Abstract
Hydrothermal vent gastropods of the genus Alviniconcha are unique among metazoans in their ability to derive their nutrition from chemoautotrophic γ- and ɛ-proteobacterial endosymbionts. Although host-symbiont relationships in Alviniconcha gastropods from the Central Indian Ridge in the Indian Ocean and the Mariana Trough in the Western Pacific have been studied extensively, host-symbiont relationships in Alviniconcha gastropods from the Southwest Pacific remain largely unknown. Phylogenetic analysis using mitochondrial cytochrome c oxidase subunit I gene sequences of host gastropods from the Manus, North Fiji, and Lau Back-Arc Basins in the Southwest Pacific has revealed a new host lineage in a Alviniconcha gastropod from the Lau Basin and the occurrence of the host lineage Alviniconcha sp. type 2 in the Manus Basin. Based on 16S rRNA gene sequences of bacterial endosymbionts, two γ-proteobacterial lineages and one ɛ-proteobacterial lineage were identified in the present study. The carbon isotopic compositions of the biomass and fatty acids of the gastropod tissues suggest that the γ- and ɛ-proteobacterial endosymbionts mediate the Calvin-Benson cycle and the reductive tricarboxylic acid cycle, respectively, for their chemoautotrophic growth. Coupling of the host and symbiont lineages from the three Southwest Pacific basins revealed that each of the Alviniconcha lineages harbors different bacterial endosymbionts belonging to either the γ- or ɛ-Proteobacteria. The host specificity exhibited in symbiont selection provides support for the recognition of each of the host lineages as a distinct species. The results from the present study also suggest the possibility that Alviniconcha sp. types 1 and 2 separately inhabit hydrothermal vent sites approximately 120 m apart in the North Fiji Basin and 500 m apart in the Manus Basin.
Of the marine invertebrates that derive their nutrition from methane-oxidizing (methanotrophic) and/or sulfur-oxidizing chemoautotrophic (thioautotrophic) bacterial endosymbionts, such as vestimentifera tubeworms and bivalve mollusks, hydrothermal vent gastropods belonging to the genus Alviniconcha from the family Provannidae differ in that they are capable of harboring primary endosymbionts that do not belong to the γ-Proteobacteria (3, 4, 24). The hydrothermal vent gastropods of the genus Alviniconcha are currently known to inhabit five tectonically distinct settings, including the Central Indian Ridge in the Indian Ocean, the Mariana Trough in the Western Pacific and the Manus, North Fiji, and Lau Back-Arc Basins in the Southwest Pacific (25).
Alviniconcha hessleri from the Mariana Trough harbors a sulfur-oxidizing chemoautotrophic γ-proteobacterial endosymbiont that mediates the Calvin-Benson cycle to fix CO2 (21), whereas Alviniconcha aff. hessleri from the Central Indian Ridge harbors a chemoautotrophic ɛ-proteobacterial endosymbiont that mediates the reductive tricarboxylic acid (rTCA) cycle for CO2 fixation (22). Phylogenetic analyses of host gastropods have revealed that two host lineages, referred to as Alviniconcha sp. types 1 and 2, inhabit deep-sea hydrothermal fields in the Manus and North Fiji Basins (14). The phylogenetic affiliation of the endosymbionts of Alviniconcha sp. types 1 and 2, as well as those of the host and endosymbionts of Alviniconcha gastropods from the Lau Basin, remain poorly defined. Considering the diversity of chemoautotrophic metabolism in prokaryotes and the ability of Alviniconcha gastropods to form endosymbiotic relationships with two phylogenetically and physiologically distinct chemoautotrophic bacteria, Alviniconcha gastropods from the Southwest Pacific might be associated with previously unknown chemoautotrophic endosymbionts.
The principle aim of the present study was to identify and clarify the host-endosymbiont lineages of the Alviniconcha gastropods from the Southwest Pacific ocean. In addition, we attempted to determine the nature of the carbon metabolism and the trophic relationships between Alviniconcha and their endosymbionts using bulk and compound-specific carbon isotopic analyses.
MATERIALS AND METHODS
Gastropod specimens.
Gastropod specimens were collected from the Manus, North Fiji, and Lau Back-Arc Basins in the South West Pacific using the Shinkai 2000 and Shinkai 6500 manned submersibles. The geographic coordinates, depth of the sampling sites, date of sampling and the submersible used for the sampling are summarized in Table 1. References containing maps of the sampling locations are also listed in Table 1.
TABLE 1.
Species of Alviniconcha gastropods sampled, number of individuals, sample locations, dive numbers, and references containing maps of the sample locations
| Species | n | Collection site | Latitude/longitude | Depth (m) | Date (yr.mo.day) | Dive | Site reference |
|---|---|---|---|---|---|---|---|
| Alviniconcha sp. type 1 | 1 | White Lady site, North Fiji Basin | 16°59.5′S/173°54.9′E | 1,970 | 1991.9.8 | Shinkai 6500 #78 | 2 |
| Alviniconcha sp. type 1 | 1 | White Lady site, North Fiji Basin | 16°59.5′S/173°54.9′E | 1,970 | 1991.9.11 | Shinkai 6500 #80 | 2 |
| Alviniconcha sp. type 2 | 3 | STARMER II site, North Fiji Basin | 16°59.3′S/173°55.0′E | 1,990 | 1991.10.21 | Shinkai 6500 #92 | 2 |
| Alviniconcha sp. type 2 | 2 | Vienna Woods site, Manus Basin | 3°9.8′S/150°16.7′E | 2,510 | 1995.10.20 | Shinkai 6500 #296 | 6,9 |
| Alviniconcha sp. type 1 | 3 | PACMANUS Field D, Manus Basin | 3°43.7′S/151°40.2′E | 1,630 | 1998.11.8 | Shinkai 2000 #1067 | 6,9 |
| Alviniconcha sp. type 2 | 1 | PACMANUS Field E, Manus Basin | 3°43.6′S/151°40.3′E | 1,676 | 1998.11.2 | Shinkai 2000 #1062 | 6,9 |
| Alviniconcha sp. | 1 | Vai Lili site, Lau Basin | 22°12.9′S/176°36.5′W | 1,722 | 2004.10.8 | Shinkai 6500 #847 | 2 |
DNA analysis of the mitochondrial cytochrome oxidase I (COI) gene sequences.
Total DNA was extracted from the head-food region of the specimens from the three back-arc basins by grinding, digestion with sodium dodecyl sulfate, and extraction with phenol and chloroform. The downstream region of the COI mitochondrial gene (approximately 690 bp) was amplified by the PCR using primers COI-B (8) and COI-6 (20). The PCR conditions were as follows: 94°C for 60 s followed by 30 to 40 cycles at 92°C for 40 s, 50°C for 60 s, and 72°C for 90 s. The nucleotide sequence of the amplified fragment was determined by using an ABI3100 sequencer and a Prism Big Dye terminator sequencing kit (Applied Biosystems, Inc., Foster City, CA) with primers COI-B, TW-2 (14), COI-3 (20), Gastro-2 (12), Gastro-3 (13) and COI-6.
To better understand the phylogenetic relationships among host lineages, the upstream region of the COI gene was amplified by PCR using primers LCO1490 and HCO2198 (5) for each of the five host lineages: A. hessleri (SMR-93-10 in reference 12), Alviniconcha sp. type 1 (MB-96-16 in reference 14), Alviniconcha sp. type 2 (FB-90-7 in reference 12), Alviniconcha aff. hessleri (the first specimen in reference 11), and Alviniconcha sp. from the Lau Basin (Fig. 1). The primers COI-7 (20) and COI-D (16) were used to amplify the COI fragment of Ifremeria nautilei, a member of the Provannidae from the North Fiji (haplotype F4 in reference 13) and the Manus Basins (haplotype M1 in reference 13). Ifremeria was used as the outgroup for phylogenetic analysis. The conditions for PCR were 94°C for 60 s, followed by 30 to 40 cycles of 92°C for 40 s, 50°C for 60 s, and 72°C for 90 s.
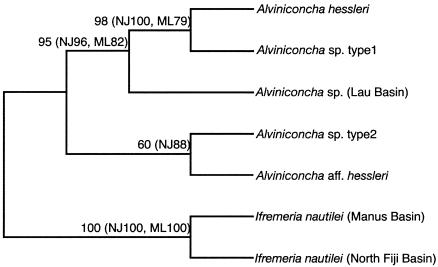
FIG. 1.
Phylogenetic relationships among the five Alviniconcha lineages known to date. The tree was constructed by using the MP method with two lineages of Ifremeria nautilei used as outgroup taxa. Numbers at the branch nodes represent bootstrap values (1,000 replicates) obtained from the MP, NJ, and the ML methods; only values greater than 50% are indicated.
Phylogenetic relationships were estimated by the maximum-parsimony (MP) method using a multiple, equally parsimonious, heuristic search with tree bisection-reconnection and 1,000 random addition sequence replicates, the maximum-likelihood (ML) method of the PAUP* package (version 4.0b10) (23), and the neighbor-joining (NJ) method (19) of the MEGA package (version 2.19 [16]).
The mitochondrial COI gene sequences from the Alviniconcha gastropods are available at the DDBJ under accession numbers AB235211 to AB235227.
DNA analysis of the endosymbiont 16S rRNA gene sequences.
Endosymbiont 16S rRNA gene sequences were analyzed as previously described (22), except that the endosymbiont DNA was extracted from the dissected gill tissue by using a DNeasy kit (QIAGEN, Valencia, CA) in accordance with the manufacturer's instructions. Endosymbiont 16S rRNA gene sequences were amplified by PCR using LA Taq polymerase (TaKaRa, Shiga, Japan) with Bac27F and Uni1492R oligonucleotide primers (17). The PCR conditions were 96°C for 120 s, followed by 27 to 30 cycles of 96°C for 20 s, 53°C for 45 s, and 72°C for 120 s. The amplified 16S rRNA gene sequence products were cloned by using an Original TA Cloning Kit (Invitrogen, Carlsbad, CA) before being sequenced with an ABI 3100 sequencer and dRhodamine Sequencing Kit according to the manufacturer's recommendations (Perkin-Elmer/Applied Biosystems).
A single phylogenetic clone type (phylotype) was obtained from the clone type analysis. The partial sequence was extended and manually aligned by using the secondary structures with ARB (18). Evolutionary analysis was performed by the NJ and MP methods using PAUP (23) based on 1,160 nucleotide positions (64 to 1417 [Escherichia coli numbering]), which have more than 50% identity across the sequences analyzed.
The bacterial 16S rRNA gene sequences from the gill endosymbionts are available at the DDBJ under the accession numbers AB235228 to AB235239.
Bulk carbon isotopic analysis.
Bulk carbon isotopic analyses were undertaken as described previously (22). The gastropod specimens were dissected into gill and mantle tissues, which were then lyophilized. The carbon isotopic compositions of the cultures and the acid-fumed gastropod tissues were analyzed by using a Thermo Electron DELTAplus Advantage mass spectrometer connected to an elemental analyzer (EA1112) through a ConFlo III interface. The measured isotopic composition was expressed as δ13C, which can be defined as follows: δ13C = [(13C/12C)sample/(13C/12C)standard − 1] × 103, where (13C/12C)sample is the 13C/12C abundance ratio for the sample and (13C/12C)standard is the 13C/12C abundance ratio for the Pee Dee Belemnite carbonate standard. The values of δ13C therefore represent the difference, in parts per thousand (per mille [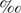 ]), between the 13C/12C value of the sample and that of the standard.
]), between the 13C/12C value of the sample and that of the standard.
Compound-specific carbon isotopic analysis.
Cellular fatty acids were extracted by using a previously described method (15). Briefly, approximately 20 mg of the gastropod tissue was incubated in 1 ml of anhydrous methanolic hydrochloric acid at 100°C for 3 h. The fatty acid methyl esters (FAMEs) were then extracted with n-hexane. The identities of the FAMEs were subsequently determined by comparison of their retention times and spectra to those of known FAME standards by gas chromatography-mass spectrometry (GCQ; Shimadzu, Tokyo, Japan). The oven temperature was set to 140°C for 3 min before being increased to 250°C at a rate of 4°C/min with He at a constant flow of 1.1 ml/min through a DB-5MS column (30 m by 0.25 μm by 0.25 mm; J&W Scientific, Folsom, CA). Standard nomenclature was used for fatty acids, which were designated in an X:Y format, where X is the number of carbon atoms and Y is the number of double bonds.
The δ13C values of the FAMEs were determined using the GC-carbon-isotope ratio MS using a Thermo Electron DELTAplus Advantage mass spectrometer connected to a gas chromatograph (Agilent 6890; Agilent, Mountain View, CA) through a GC/C/C/III interface as described previously (22). The oven temperature was set to 120°C for 3 min before being increased to 300°C at a rate of 4°C/min with He at a constant flow of 1.1 ml/min through an HP-5 column (30 m by 0.25 μm by 0.25 mm; Agilent). The isotopic compositions of the FAMEs were measured with an internal isotopic standard (19:0, δ13C = −29.80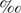 ) with a correction made for the additional carbon atom from the methanol-derivatizing reagent (δ13C = −39.04
) with a correction made for the additional carbon atom from the methanol-derivatizing reagent (δ13C = −39.04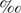 ). The internal isotopic standard reduced measurement errors to within 1
). The internal isotopic standard reduced measurement errors to within 1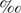 for all isotopic analyses.
for all isotopic analyses.
RESULTS AND DISCUSSION
Genetic differentiation of the host gastropods.
In order to reveal the host lineages of Alviniconcha gastropods and their distribution in the South West Pacific basins, a nucleotide sequence for the downstream region of the COI mitochondrial gene (696 bp) was determined for a single specimen from the Vai Lili site in the Lau Basin, as well as several sites in the Manus and North Fiji Basins. As summarized in Table 1, Alviniconcha sp. type 1 inhabited PACMANUS field D in the Manus Basin and the White Lady site in the North Fiji Basin, whereas Alviniconcha sp. type 2 occurred in PACMANUS field E and at the Vienna Woods site in the Manus Basin and in the STARMER II site in the North Fiji Basin. Consequently, it appears that the distribution of Alviniconcha spp. types 1 and 2 within the fields and sites examined in the present study does not overlap. Instead, despite the relatively close geographic proximity of Alviniconcha sp. types 1 and 2—120 m between the White Lady and STARMER II sites in the North Fiji Basin, as well as the 500 m between the PACMANUS fields D and E in the Manus Basin—an allopatric distribution was assumed for the two Alviniconcha spp. This is the first report of Alviniconcha sp. type 2 in the Manus Basin.
Phylogenetic analysis also revealed that the sequence identities between the host lineage from the Lau Basin specimen and the four other host lineages ranged from 88.3 to 91.6%, which is markedly lower than the identities among these lineages themselves, which ranged from 98.8 to 100%. It is therefore proposed that the gastropod from the Lau Basin represent a new distinct lineage in the genus Alviniconcha. The finding of marked genetic differentiation in the Lau Basin specimen is consistent with a previous study in which 20 enzymatic systems in Alviniconcha gastropods from the Lau and North Fiji Basins were analyzed (1).
To better understand phylogenetic relationships among the five Alviniconcha lineages, the nucleotide sequence from the upstream COI gene region (517 bp) was then determined and combined to that from the downstream COI gene region for the specimen from the Lau Basin, as well as each of the four other Alviniconcha lineages, for which the nucleotide sequence of the downstream region had been determined previously (11-13). On the basis of the combined COI nucleotide sequences (1,231 bp) from the five Alviniconcha lineages and two confamilial Ifremeria gastropods, it was shown that the three host lineages of A. hessleri, Alviniconcha sp. type 1, and Alviniconcha spp. from the Lau Basin formed a clade with high bootstrap probabilities (>82%). The remaining two lineages, Alvinoconcha sp. type 2 and Alvinoconcha aff. hessleri, were found to cluster together in NJ and MP trees with relatively high (88%) and low (60%) bootstrap probabilities, respectively. With the exception of Alviniconcha sp. type 2, the four lineages clustered with a bootstrap probability of 60% on the ML tree.
Phylogenetic affiliations of the Alviniconcha endosymbionts.
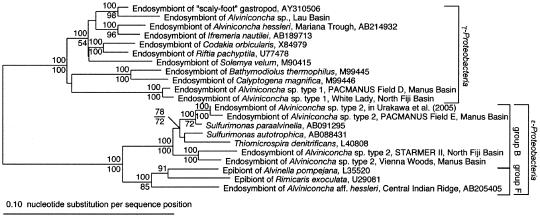
To determine the phylogenetic affiliations of gill endosymbionts, the host lineages of which were identified above, were analyzed based on their 16S rRNA gene sequences. The examination of at least eight clones generated from a 16S rRNA gene-sequence library from the gill filaments of each gastropod showed only one 16S rRNA gene sequence. As shown in Fig. 2 and Table 2, phylogenetic analysis placed the endosymbiont phylotypes within either the γ- or ɛ-Proteobacteria.
FIG. 2.
Distance tree of the members of the γ- and ɛ-Proteobacteria, as well as the Alviniconcha endosymbionts based on near-complete 16S rRNA gene sequences (1,160 nucleotides). Bootstrap values (in percent values) are based on 1,000 replicates (NJ and MP) and are shown for branches with more than 50% bootstrap support.
TABLE 2.
Carbon isotopic compositions of total biomass and FAMEs in gastropod tissues
| Host species and host tissue | Sampling site; symbiont lineage | Mean total biomass ± SD | Mean carbon isotopic composition ( |
||||
|---|---|---|---|---|---|---|---|
| 16:1 | 16:0 | 18:1 | 18:0 | 20:2 | |||
| Alviniconcha sp. type 1, Gill | White Lady site, North Fiji Basin; γ-Proteobacteria | −30.7 ± 0.6 | −38.4 ± 0.8 | −38.2 ± 0.7 | −37.4 ± 0.4 | −36.9 ± 0.1 | −37.0 ± 0.4 |
| Alviniconcha sp. type 2, | STARMER II site, North Fiji Basin; ɛ-Proteobacteria | ||||||
| Gill | −10.8 ± 0.2 | −4.7 ± 0.2 | −4.7 ± 0.2 | −5.7 ± 0.4 | −4.6 ± 0.2 | −6.3 ± 0.2 | |
| Mantle | −11.4 ± 0.3 | −5.1 ± 0.2 | −5.4 ± 0.1 | −6.0 ± 0.4 | −6.3 ± 0.3 | −5.9 ± 0.9 | |
| Alviniconcha sp. type 2 | Vienna Woods site, Manus Basin; ɛ-Proteobacteria | ||||||
| Gill | −13.3 ± 0.8 | −6.6 ± 1.4 | −8.9 ± 0.2 | −7.6 ± 0.5 | −10.3 ± 1.2 | −9.3 ± 0.0 | |
| Mantle | −13.4 ± 0.2 | −6.1 ± 0.2 | −8.4 ± 0.9 | −8.2 ± 0.6 | −9.0 ± 1.0 | −9.8 ± 0.7 | |
| Alviniconcha sp. type 1 | PACMANUS field D, Manus Basin; γ-Proteobacteria | ||||||
| Gill | −30.5 ± 0.3 | −37.5 ± 1.0 | −38.0 ± 0.7 | −36.6 ± 0.9 | −36.6 ± 0.0 | −36.8 ± 0.6 | |
| Mantle | −30.0 ± 0.1 | −36.7 ± 0.4 | −37.1 ± 0.2 | −37.6 ± 0.1 | −36.9 ± 0.3 | −37.3 ± 0.1 | |
| Alviniconcha sp. type 2 | PACMANUS field E, Manus Basin; ɛ-Proteobacteria | ||||||
| Gill | −10.6 | −5.7 | −5.7 | −5.7 | −5.4 | −7.1 | |
| Mantle | −10.3 | −6.4 | −6.5 | −7.3 | −6.3 | −7.4 | |
| Alviniconcha sp. | Vai Lili site, Lau Basin; γ-Proteobacteria | ||||||
| Gill | −30.5 | −36.1 | −36.8 | −36.1 | −35.8 | −36.0 | |
| Mantle | −30.0 | −35.6 | −36.3 | −36.1 | −36.0 | −35.7 | |
| Alviniconcha hessleri | Alice Springs, Mariana Basina; γ-Proteobacteria | ||||||
| Gill | −29.7 ± 0.4 | −38.6 ± 0.9 | −39.5 ± 0.6 | −37.7 ± 0.5 | −36.5 ± 0.7 | −36.9 ± 0.4 | |
| Mantle | −28.4 ± 0.5 | −36.1 ± 0.4 | −36.8 ± 0.1 | −36.4 ± 0.5 | −35.7 ± 0.4 | −36.5 ± 0.9 | |
| Alviniconcha aff. hessleri | Kairei Field, Central Indian Ridgeb; ɛ-Proteobacteria | ||||||
| Gill | −11.0 ± 0.1 | −5.1 ± 0.9 | −6.5 ± 0.9 | −7.7 ± 0.8d | −8.5 ± 1.4 | −7.6 ± 0.8 | |
| Mantle | −10.7 ± 0.1 | NDc | −8.3 ± 0.9 | −9.1 ± 1.4d | −9.2 ± 0.9 | −8.6 ± 2.0 | |
Specimens of Alviniconcha sp. type 1 from the PACMANUS field D in the Manus Basin and the White Lady site in the North Fiji Basin were found to harbor closely related γ-proteobacterial endosymbionts (Fig. 2). Similarly, the sequence identities among γ-proteobacterial endosymbionts within the Manus and North Fiji Basins were greater than 99.3 and 97.8%, respectively. Sequence identities between specimens from the two basins were greater than 97.7%. The endosymbionts of Alviniconcha sp. type 1 were related to free-living Thiomicrospira spp. with sequence identities of less than 90%.
Conversely, specimens of Alviniconcha sp. type 2 from the Vienna Woods site in the Manus Basin and the STARMER II site in the North Fiji Basin both harbor ɛ-proteobacterial endosymbionts (Fig. 2). The sequence identities of the ɛ-proteobacterial endosymbionts within the Manus and North Fiji Basins were greater than 99.6%, and the sequence identities between the two basins were greater than 95.0%. The ɛ-proteobacterial endosymbionts belong to a subgroup of the ɛ-Proteobacteria called group B that includes Thiomicrospira denitrificans and Sulfurimonas spp. which have been cultured previously. Although Urakawa et al. (24) reported that the Alviniconcha sp. type 1 from at the PACMANUS field E harbors an ɛ-proteobacterial endosymbiont belonging to group B (24), we subsequently determined that the gastropod specimens examined earlier (24) were affiliated with Alviniconcha sp. type 2. We also examined one specimen from the PACMANUS field E and determined that it should be regarded as Alviniconcha sp. type 2 and that, as shown in Fig. 2, the endosymbiont is closely related to that reported previously (24). Furthermore, the endosymbiont lineage from Alviniconcha sp. type 2 from at PACMANUS field E is clearly different from those found at the Vienna Woods site in the Manus Basin and the STARMER II site in the North Fiji Basin (identities of less than 93.5%).
The Alviniconcha sp. from the Lau Basin harbors a γ-proteobacterial endosymbiont that is phylogenetically distinct from those found in other Alviniconcha lineages (Fig. 2). The γ-proteobacterial endosymbiont is closely related to the endosymbiont of the “scaly-footed” hydrothermal gastropod from the Central Indian Ridge with which it shares a sequence identity of ca. 95%.
Carbon metabolism in the Alviniconcha endosymbionts.
The carbon isotopic compositions of the biomass and fatty acids of an organism are correlated with the pathways involved in CO2 fixation and subsequent acetyl-coenzyme A (acetyl-CoA) synthesis by the organism (10). Previously, enzymatic analysis has revealed that the γ-proteobacterial endosymbiont of A. hessleri from the Mariana Trough utilizes the Calvin-Benson cycle and ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) for CO2 fixation (21); the ɛ-proteobacterial endosymbiont of Alvinoconcha aff. hessleri from the Central Indian Ridge mediates the rTCA cycle for the conversion of CO2 into organic matter (22). The biomass of A. hessleri has a δ13C value of approximately −29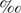 , and the fatty acids of the gastropod tissues are depleted in 13C relative to the gastropod biomass by 6.8 to 9.8
, and the fatty acids of the gastropod tissues are depleted in 13C relative to the gastropod biomass by 6.8 to 9.8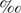 (Table 2). Conversely, the biomass of Alvinoconcha aff. hessleri has a δ13C value of approximately −11
(Table 2). Conversely, the biomass of Alvinoconcha aff. hessleri has a δ13C value of approximately −11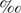 , and the fatty acids of the gastropod tissues are enriched in 13C relative to the gastropod biomass by 1.5 to 5.9
, and the fatty acids of the gastropod tissues are enriched in 13C relative to the gastropod biomass by 1.5 to 5.9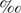 (Table 2). Since the δ13C values of CO2 in hydrothermal fluids from globally distributed deep-sea hydrothermal fields exhibit relatively small variation (−1 to −7
(Table 2). Since the δ13C values of CO2 in hydrothermal fluids from globally distributed deep-sea hydrothermal fields exhibit relatively small variation (−1 to −7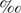 [7]), the carbon isotopic composition of the biomass and fatty acids of the gastropod tissues can be used to elucidate nature of the pathways through which hydrothermal CO2 was fixed, and fatty acids were subsequently synthesized by the bacterial endosymbionts.
[7]), the carbon isotopic composition of the biomass and fatty acids of the gastropod tissues can be used to elucidate nature of the pathways through which hydrothermal CO2 was fixed, and fatty acids were subsequently synthesized by the bacterial endosymbionts.
The Alviniconcha gastropods that harbor γ-proteobacterial endosymbionts have biomass δ13C values ranging from −30.0 to −30.7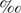 with fatty acids depleted of 13C relative to the biomass by 5.3 to 7.7
with fatty acids depleted of 13C relative to the biomass by 5.3 to 7.7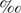 (Table 2); these findings are consistent with the chemoautotrophy of the Calvin-Benson cycle and the subsequent synthesis of fatty acids from 13C-depleted acetyl-CoA (21). As with Alvinoconcha aff. hessleri, the biomass δ13C value of other Alviniconcha gastropods that harbor ɛ-proteobacterial endosymbionts have values ranging from −10.3 to −13.4
(Table 2); these findings are consistent with the chemoautotrophy of the Calvin-Benson cycle and the subsequent synthesis of fatty acids from 13C-depleted acetyl-CoA (21). As with Alvinoconcha aff. hessleri, the biomass δ13C value of other Alviniconcha gastropods that harbor ɛ-proteobacterial endosymbionts have values ranging from −10.3 to −13.4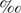 with fatty acids enriched in 13C relative to the biomass by 2.9 to 7.3
with fatty acids enriched in 13C relative to the biomass by 2.9 to 7.3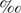 (Table 2). Since the 13C enrichment of fatty acids relative to biomass is a characteristic of fatty acid synthesis from 13C-enriched acetyl-CoA produced through the rTCA cycle, the observed isotope fractionation patterns are most likely to reflect the chemoautotrophy typical of the rTCA cycle. Although the carbon isotopic composition of hydrothermal CO2 from the sites and enzymatic activity of key enzymes involved in the two chemoautotrophic cycles were not examined in the present study, it seems likely that the γ- and ɛ-proteobacterial Alviniconcha endosymbionts assayed in the present study are chemoautotrophs that convert CO2 into organic matter via the Calvin-Benson and rTCA cycles, respectively.
(Table 2). Since the 13C enrichment of fatty acids relative to biomass is a characteristic of fatty acid synthesis from 13C-enriched acetyl-CoA produced through the rTCA cycle, the observed isotope fractionation patterns are most likely to reflect the chemoautotrophy typical of the rTCA cycle. Although the carbon isotopic composition of hydrothermal CO2 from the sites and enzymatic activity of key enzymes involved in the two chemoautotrophic cycles were not examined in the present study, it seems likely that the γ- and ɛ-proteobacterial Alviniconcha endosymbionts assayed in the present study are chemoautotrophs that convert CO2 into organic matter via the Calvin-Benson and rTCA cycles, respectively.
Host-symbiont relationships among the Alviniconcha gastropods.
In this and previous studies, the phylogenetic relationships of host gastropods and their bacterial endosymbionts were conducted in conjunction with bulk and compound-specific carbon isotopic analyses at a global scale across five tectonic settings. Analysis of fatty acid profiles of gastropod tissues revealed that the symbiont-free mantle tissue contained substantially more monosaturated C16 and C18 fatty acids and that these were derived from the endosymbionts (21, 22). Furthermore, the carbon isotopic composition of the biomass and fatty acid content of the gill and symbiont-free gastropod tissues examined in the present study were similar (Table 2). This finding suggests that endosymbiont cells are consumed by, and incorporated into, the host gastropods.
Coupling of the host and endosymbiont lineages of Alviniconcha gastropods showed that, although Alviniconcha sp. type 2 is associated with bacterial endosymbionts from two related ɛ-proteobacterial lineages, each of the other Alviniconcha lineages harbors bacterial endosymbionts from one distinct lineage within either the γ- or ɛ-Proteobacteria. These host-symbiont relationships in Alviniconcha gastropods could potentially be used to motivate for the recognition each host lineage as a separate species. Furthermore, since Alviniconcha sp. types 1 and 2 harbor γ- and ɛ-proteobacterial endosymbionts, respectively, and, given that they appear to exclusively inhabit adjacent hydrothermal vents in the Manus and North Fiji Basins, then the possibility that they occupy separate ecological niches should be explored further.
In the present study, the symbiotic relationship in Alviniconcha gastropods from the Southwest Pacific was defined more clearly by using molecular phylogenetic analyses and carbon isotopic characterizations. Further ecological and anatomical studies of these uniquely evolved gastropod assemblages might increase our understanding of the gastropod-proteobacterial endosymbioses that have been discovered in hydrothermal vent ecosystems around the globe.
Acknowledgments
We thank the captains and crews of the R/V Yokosuka and Natsushima and the Shinkai 2000 and 6500 for their technical expertise. We also thank Toshiyuki Yamaguchi and Yasunori Kano for helpful discussions.
REFERENCES
- 1.Denis, F., D. Jollivet, and D. Moraga. 1993. Genetic separation of two allopatric populations of hydrothermal snails Alviniconcha spp. (Gastropoda) from two South Western Pacific back-arc basins. Biochem. Syst. Ecol. 21:431-440. [Google Scholar]
- 2.Desbruyeres, D., A.-M. Alayse, S. Ohta, et al. 1994. Deep-sea hydrothermal communities in Southwestern Pacific back-arc basins (the North Fiji and Lau Basins): composition, microdistribution, and food web. Mar. Geol. 116:227-242. [Google Scholar]
- 3.Distel, D. L. 1998. Evolution of chemoautotrophic endosymbioses in bivalves. Bioscience 48:277-286. [Google Scholar]
- 4.Fisher, C. R. 1990. Chemoautotrophic and methanotrophic symbioses in marine invertebrates. Rev. Aquat. Sci. 2:399-436. [Google Scholar]
- 5.Folmer, O., M. Black, W. Hoeh, R. A. Lutz, and R. C. Vrijenhoek. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3:294-299. [PubMed] [Google Scholar]
- 6.Galkin, S. V. 1997. Megafauna associated with hydrothermal vents in the Manus Back-Arc Basin. Mar. Geol. 142:197-206. [Google Scholar]
- 7.Gamo, T. 1995. Wide variation of chemical characteristics of submarine hydrothermal fluids due to due to secondary modification processes after high temperature water-rock interaction: a review, p. 425-451. In H. Sakai and Y. Nozaki (ed.), Biogeochemical processes and ocean flux in the Western Pacific. Terra Scientific Publishing Company, Tokyo, Japan.
- 8.Hasegawa, T., T. Yamaguchi, S. Kojima, and S. Ohta. 1996. Phylogenetic analysis among three species of intertidal barnacles of the genus Tetraclita (Cirripedia: Balanomorpha) by nucleotide sequences of a mitochondrial gene. Benthos. Res. 51:33-39. (In Japanese with English abstract.) [Google Scholar]
- 9.Hashimoto, J., S. Ohta, A. Fialamedioni, J.-M. Auzende, S. Kojima, M. Segonzac, Y. Fujiwara, J. C. Hunt, K. Gena, T. Miura, T. Kikuchi, T. Yamaguchi, T. Toda, H. Chiba, S. Tsuchida, J. Ishibashi, K. Henry, M. Zbinden, A. Pruski, A. Inoue, H. Kobayashi, J.-L. Birrien, J. Naka, T. Yamanaka, C. Laporte, K. Nishimura, C. Yeats, S. Malagun, P. Kia, M. Oyaizu, and T. Katayama. 1999. Hydrothermal vent communities in the Manus Basin, Papua New Guinea: results of the BIOACCESS cruises '96 and '98. InterRidge News 8:12-18. [Google Scholar]
- 10.Hayes, J. M. 2001. Fractionation of the isotopes of carbon and hydrogen in biosynthetic processes, p. 225-277. In J. Vally and D. Cole (ed.), Stable isotope geochemistry, vol. 43. Mineralogical Society of America, Washington, D.C. [Google Scholar]
- 11.Kojima, S., K. Fujikura, T. Okutani, and J. Hashimoto. 2004. Phylogenetic relationship of Alviniconcha gastropods from the Indian Ocean to those from the Pacific Ocean (Provannidae: Mollusca) revealed by nucleotide sequences of mitochondrial DNA. Venus 63:65-68. [Google Scholar]
- 12.Kojima, S., R. Segawa, Y. Fujiwara, K. Fujikura, S. Ohta, and J. Hashimoto. 2001. Phylogeny of hydrothermal-vent-endemic gastropods Alviniconcha spp. from the Western Pacific revealed by mitochondrial DNA sequences. Biol. Bull. 200:298-304. [DOI] [PubMed] [Google Scholar]
- 13.Kojima, S., R. Segawa, Y. Fujiwara, J. Hashimoto, and S. Ohta. 2000. Genetic differentiation of populations of a hydrothermal vent-endemic gastropod, Ifremeria nautilei, between the North Fiji Basin and the Manus Basin revealed by nucleotide sequences of mitochondrial DNA. Zool. Sci. 17:1167-1174. [DOI] [PubMed] [Google Scholar]
- 14.Kojima, S., R. Segawa, J. Hashimoto, and S. Ohta. 1997. Molecular phylogeny of vestimentiferans collected around Japan revealed by the nucleotide sequences of mitochondrial DNA. Mar. Biol. 127:507-513. [Google Scholar]
- 15.Komagata, K., and K. Suzuki. 1987. Lipid and cell-wall analysis in bacterial systematics. Methods Microbiol. 19:161-207. [Google Scholar]
- 16.Kumar, S., K. Tamura, I. B. Jacobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Arizona State University, Tempe. [DOI] [PubMed]
- 17.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Inc., New York, N.Y.
- 18.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saitoh, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 10:471-483. [DOI] [PubMed] [Google Scholar]
- 20.Shimayama, T., H. Himeno, J. Sasuga, S. Yokobori, T. Ueda, and K. Watanabe. 1990. The genetic code of a squid mitochondrial gene. Nucleic Acids Symp. Ser. 22:77-78. [PubMed] [Google Scholar]
- 21.Suzuki, Y., T. Sasaki, M. Suzuki, K. H. Nealson, and K. Horikoshi. 2005. Molecular phylogenetic and isotopic evidence of two lineages of chemoautotrophic endosymbionts distinct at the subdivision level harbored in one host-animal type: the genus Alviniconcha (Gastropoda: Provannidae). FEMS Microbiol. Lett. 249:105-112. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki, Y., T. Sasaki, M. Suzuki, Y. Nogi, T. Miwa, K. Takai, K. H. Nealson, and K. Horikoshi. 2005. Novel chemoautotrophic endosymbiosis between a member of the Epsilonproteobacteria and the hydrothermal-vent gastropod Alviniconcha aff. hessleri (Gastropoda: Provannidae) from the Indian Ocean. Appl. Environ. Microbiol. 71:5440-5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swofford, D. L. 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates, Sunderland, Mass.
- 24.Urakawa, H., N. Dubilier, Y. Fujiwara, D. E. Cunningham, S. Kojima, and D. A. Stahl. 2005. Hydrothermal vent gastropods from the same family (Provannidae) harbour ε- and γ-proteobacterial endosymbionts. Environ. Microbiol. 7:750-755. [DOI] [PubMed] [Google Scholar]
- 25.Waren, A., and P. Bouchet. 2001. Gastropoda and monoplacophora from hydrothermal vents and seeps; new taxa and records. Veliger 44:116-231. [Google Scholar]