Abstract
In soil, fungal colonization of plant roots has been traditionally studied by indirect methods such as microbial isolation that do not enable direct observation of infection sites or of interactions between fungal pathogens and their antagonists. Confocal laser scanning microscopy was used to visualize the colonization of tomato roots in heat-treated soil and to observe the interactions between a nonpathogenic strain, Fo47, and a pathogenic strain, Fol8, inoculated onto tomato roots in soil. When inoculated separately, both fungi colonized the entire root surface, with the exception of the apical zone. When both strains were introduced together, they both colonized the root surface and were observed at the same locations. When Fo47 was introduced at a higher concentration than Fol8, it colonized much of the root surface, but hyphae of Fol8 could still be observed at the same location on the root. There was no exclusion of the pathogenic strain by the presence of the nonpathogenic strain. These results are not consistent with the hypothesis that specific infection sites exist on the root for Fusarium oxysporum and instead support the hypothesis that competition occurs for nutrients rather than for infection sites.
Fusarium oxysporum is commonly found in soil, where it survives as dormant propagules (chlamydospores) and grows saprophytically on organic matter. This fungal species also includes many important plant pathogens that can induce necroses or wilts in crops of economic importance, even though strains of F. oxysporum also are commonly isolated from healthy roots. Strains from apparently healthy plants are termed nonpathogenic and are interesting, since some of them can protect plants against the pathogenic strains. Several nonpathogenic strains of F. oxysporum, isolated from soils suppressive to fusarium wilts, have been selected as potential biological control agents (3, 13, 17, 24). Characterizing the diverse interactions between the fungus and plant roots could help define the differences between pathogenic and nonpathogenic strains. Until recently, observing fungi in soil was very difficult, and most of the studies describing the colonization of root surfaces by F. oxysporum were carried out using hydroponic systems(18, 19) or with a substrate such as sand or vermiculite (11), but not with soil.
To facilitate the observation of hyphae on the root surface, transformed strains expressing reporter genes have been used (25). The β-glucuronidase gene has been used to mark both pathogenic and nonpathogenic strains of F. oxysporum (2, 18, 19, 26). Unfortunately, this reporter gene, which needs a substrate to stain hyphae, is not easy to use in studies of root colonization in soil. Other reporter genes, such as the green fluorescent protein (GFP) gene or the DsRed2 gene, have also been used to transform strains of pathogenic fungi and antagonistic bacteria or fungi (4, 10, 14, 16, 22, 23). When strains marked with different reporter genes are used simultaneously, both microorganisms can be observed simultaneously on the same root (5). Simultaneous observations of a pathogenic and a nonpathogenic strain of F. oxysporum would be very useful to study their interactions at the root surface in soil.
The nonpathogenic strain Fo47 utilizes several modes of action to generate its biocontrol capability: competition for nutrients in soil, competition for root colonization, and induced systemic resistance. The relative importance of these modes of action is not clear (8, 12), since competition, especially competition at the root surface in soil, is difficult to demonstrate. Using β-glucuronidase-transformed strains to assess the fungal activity and either antibodies (7) or microbial isolations (2) to quantify the fungal biomass, competition for root colonization was shown to occur between pathogenic and nonpathogenic strains of F. oxysporum. There have been no direct observations of interactions, however, at the root surface in soil. In this study, a pathogenic strain of F. oxysporum f. sp. lycopersici was transformed with the DsRed2 gene and the biocontrol strain, Fo47, was transformed with the GFP gene so that we could observe the simultaneous colonization of a root by these two strains of F. oxysporum. The objectives of this study were to (i) describe the pattern of tomato root colonization by F. oxysporum in soil, (ii) study interactions between a pathogenic strain and a biocontrol strain of F. oxysporum in soil and at the root surface, (iii) examine the hypothesis of competition for infection sites, and (iv) contribute to improve our knowledge of the modes of action of Fo47.
MATERIALS AND METHODS
Fungal strains and inoculum preparation.
The pathogenic strain Fusarium oxysporum f. sp. lycopersici (Fol8) isolated from a diseased tomato plant was transformed with the DsRed2 gene as described previously by Nahalkova and Fatehi (16). The nonpathogenic strain F. oxysporum (Fo47), isolated from a suppressive soil (1), was transformed with the GFP gene by the same methodology as for Fol8. Both fungal strains were cryopreserved by freezing a suspension of conidia in 25% (vol/vol) glycerol at −80°C. Before being used, the fungi were transferred to potato dextrose agar (Sigma-Aldrich, Saint Quentin Fallavier, France) at 25°C and grown for 7 days. The Fol8-DsRed2 and Fo47-GFP strains were transferred to 150 ml of a minimal liquid medium (6) in which sucrose was replaced by glucose (5 g liter−1). They were cultivated at 25°C on a rotary shaker at 125 rpm (AJ 110; INFORS, Switzerland). After 5 days of growth, fungal cultures were filtered through a sterile number 2 sintered-glass funnel (40- to 100-μm-pore-size mesh) to retain the mycelia. The microconidia remaining in the filtrate were harvested by centrifugation (5,000 × g; 20 min at 15°C) and washed twice in sterile distilled water. The concentration of the conidial suspension was estimated under the microscope with a hemocytometer and adjusted as necessary with sterile distilled water.
Plant inoculation and cultivation.
The tomato variety hybrid F1 Montfavet 63-5, susceptible to fusarium wilt, was used in this experiment. Seeds were surface sterilized by immersion in 1.25% sodium hypochlorite for 20 min and rinsed three times in sterile distilled water. Seeds were germinated on malt extract agar (10 g liter−1 malt extract; Biokar Diagnostic, Beauvais, France) in petri dishes kept at an inclination of 60° and incubated in the dark at 22°C for 4 days. Seedlings of the same size (1 cm long) were transplanted directly from the petri dish into the infested soil, one seedling per pot, and cultivated in a growth chamber at 25°C during the day period of 16 h and at 22°C during the night period of 8 h. Noninoculated controls were made by transplanting seedlings into noninfested soil.
Inoculum concentrations and ratios.
To correlate observations at the root surface with known conditions leading to effective control of the disease, two inoculum concentration ratios were evaluated, 1/1 and 1/100, which correspond to ratios for which biological control was, respectively, not effective or effective (20). The following inoculum concentrations were applied: single inoculations of Fol8 and Fo47 at 103 and 105 conidia ml−1 and mixed inoculations of Fol8 and Fo47 at 105:105 and 103:105 conidia ml−1, respectively. Soil from a vegetable field (sand, 86%; silt, 6%; clay 8%; pH 5.5; C/N ratio, 8.1) was heat treated at 110°C for 1 h. It was distributed in small pots, each 20 ml, and infested by the addition of 1 ml of an appropriate conidial suspension. Seedlings were transplanted into the soil immediately after infestation; there were 10 plants per treatment and per date of observation.
Microscopic observations.
Observations were made 18 h and 2, 3, 4, and 7 days after transplantation of the seedlings into the infested soil. The aerial parts of the young plants were eliminated by cutting the hypocotyls at the soil surface, and the roots were removed from the soil after dipping the soil clod in water. Roots with adhering soil particles were placed on a “deep glass slide” (laboratory made) in a drop of 0.1% water agar. The full length of each root was observed under the microscope, and the most interesting spots were observed by confocal laser microsccopy. A few root samples were stained by immersion in propidium iodide at 10 μg ml−1 for 10 min and then rinsed in sterile distilled water to better observe the plant cell walls.
Confocal observations were made with a confocal microscope (LEICA TCS SP2 AOBS; Leica Microsystems, Germany). A dry objective (×10/0.40; working distance, 2,200 μm) was used for most images. Each fluorescent image corresponds to the maximum projection of optical sections from a z series, using Leica Confocal software. The resultant depth (z) of each projection is between 60 and 430 μm, depending on the diameter of the root and the magnification. The optical section number of each projection was between 25 and 80. Each figure is a superposition of the fluorescent projection and a transmitted, nonconfocal image (see Fig. 3d for an exception; in this figure, the transmitted image is not presented).
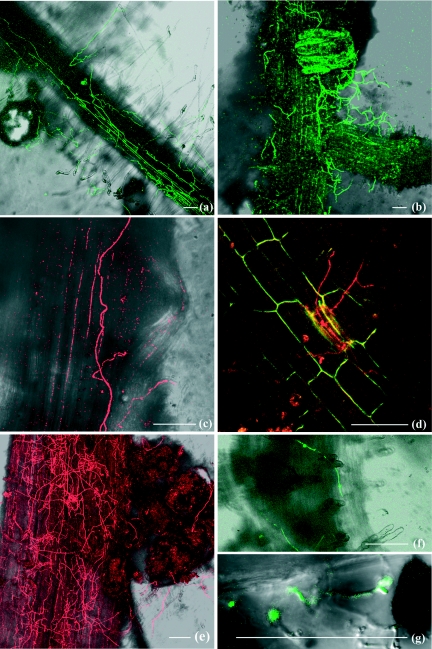
FIG. 3.
Colonization pattern of tomato roots by strains of pathogenic F. oxysporum f. sp. lycopersici (Fol8) expressing the DsRed2 gene (red) or nonpathogenic F. oxysporum (Fo47) expressing the GFP gene (green). Confocal laser scanning microscopic analysis of tomato seedling roots grown in soil infested with 105 conidia ml−1 was carried out. Three days after transplantation, hyphae of Fo47 growing along or transversally to the main axis of the root (a) were observed. Four days after transplantation, heavy colonization of the base of a lateral root by Fo47 (b) and a lateral root primordium emerging from a taproot colonized by hyphae of Fol8 (c) were observed. Six days after transplantation, hyphae of Fol8 penetrating into epidermis cells of a lateral root (d) were observed. Four days after transplantation, heavy colonization of the root surface by Fol8 (e) and chlamydospores of Fo47 at the root surface (f) and in soil (g) were observed. Bars, 100 μm.
RESULTS
Plant growth.
Eighteen hours after transplantation and at the time of the first observation, the taproots were ∼1.5 cm in length. Their growth was important for the first 2 days but was then limited by the narrow layer of soil in the 4-cm-deep pots. Lateral roots were observed beginning on day 3 (Table 1). An apical zone including the apex itself and the elongation zone, which corresponded to the newly formed root tissue, was observed on both lateral and taproots, as well as a zone where the root hairs were present and a mature root zone where lateral roots were present (Fig. 1).
TABLE 1.
Length of the tap root and number of lateral roots (mean, 40 roots) and length of the root colonized by Fo47 (mean, 10 roots, corresponding to plants inoculated by Fo47 at the higher inoculum concentration)
| Time post- transplantation | Tap root length (cm) | Mean no. of lateral roots | Plants inoculated with Fo47 at 105 ml−1 soil
|
|
|---|---|---|---|---|
| Tap root length (cm) | Tap root length colonized (cm) | |||
| 18 h | 1.5 ± 0.3 | 1.6 ± 0.4 | 0.9 ± 0.3 | |
| 2 days | 2.5 ± 0.6 | 2.7 ± 0.3 | 2.4 ± 0.3 | |
| 3 days | 3.3 ± 0.5 | 7.9 ± 3 | 3.4 ± 0.5 | 2.9 ± 0.5 |
| 4 days | 3.7 ± 0.5 | 11 ± 4 | 3.6 ± 0.5 | 2.3 ± 0.5 |
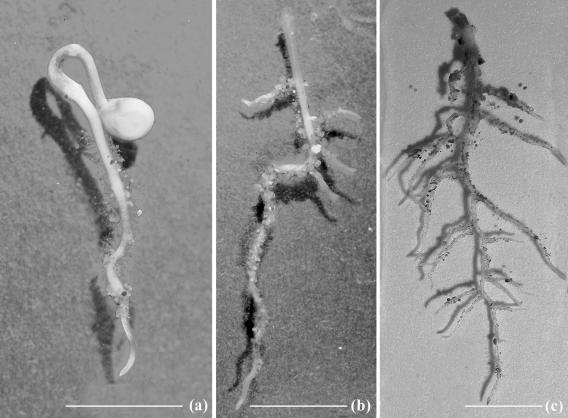
FIG. 1.
Roots of tomato seedlings 18 h (a), 3 days (b), and 6 days (c) after transplantation into infested soil. Pictures show the soil particles adhering to the roots ready to be observed. Bars, 1 cm.
Frequency of roots colonized by fusaria.
Both Fol8 and Fo47 could be detected 18 h after plants were transplanted into the infested soil. The frequency of observed root colonization increased with time. Fungi were detected on at least 7/10 roots and observed in all combinations except for Fol8 in the 103:105 Fol8:Fo47 ratio.
Pattern of soil and root colonization.
In soil infested with 105 conidia ml soil−1, the general pattern of colonization of the rhizosphere soil and of the root was similar irrespective of the strain. This general pattern is described first, and differences between strains and concentrations are then detailed in succeeding paragraphs.
Eighteen hours after the seedlings were transplanted into the infested soil, germinated conidia attached to soil particles were observed. There was no obvious chemotactic growth towards the root, and only a few germ tubes reached the root surface. Most of the germinated conidia were observed in the soil explored by root hairs (Fig. 2a and b).
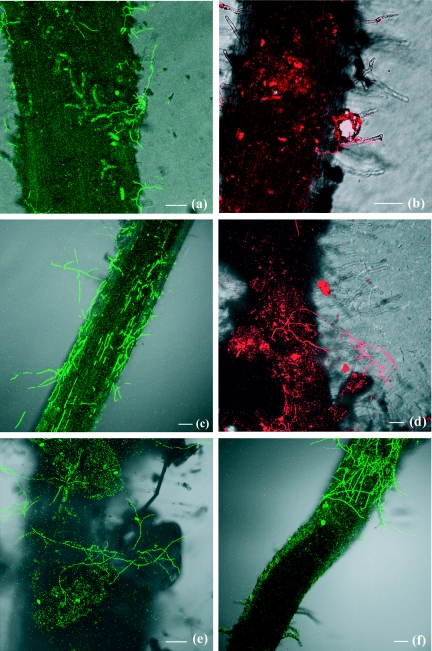
FIG. 2.
Colonization pattern of tomato roots by strains of pathogenic F. oxysporum f. sp. lycopersici (Fol8) expressing the DsRed2 gene (red) or nonpathogenic F. oxysporum (Fo47) expressing the GFP gene (green). Confocal laser scanning microscopic analysis of tomato seedling roots grown in soil infested with 105 conidia ml−1 is shown. Germinated conidia of Fo47 (a) or Fol8 (b) attached to soil particles and reached the root surface 18 h after the seedlings were transplanted. Two days after transplantation, hyphae of Fo47 (c) or Fol8 (d) colonizing the root hair zone (d) and the root surface (c) and hyphae of Fo47 running from soil particles to soil particles (e) were seen. A dense network of hyphae of Fo47 was observed on the root surface, but there was no colonization of the apical zone (f). Bars, 100 μm.
Two days after the seedlings were transplanted into the infested soil, fungal development was obvious in the soil adhering to the root. Some hyphae connected several soil particles (Fig. 2e), while others reached the root surface, where they created small networks (Fig. 2c and d). Fungal colonization was never observed in the apical zone of the root (Fig. 2f), which limited the length of the taproot colonized by the fungi (Table 1).
Three days after the seedlings were transplanted into the infested soil, the hyphal networks on the root surface became denser and began to merge. There was obvious continuity between some hyphae adhering to soil particles and hyphae growing on the root surface (Fig. 3a and b). Lateral roots developed, and approximately two of seven roots were colonized by either Fo47 or Fol8. The fungi were observed mostly at the base of the lateral roots and never at the apex (Fig. 3b). These roots were colonized either from a network of hyphae already present on the surface of the taproot (Fig. 3c) or from hyphae present in the soil (Fig. 3d).
Between the fourth and seventh days after the seedlings were transplanted, the lateral roots colonized most of the limited volume of soil. The root surface appeared heavily colonized by the fungi (Fig. 3e). There was no preferential growth of hyphae along the intercellular junctions. Some hyphae followed the main axis of the root (Fig. 3a), but others grew transversally to the main axis of the root (Fig. 2f and 3a and e). The pattern of fungal colonization of lateral roots was the same as that described for the taproot, with the apical zone never colonized by fungal hyphae. At various times, fungi were observed penetrating epidermal cells (Fig. 3d).
Differences in root colonization based on the inoculum concentration.
The behavior of the fungi was similar regardless of inoculum concentration; however, at the lower inoculum concentration, the fungi were more difficult to observe. Usually, they were not detected until 2 days after transplantation. With time, the intensity of root colonization increased, but plants grown in soil with higher inoculum densities always had more intense root colonization. Fo47 grew faster than Fol8, so the difference in colonization intensity due to differences in the initial inoculum concentration was more evident for Fol8 than for Fo47.
Differences of root colonization between Fo47 and Fol8.
Qualitatively, the saprophytic development of Fo47 was faster than that of pathogenic strain Fol8. After 18 h, the development of Fo47 was greater than that of Fol8 (Fig. 2a and b), but the difference was even clearer after 2 days of culture. The networks of hyphae of Fol8 at the root surface were still distinct (Fig. 2d), and an average of 24 networks per root could be counted, while networks of Fo47 had already merged and could no longer be counted (Fig. 2c). Three days after transplantation, Fol8 had a clear pattern of decreasing density of colonization from the upper part of the root towards the elongation zone, while Fo47 had more evenly colonized the entire root surface and begun to form chlamydospores (Fig. 3f and g). At this time, observation of Fo47 under fluorescent light was more difficult than that of Fol8, and it was necessary to utilize the laser light to observe it.
Interactions between Fo47 and Fol8 at the root surface of tomato.
When the two strains were both inoculated together at 105 conidia, they were readily observed on the same root in the same microscope field. After 18 h of culture, germinated conidia of Fo47 and Fol8 were observed in the soil explored by root hairs (Fig. 4a); after 2 days, these germ tubes reached similar locations on the root surface (Fig. 4b). Both fungi were observed together on older portions of the roots, but Fo47 was found alone on younger portions of the root. For example, on one root measuring 2.1 cm, Fol8 and Fo47 were observed colonizing from the hypocotyls towards the apex at 0.5 and 1.2 cm, respectively. After 3 days of culture, the root surface was more intensively colonized by hyphae of Fo47 than by hyphae of Fol8 (Fig. 4c). The intensity of colonization by Fol8 in interaction with Fo47 was lower than colonization by Fol8 when inoculated alone. However, the colonization of the root by Fol8 did not stop, since converging networks of Fol8 were later observed (Fig. 4d).
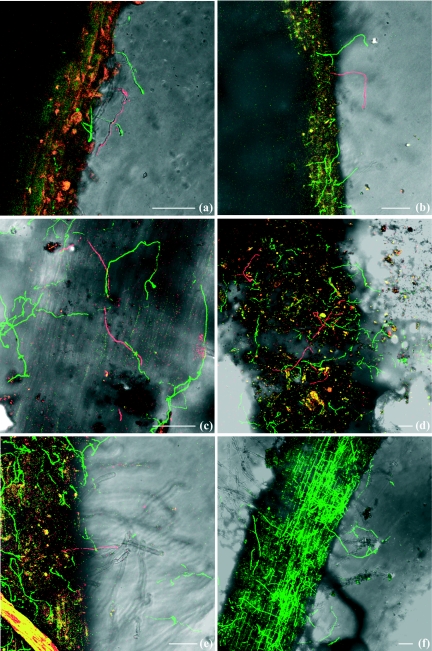
FIG. 4.
Confocal laser scanning microscopic analysis of tomato seedling roots grown in soil coinoculated by strains of pathogenic F. oxysporum f. sp. lycopersici (Fol8) expressing the DsRed2 gene (red) and nonpathogenic F. oxysporum (Fo47) expressing the GFP gene (green). Soil infested by both fungi at the concentration of 105 conidia ml−1 is shown (a to d), with germinated conidia and hyphae of Fol8 and Fo47 reaching the root surface at the same location 18 h (a) and 2 days (b) after transplantation. More intense colonization of the root surface by Fo47 than by Fol8 3 days (c) and 4 days (d) after transplantation was observed. (e and f) Soil infested by Fol8 at 103 conidia ml−1 and Fo47 at 105 conidia ml−1. A single hypha of Fol8 reaching the root surface colonized by Fo47 is shown 2 days after transplantation (e). Intense colonization of the root surface by Fo47 only at 3 days after transplantation (f) is shown. Bars, 100 μm.
When Fo47 was introduced at a higher inoculum concentration (105) than Fol8 (103), it was dominant (Fig. 4f) and Fol8 was much more difficult to detect. However, it was possible to observe some places where both Fol8 and Fo47 were present simultaneously (Fig. 4e). Fo47 never totally excluded Fol8.
DISCUSSION
Fungal strains expressing fluorescent proteins have been used mainly to characterize their interactions with a plant (22). Even when soilborne pathogens have been examined, most of the studies were conducted with hydroponic systems or on artificial substrates. Our objective in this study was to use marked strains of pathogenic and nonpathogenic F. oxysporum strains to observe their interactions at the root surface in soil.
Eigtheen hours after transplantation, young hyphae emerging from conidia attached to soil particles colonized the rhizosphere soil. Germ tubes did not show an obvious tropism towards the root surface. Some hyphae grew from one soil particle to the next without ever reaching the root surface. Other hyphae reached the root surface, where they formed small mycelial networks. They colonized the taproot and the lateral roots as soon as they emerged, but they never colonized the elongation zone or the apex of the roots. The general pattern of root colonization by pathogenic and nonpathogenic strains in soil was similar but differed greatly from that previously described for hydroponic systems (18, 19). Indeed, in hydroponic systems, the apical zone was heavily colonized by either the pathogenic or the nonpathogenic strain, and the apex was supposed to be a penetration zone for the pathogenic F. oxysporum strain (26). Differences in experimental design could explain these differences in fungal behavior. In hydroponics, the root system was dipped into a conidial suspension; thus, fungal development resulted from a relatively small number of conidia attached to the root surface. In soil, as the radicle grew it came into contact with conidia adhering to soil particles. The effective inoculum concentrations resulting from these procedures were much lower in hydroponic systems than in soil. In soil, nongerminated conidia were induced to germinate by root exudates released within a short distance of the growing apex of the root. Some hyphae colonized the rhizosphere soil, and others reached the root surface but always behind the elongation zone, probably due to the faster growth of the roots than of the hyphae. This pattern of root colonization in soil fits with observations by Rovira et al. (25) that the main zone of root exudation is located behind the apex. Moreover, in heat-treated soil, the organic matter provided a substrate for saprophytic growth of the fungi; but in hydroponic systems, fungal development resulted from conidia bound to the root surface, which is the only source of carbon to support the growth of the fungus in the mineral nutrient solution.
In soil, Fo47 grew more rapidly than did Fol8. It also formed denser networks at the root surface and colonized roots closer to the elongation zone than did Fol8, which more intensively colonized the older portions of the young root. The staining quality of Fo47 hyphae decreased with time. Many of the hyphae observable by laser confocal scanning microscopy were not observable under fluorescent light. This might be due to the low level of fluorescence emitted when the hyphae stopped active growth. Expression of the GFP gene under the control of the glyceraldehyde phosphate dehydrogenase promoter is related to the metabolic activity of the fungus. Four days after the seedlings were transplanted, hyphae present on the older portions of the root were no longer actively growing and were forming chlamydospores, which are survival structures produced when nutrients become limiting. Under these conditions, it is expected that GFP production will be reduced. We have reported such phenomena before (18), but others (2) have insisted that there was no “reduction in staining quality” even 10 days after inoculation. Fo47, being a nonpathogenic strain isolated from a suppressive soil, might be better adapted to the soil environment than Fol8, which is a wilt pathogen, better adapted to a plant environment. In the 1960s, Garrett (9) had already discussed the role of the saprophytic ability among root-infecting fungi and distinguished between those with a high competitive saprophytic ability and those more adapted to growth at plant expense.
When the two strains were coinoculated, both were observed on the root. The presence of one strain on the root did not prevent its colonization by the other strain. The amount of root colonization by either strain was reduced relative to that colonized in a single inoculation with either strain alone at the same inoculum concentration. This reduced colonization is consistent with a reciprocal competitive interaction for nutrients (7). Fo47 always dominated, regardless of inoculum level, and consistently formed a dense network of hyphae in the rhizosphere and at the root surface. Hyphae of Fol8 were consistently detected among those of Fo47.
The apical zone of roots growing in soil was never colonized by the fungi. This means that the new root tissues formed behind the apex are not colonized by hyphae growing from the upper part of the root but by hyphae present in the rhizosphere soil. Thus, root colonization by F. oxysporum is a dynamic process, taking place continuously behind the apex of the growing roots. Consequently, to protect the plant efficiently, the nonpathogenic strain must constantly succeed in its competition with the pathogen at the apical zone of the root.
Both strains colonized the entire root surface, except the apical zone, and could be found at the same locations on the root surface. These observations are not consistent with the hypothesis that there are only a limited number of infection sites that can be specifically colonized by one or the other of the two fungal strains. On the contrary, in the upper portions of the roots, both fungi could be observed simultaneously in the same microscope field. Even when Fo47 intensively colonized the root surface, it never totally excluded Fol8. These observations contradict the hypothesis that competition for infection sites occurs on the root surface. Mandeel and Baker (15) and Bao and Lazarovits (2) used serial dilutions to quantify colonization of the root by the fungus. They found reduced colonization of the roots by the pathogen in the presence of the nonpathogen. Bao and Lazarovits (2) observed the pathogen inside the xylem vessels and the nonpathogenic strain on the root surface and in the upper layers of the cortical cells and concluded that these strains can exclude each other from the same ecological niche. Such partitioning might be, but is not necessarily, in response to competition for space and the occupation of particular infection sites.
Competition between strains of F. oxysporum does occur, since the proportion of the root surface colonized by either strain is reduced in the presence of the other. This reduction could result from competition for nutrients or for space. The proportion of the root colonized and the efficacy of biological control depend on the inoculum ratio of the pathogenic and the nonpathogenic strains. This dose-response relationship has been used by Larkin and Fravel (12) to characterize the mechanism(s) of action by nonpathogenic strains. Better saprophytic competition for nutrients was a mechanism of action used by strain Fo47 (12), as was the induction of early defense reactions (21). It is difficult to distinguish between competition for root colonization and locally induced resistance, since both mechanisms contribute to decrease the intensity of root colonization.
The present study, which is the first report of root colonization by F. oxysporum in soil, tended to minimize the importance of competition for infection sites, if it even exists, and reemphasizes competition for nutrients and induced resistance as the main mechanisms of action of Fo47.
Acknowledgments
The confocal photographs were made at the Centre de Microscopie Appliquée à la Biologie, University of Burgundy, Dijon, France. The GFP-transformed strain of Fo47 was provided by M. J. Daboussi, Institut de Génétique et Microbiologie, CNRS, Orsay, France.
This work was supported in part by European Project 2E—BCAs in Crops (Food-CT-2003-001687). The third and fourth authors (J.N. and J.F.) were supported in part by Stiftelsen Oscar och Lili Lamms Minne, MISTRA, and Formas, Sweden.
REFERENCES
- 1.Alabouvette, C., D. de la Broise, P. Lemanceau, Y. Couteaudier, and J. Louvet. 1987. Utilisation de souches non pathogènes de Fusarium pour lutter contre les fusarioses: situation actuelle dans la pratique. Bull. OEPP 17:665-674. [Google Scholar]
- 2.Bao, J. R., and G. Lazarovits. 2001. Differential colonization of tomato roots by nonpathogenic and pathogenic Fusarium oxysporum strains may influence fusarium wilt control. Phytopathology 91:449-456. [DOI] [PubMed] [Google Scholar]
- 3.Biles, C. L., and R. D. Martyn. 1989. Local and systemic resistance induced in watermelons by formae speciales of Fusarium oxysporum. Phytopathology 79:856-860. [Google Scholar]
- 4.Bloemberg, G. V., A. H. M. Wijfjes, G. E. M. Lamers, N. Stuurman, and B. J. J. Lugtengerg. 2000. Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: new perspectives for studying microbial communities. Mol. Plant-Microbe Interact. 13:1170-1176. [DOI] [PubMed] [Google Scholar]
- 5.Bolwerk, A., A. L. Lagopodi, A. H. M. Wijfjes, G. E. M. Lamers, T. F. C. Chin-A-Woeng, B. J. J. Lugtenberg, and G. V. Bloemberg. 2003. Interactions in the tomato rhizosphere of two Pseudomonas biocontrol strains with the phytopathogenic fungus Fusarium oxysporum f. sp. radicis-lycopersici. Mol. Plant-Microbe Interact. 16:983-993. [DOI] [PubMed] [Google Scholar]
- 6.Correll, J. C., C. J. R. Klittich, and J. F. Leslie. 1987. Nitrate non-utilizing mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology 77:1640-1646. [Google Scholar]
- 7.Eparvier, A., and C. Alabouvette. 1994. Use of ELISA and GUS-transformed strains to study competition between pathogenic and non-pathogenic Fusarium oxysporum for root colonization. Biocontrol Sci. Technol. 4:35-47. [Google Scholar]
- 8.Fravel, D., C. Olivain, and C. Alabouvette. 2003. Fusarium oxysporum and its biocontrol. New Phytol. 157:271-279. [DOI] [PubMed] [Google Scholar]
- 9.Garrett, S. D. 1963. Soil fungi and soil fertility. Pergamon Press, Oxford, United Kingdom.
- 10.Horowitz, S., S. Freeman, and A. Sharon. 2002. Use of green fluorescent protein-transgenic strains to study pathogenic and nonpathogenic lifestyles in Colletotrichum acutatum. Phytopathology 92:743-749. [DOI] [PubMed] [Google Scholar]
- 11.Lagopodi, A. L., A. F. L. Ram, G. E. M. Lamers, P. J. Punt, C. A. M. J. J. Van den Hondel, B. J. J. Lugtenberg, and G. V. Bloemberg. 2002. Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol. Plant-Microbe Interact. 15:172-179. [DOI] [PubMed] [Google Scholar]
- 12.Larkin, R. P., and D. R. Fravel. 1999. Mechanisms of action and dose-response relationships governing biological control of fusarium wilt of tomato by nonpathogenic Fusarium spp. Phytopathology 89:1152-1161. [DOI] [PubMed] [Google Scholar]
- 13.Larkin, R. P., D. L. Hopkins, and F. N. Martin. 1996. Suppression of fusarium wilt of watermelon by nonpathogenic Fusarium oxysporum and other microorganisms recovered from disease suppressive soil. Phytopathology 86:812-819. [Google Scholar]
- 14.Lübeck, M., M. B. Knudsen, B. Jensen, U. Thrane, C. Janvier, and D. F. Jensen. 2002. GUS and GFP transformation of the biocontrol strain Clonostachys rosea IK726 and the use of these marker genes in ecological studies. Mycol. Res. 106:815-826. [Google Scholar]
- 15.Mandeel, Q., and R. Baker. 1991. Mechanisms involved in biological control of fusarium wilt of cucumber with strains of nonpathogenic Fusarium oxysporum. Phytopathology 81:462-469. [Google Scholar]
- 16.Nahalkova, J., and J. Fatehi. 2003. Red fluorescent protein (DsRed2) as a novel reporter in Fusarium oxysporum f. sp. lycopersici. FEMS Microbiol. Lett. 225:305-309. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa, K., and H. Komada. 1984. Biological control of fusarium wilt of sweet potato by non-pathogenic Fusarium oxysporum. Ann. Phytopathol. Soc. Japan 50:1-9. [Google Scholar]
- 18.Olivain, C., and C. Alabouvette. 1997. Colonization of tomato root by a non-pathogenic strain of Fusarium oxysporum. New Phytol. 137:481-494. [DOI] [PubMed] [Google Scholar]
- 19.Olivain, C., and C. Alabouvette. 1999. Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici discussed in comparaison to a non-pathogenic strain. New Phytol. 141:497-510. [DOI] [PubMed] [Google Scholar]
- 20.Olivain, C., C. Alabouvette, and C. Steinberg. 2004. Production of a mixed inoculum of Fusarium oxysporum Fo47 and Pseudomonas fluorescens C7 to control fusarium diseases. Biocontrol Sci. Technol. 14:227-238. [Google Scholar]
- 21.Olivain, C., S. Trouvelot, M. N. Binet, C. Cordier, A. Pugin, and C. Alabouvette. 2003. Colonization of flax roots and early physiological responses of flax cells inoculated with pathogenic and non-pathogenic strains of Fusarium oxysporum. Appl. Environ. Microbiol. 69:5453-5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oren, L., S. Ezrati, D. Cohen, and A. Sharon. 2003. Early events in the Fusarium verticillioides-maize interaction characterized by using a green fluorescent protein-expressing transgenic isolate. Appl. Environ. Microbiol. 69:1695-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr, K. A., and G. R. Knudsen. 2004. Use of green fluorescent protein and image analysis to quantify proliferation of Trichoderma harzianum in nonsterile soil. Phytopathology 94:1383-1389. [DOI] [PubMed] [Google Scholar]
- 24.Postma, J., and H. Rattink. 1992. Biological control of fusarium wilt of carnation with a nonpathogenic isolate of Fusarium oxysporum. Can. J. Bot. 70:1199-1205. [Google Scholar]
- 25.Rovira, A. D., E. I. Newman, H. J. Bowen, and R. Campbell. 1974. Quantitative assessment of the rhizoplane microflora by direct microscopy. Soil Biol. Biochem. 6:211-216. [Google Scholar]
- 26.Turlier, M. F., A. Eparvier, and C. Alabouvette. 1994. Early dynamic interactions between Fusarium oxysporum f. sp. lini and the roots of Linum usitatissimum as revealed by transgenic GUS-marked hyphae. Can. J. Bot. 72:1605-1612. [Google Scholar]