4OH-treated and nontreated fruits on each sampling day and was analyzed statistically with a Student t test. Averages for points on the same day labeled with different letters were statistically different (P < 0.05).
DISCUSSION
C. gloeosporioides is a broad-host-range plant-pathogenic fungus that secretes ammonia as a mechanism of tissue alkalinization (8). An environmental pH of 6.0 significantly increases both pelB expression and the quantity/activity of the specific protein species secreted compared to those secreted at pH 4.0 (8, 28). The finding that, during alkalinization, pelB and pac1 transcript levels increase in parallel suggests that an ambient pH signal transduction pathway (regulated by the pacC homolog) exists in C. gloeosporioides and modulates pelB (6, 8, 17, 20).
Drori et al. (8) suggested that the secretion of PL is also subject to nutritional signals, such as the presence of nitrogen. The regulation of nitrogen assimilation is both complex and important for disease development (13). In Colletotrichum acutatum, nitrogen starvation stimulates synchronous preinfection development (11). In Magnaporthe grisea, nitrogen starvation enhances the expression of PTH11 and MPG1, genes affecting appressorium differentiation and formation, respectively (7, 21). In Colletotrichum lindemuthianum, however, mutants of the AREA fungal nitrogen regulators are not affected during the biotrophic stage but must function during the necrotrophic stage (15).
For C. gloeosporioides, the presence of a nitrogen source seems to be a key element in the expression of virulence factors. Nutritional deprivation of primary nitrogen sources has shown that they are critical for PL secretion. PL secretion was observed only with the combination of a suitable environment (pH 6.0) and ammonia production. Increasing the amount of ammonia added to the secondary growth medium also increased the amount of PL secreted. Differential rates of ammonia accumulation at pH 6.0 by C. gloeosporioides in the presence of KNO3 and glutamate resulted in differential activation of pelB expression and PL secretion. In vivo, it is possible that the nitrogen source nutrients for the pathogen's initial growth are provided by protease activity breaking down structural glycoproteins in the plant cell wall (18). C. gloeosporioides, C. acutatum, and Colletotrichum coccodes probably utilize these degraded proteins to secrete up to 3,350 μM ammonia into decayed tissue (16). Ammonia may therefore have the following two key functions: (i) alkalinization of tissue and (ii) direct activation of pelB expression and PL secretion (16, 28). The effect of ammonia on alkalinization of the medium in the presence of NO3 resulted in an increase in the pH level from 4 to 6.5 (16). Alkalinization of the host's decayed tissue also has been observed for apples, tomatoes, and other infected hosts (16). This alkalinization represents the basic signal for activation of the pH-dependent transcriptional regulator pac1, which modulates the activation of pelB and the secretion of PL.
Several experiments were performed to demonstrate the importance of ammonia accumulation and pH on the expression of pathogenicity factors. When the pathogen was grown at pH 6.0 without nitrogen sources, ammonia did not accumulate and pelB expression and PL secretion were not detected; only the pH-dependent transcription activator pac1 was expressed. The addition of a nitrogen source to the same secondary medium resulted in the accumulation of ammonia, pelB expression, and PL secretion. However, the growth of C. gloeosporioides in nitrogen-containing medium at pH 4.0 prevented pac1 and pelB transcript expression and, consequently, PL secretion.
In a second set of experiments in which C. gloeosporioides Nit mutants were grown in the presence of the easily assimilated nitrogen source glutamate, the mutants secreted ammonia, alkalinized the medium, and secreted PL at pH 6.0 as well. Growth of the mutants under similar conditions in the presence of NO3 as the nitrogen source did not enhance the secretion of ammonia or PL or increase the pH.
A second postulated function for ammonia is the direct activation of pelB expression and PL secretion, as observed when ammonia was included as the sole nitrogen source. This hypothesis was supported by the differential accumulation of pelB transcripts and PL secretion when the fungus, grown in the presence of two other nitrogen sources (KNO3 and glutamate), produced different amounts of ammonia. These results suggest that the accumulation of large amounts of ammonia at the infection site during decay development represents a specific condition that is recognized by the pathogen and that activates virulence factors such as pelB expression and PL secretion. Furthermore, infiltration of ammonia into ripening avocado fruits caused a clear increase in decay development by C. gloeosporioides, supporting the importance of ammonia during fungal pathogenicity.
The signal that activates the mechanism of ammonia accumulation and the activation of pelB expression and PL secretion is still not known. The mechanism responsible for reduced fungal sensitivity to high levels of ammonia during fungal attack is also not known. Our present results suggest that one of the signals affecting the process of ammonia secretion is a low environmental pH (4.0). Ammonium is an important nitrogen source for bacteria, fungi, and plants, but it is toxic to animals (22). The ammonium transport proteins (methylamine permeases/ammonium transporters) are ubiquitous in bacteria, archaea, and eukarya (12), are specific for ammonium, and function in the utilization of ammonium as a source of nitrogen (14), but their amounts and activities during fungal pathogenicity have not been described. Members of the MEP/amount family may transport NH4+ across the cytoplasmic membrane and concentrate it in an energy-dependent manner (24). Cell membrane channels with high affinities for ammonium transport that function in both the uptake and excretion of ammonium have recently been described (22). Further studies of these transporters could help explain the mechanism underlying ammonia accumulation by Colletotrichum species.
Our results support the hypothesis that both ambient pH and nitrogen availability are major regulators of C. gloeosporioides pelB transcriptional activation and PL secretion. PL secretion is transcriptionally regulated by pH, and at least one component of a conserved regulatory pathway mediating pH-regulated gene expression, pac1, exists in this fungus (8). Our data suggest that alkalinization by the presence of ammonia is also important for the activation of quiescent C. gloeosporioides infection and for its transformation from the biotrophic to the necrotrophic stage of fungal colonization in the fruit host (16). The accumulation of specific amino acids during fruit ripening (1) could contribute to substrate availability to the pathogen to ensure ammonia production. The ability of the pathogen to accumulate large amounts of ammonia in decaying tissue ensures the activation of pelB expression under optimal pH and nutritional conditions for PL secretion. Altering ammonia secretion and pH-regulated processes, such as pelB expression, could form the basis for a viable strategy to delay, reduce, or prevent disease development by this plant-pathogenic fungus.
Acknowledgments
This research was supported by grants to D.P. from BARD. H.K.-H. and E.S. were awarded personal scholarships from the Israel Fruit Marketing Board/Ministry of Agriculture & Rural Development.
REFERENCES
- 1.Boggio, S. B., J. F. Palatnik, H. W. Heldt, and E. M. Valle. 2000. Changes in amino acid composition and nitrogen metabolizing enzymes in ripening fruits of Lycopersicon esculentum Mill. Plant Sci. 159:125-133. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Caddick, M. X., A. G. Brownlee, and H. N. Arst, Jr. 1986. Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Mol. Gen. Genet. 203:346-353. [DOI] [PubMed] [Google Scholar]
- 4.Collmer, A., L. J. Ried, and S. M. Mount. 1988. Assay methods for pectic enzymes. Methods Enzymol. 161:329-335. [Google Scholar]
- 5.Dean, R. A., and W. E. Timberlake. 1989. Regulation of the Aspergillus nidulans pectate lyase gene (pelA). Plant Cell 1:275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denison, S. H. 2000. pH regulation of gene expression in fungi. Fungal Genet. Biol. 29:61-71. [DOI] [PubMed] [Google Scholar]
- 7.Donofrio, N., and R. A. Dean. 2005. Gene regulation during nitrogen stress and invasive plant growth in the rice blast pathosystem. Presented at the 23rd Fungal Genetics International Conference, Asilomar, Calif.
- 8.Drori, N., H. Kramer-Haimovich, J. Rollins, A. Dinoor, Y. Okon, O. Pines, and D. Prusky. 2003. A combination of external pH and nitrogen assimilation affects secretion of the virulence factor pectate lyase by Colletotrichum gloeosporioides. Appl. Environ. Microbiol. 69:3258-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Free, S. J., P. W. Rice, and R. L. Metzenberg. 1979. Arrangement of genes coding for ribosomal ribonucleic acids in Neurospora crassa. J. Bacteriol. 137:1219-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman, S., E. Shabi, and T. Katan. 2000. Characterization of Colletotrichum acutatum causing anthracnose of anemone (Anemone coronaria L.). Appl. Environ. Microbiol. 66:5267-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horowitz, S., S. Freeman, A. Zveibil, and O. Yarden. 2005. A defect in a NirA-like transcription factor confers morphological abnormalities and lack of pathogenicity in Colletotrichum acutatum. Presented at the 23rd Fungal Genetics International Conference, Asilomar, Calif.
- 12.Howitt, S. M., and M. K. Udvardi. 2000. Structure, function and regulation of ammonium transporters in plants. Biochim. Biophys. Acta 1465:152-170. [DOI] [PubMed] [Google Scholar]
- 13.Marzluf, G. A. 1997. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 61:17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellier, A. L., R. Lauge, C. Veneault-Fourrey, and T. Langin. 2003. CLNR1, the AREA/NIT2-like global nitrogen regulator of the plant fungal pathogen Colletotrichum lindemuthianum is required for the infection cycle. Mol. Microbiol. 48:639-655. [DOI] [PubMed] [Google Scholar]
- 16.Prusky, D., J. L. McEvoy, B. Leverentz, and W. S. Conway. 2001. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol. Plant-Microbe Interact. 14:1105-1113. [DOI] [PubMed] [Google Scholar]
- 17.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181:7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauscher, M., K. Mendgen, and H. Deising. 1995. Extracellular proteases of the rust fungus Uromyces viciae-fabae. Exp. Mycol. 19:26-34. [Google Scholar]
- 19.Sambrook, J., E. F. Fritsch, and T. A. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Shah, A. J., J. Tilburn, M. W. Aldard, and H. N. Arst, Jr. 1991. pH regulation of penicillin production in Aspergillus nidulans. FEMS Microbiol. Lett. 77:209-212. [DOI] [PubMed] [Google Scholar]
- 21.Soanes, D. M., M. J. Kershaw, R. N. Cooley, and N. J. Talbot. 2002. Regulation of the MPG1 hydrophobin gene in the rice blast fungus Magnaporthe grisea. Mol. Plant-Microbe Interact. 15:1253-1267. [DOI] [PubMed] [Google Scholar]
- 22.Soupene, E., H. Lee, and S. Katsu. 2002. Ammonium/methylammonium transporter (Amt) proteins facilitate diffusion of NH3 bidirectionally. Proc. Natl. Acad. Sci. USA 99:3926-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu, J. C. 1985. An improved Mathur's medium for growth, sporulation and germination of spores of Colletotrichum lindemuthianum. Microbiosis 44:87-93. [Google Scholar]
- 24.von Wiren, N., S. Gazzarrini, A. Gojon, and W. B. Frommer. 2000. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 3:254-261. [PubMed] [Google Scholar]
- 25.Wattad, C., S. Freeman, A. Dinoor, and D. Prusky. 1995. A nonpathogenic mutant of Colletotrichum magna is deficient in extracellular secretion of pectate lyase. Mol. Plant-Microbe Interact. 8:621-626. [Google Scholar]
- 26.Wattad, C., D. Kobiler, A. Dinoor, and D. Prusky. 1997. Pectate lyase of Colletotrichum gloeosporioides attacking avocado fruits: cDNA cloning and involvement in pathogenicity. Physiol. Mol. Plant Pathol. 50:197-212. [Google Scholar]
- 27.Yakoby, N., D. Beno-Moualem, N. T. Keen, A. Dinoor, O. Pines, and D. Prusky. 2001. Colletotrichum gloeosporioides pelB is an important virulence factor in avocado fruit-fungus interaction. Mol. Plant-Microbe Interact. 14:988-995. [DOI] [PubMed] [Google Scholar]
- 28.Yakoby, N., I. Kobiler, A. Dinoor, and D. Prusky. 2000. pH regulation of pectate lyase secretion modulates the attack of Colletotrichum gloeosporioides on avocado fruits. Appl. Environ. Microbiol. 66:1026-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]