Abstract
The principal nutrient source for forest trees derives from the weathering of soil minerals which results from water circulation and from plant and microbial activity. The main objectives of this work were to quantify the respective effects of plant- and root-associated bacteria on mineral weathering and their consequences on tree seedling growth and nutrition. That is why we carried out two column experiments with a quartz-biotite substrate. The columns were planted with or without pine seedlings and inoculated or not with three ectomycorrhizosphere bacterial strains to quantify biotite weathering and pine growth and to determine how bacteria improve pine growth. We showed that the pine roots significantly increased biotite weathering by a factor of 1.3 for magnesium and 1.7 for potassium. We also demonstrated that the inoculation of Burkholderia glathei PML1(12) significantly increased biotite weathering by a factor of 1.4 for magnesium and 1.5 for potassium in comparison with the pine alone. In addition, we observed a significant positive effect of B. glathei PMB1(7) and PML1(12) on pine growth and on root morphology (number of lateral roots and root hairs). We demonstrated that PML1(12) improved pine growth when the seedlings were supplied with a nutrient solution which did not contain the nutrients present in the biotite. No improvement of pine growth was observed when the seedlings were supplied with all the nutrients necessary for pine growth. We therefore propose that the growth-promoting effect of B. glathei PML1(12) mainly resulted from the improved plant nutrition via increased mineral weathering.
In ecosystems with low inputs and without any fertilization or soil amendment by humans, the nutrients available to plants come from atmospheric inputs and weathering of soil minerals. This is mainly the case with forest ecosystems which, in addition, are frequently located on poor and acidic soils (3). Plants developing on acidic soils are subjected to two major constraints: (i) high concentrations of ions like Al3+ and H+ which inhibit root growth and (ii) low mineral nutrient availability as a result of low reserves and impaired uptake due to high H+ concentrations (40).
In temperate and boreal forest ecosystems, the vast majority of trees live in close association with symbiotic fungi, the ectomycorrhizal fungi, which connect tree roots to the soil environment via a broad network of hyphae. These fungi contribute to plant nutrition by carrying far away from the roots and from very small pores, the water and the nutrients they released by weathering the primary minerals (30, 59). In addition, the ectomycorrhizal symbiosis modifies root exudation qualitatively and quantitatively by changing the metabolic functions of the roots (34, 51, 56). It creates a special environment called mycorrhizosphere, where the bacterial equilibrium is different from that of the bulk soil (soil without any influence of roots) (36). These modifications of the bacterial equilibrium in the mycorrhizosphere are likely to contribute to plant nutrition (15). However, the mechanisms involved still remain to be clarified, especially the role of mycorrhizosphere bacteria on the weathering of soil primary minerals.
It is already known that the physicochemical changes in the rhizospheric soil, induced by roots and their associated microflora, influence the weathering processes and nutrient uptake by plants (23, 24, 26, 30, 39). However, the respective quantitative contributions of plant and rhizosphere microorganisms on mineral weathering processes and their consequences on plant nutrition still remain to be determined, especially in the case of nutrient-poor forest ecosystems. This study presents the first part of a project, the purpose of which is to quantify the contribution of the different microbial components of the mycorrhizosphere to mineral weathering processes and tree nutrition. It is focused on the bacterial component, the contribution of which to mineral weathering in forest soil has been completely neglected so far.
To determine the respective contribution of tree roots and mycorrhizosphere bacteria to mineral weathering, we carried out a column experiment in a growth chamber, associating pine seedlings with different combinations of three strains of Burkholderia glathei. The inputs and outputs of potassium and magnesium in solution, as well as the immobilization of these elements by pine seedlings, were measured to compare the weathering budget in the different treatments. Because the bacterial strain B. glathei PML1(12) simultaneously promoted mineral weathering and plant growth, we carried out a complementary experiment in a greenhouse to confirm these effects and to test whether both effects were linked. For that purpose, we compared the effect of this bacterial strain on the growth of pine seedlings supplied either with a deficient or a complete nutrient solution.
MATERIALS AND METHODS
Bacterial strains. (i) Origin.
A collection of 140 bacterial strains was isolated from oak (Quercus petreae)-Scleroderma citrinum ectomycorrhizas and bulk soil, sampled in the mineral soil horizon in an experimental forest site located at Breuil, in central France. The ecosystem is oligotrophic and very sensitive to nutrient deficiency and acidification. The soil is an alocrisol with acid mull type humus developed in eolian silt over a granite parent material containing two micas (12). The bacterial isolates were preserved at −80°C in nutrient broth glycerol medium (8 g liter−1 nutrient broth from Difco, 850 ml of demineralized water, and 150 ml of glycerol).
(ii) Selection of three bacterial strains.
Among our collection of 140 bacterial strains, the three strains PMB1(7), PML1(4), and PML1(12), which were isolated from oak-S. citrinum ectomycorrhizas, were chosen because they showed the highest potential for mineral weathering in three different biotests (Table 1). The first two biotests (CAS and TCP petri dish tests), which measure the bacterial efficacy for phosphorus and iron mobilization, were performed according to the method of Frey-Klett et al. (15). The third biotest, which allows quantification of the bacterial impact on biotite weathering, consisted of incubating each bacterial suspension (20 μl, A600 = 0.9) with 160 μl of nutrient solution (1.5 mg liter−1 of sodium, 2 mg liter−1 of phosphorus [10 mg liter−1 of NaH2PO4 · 2H2O], 2.3 mg liter−1 of calcium, 1.9 mg liter−1 of sulfur [10 mg liter−1 of CaSO4 · 2H2O], 11.2 mg liter−1 of nitrogen [32 mg liter−1 of NO3NH4], and 20 μl of glucose [20 g liter−1 of glucose]) and 10 mg of biotite (200 to 500 μm) for 65 h in shaken cultures (350 rounds per minute). In each of these three biotests, strain PN4(4), which was isolated from bulk soil, was used as a control because it presents a very low efficacy for P and Fe mobilization and biotite weathering. The results presented in Table 1 underline the great diversity of behaviors obtained in the different biotests for the four bacterial strains.
TABLE 1.
Capacity of three bacterial strains selected for column experiments to mobilize iron and phosphorus in three in vitro testse
| Strain | Petri dish test results
|
Solution pH | Results of biotest with mineral
|
||
|---|---|---|---|---|---|
| Efficacy of Fe mobilizationa | Efficacy of P mobilizationb | [Fe] released after biotite weathering | [K] released after biotite weathering | ||
| PMB1(7) | 0.23‡ | 0.70‡ | 2.93‡ | 2.26‡ | 2.59‡ |
| PML1(4) | 0.15† | 0.44†‡ | 2.68§ | 3.32§ | 3.37§ |
| PML1(12) | 0.27‡ | 0.26† | 3.05‡ | 2.29‡ | 2.55‡ |
| PN4(4)c | 0.00* | 0.00* | 4.05† | 1.28† | 1.90† |
| Nutrient solutiond | 5.15* | 0.94* | 1.44* | ||
The efficacy of iron mobilization was determined by the diameter of the discoloration zone on CAS medium corresponding to the zone of iron solubilization/bacterial concentration (CFU/ml).
The efficacy of phosphorus mobilization was determined by the diameter of the discoloration zone on TCP medium corresponding to the zone of phosphorus solubilization/bacterial concentration (CFU/ml).
A reference strain which presents low efficacy to mobilize nutrients in the different biotests.
effect of the noninoculated complete solution (same composition as the deficient nutrient solution used for the column experiments plus 2 g liter−1 of glucose) on the mobilization of potassium and iron in the biotest with mineral.
For each variable (efficacy of Fe and P mobilization, solution pH, and [Fe] and [K] released after biotite weathering), treatments associated with the same symbol are not significantly different according to a one-factor (bacterial treatment) ANOVA (P = 0.05) and the Bonferroni-Dunn test.
(iii) Molecular identification.
Partial sequencing of the 16S ribosomal DNA genes of the bacterial isolates was carried out as described by Bertaux et al. (7). After purification, the 16S ribosomal DNA gene products were sequenced with the eubacterial primer 27F (60). The sequences were compared with those in the GenBank databases (www.ncbi.nlm.nih.gov/BLAST) using the BLAST program (1) (Table 2).
TABLE 2.
Molecular identification of three bacterial strains
| Isolate | rrs sequence length (nudeotides) | Top match (GenBank accession no.)a | % Nucleotide identity |
|---|---|---|---|
| PMB1(7) | 453 | Burkholderia glathei (AY154378) | 98 |
| PML1(4) | 764 | Burkholderia glathei (AY154374) | 99 |
| PML1(12) | 677 | Burkholderia glathei (AY154378) | 98 |
Top match as determined by the BLAST method. The BLAST program was described by Altschul et al. (1).
Preparation of the bacterial inocula.
Bacteria were grown on 10% TSA medium (3 g liter−1 tryptic soy broth from Difco and 15 g liter−1 agar) at 25°C for 36 h. Bacteria were suspended in sterile ultrapure water and washed twice. The inoculum concentrations were then adjusted to 4 × 107 CFU ml−1 according to an A600 standard curve calibrated by plate enumeration.
Plant material.
Nonmycorrhizal Scots pine seedlings (Pinus sylvestris; provenance: Haguenau forest, France) were grown in a nonsterile peat-vermiculite substrate in a greenhouse with the following conditions: 60% humidity, night temperature of 15°C, day temperature of 22°C, a 16-h period of daylight, and watering two times for 2 min by day. After 6 weeks, the seedling roots were washed very carefully with a brush in sterile ultrapure water, four times successively, to remove remaining peat and vermiculite particles. The excess water was then removed using absorbent paper, and the seedlings were weighed individually in the case of the growth chamber experiment.
Mineral material.
The mineral chosen was a biotite from Bancroft (Canada), a 2:1 phyllosilicate, frequently present in acid soils, which weathers relatively quickly and holds K, Mg, and Fe nutrient elements (which are indispensable for plants). It is a pure homogeneous mineral and its composition is 41.01% SiO2, 10.9% Al2O3, 2.21% Fe2O3, 10.05% FeO, 0.27% MnO, 18.9% MgO, 0.41% Na2O, 9.46% K2O, 2.28% TiO2, 4.42% F, and 824 ppm Zn. Its structural formula is (Si3Al1) (Fe3+0.12 Fe2+0.61 Mg2.06 Mn0.02 Ti0.13) and K0.88 Na0.06 O10 (OH0.98 F1.02).
The biotite crystals were ground, washed with distilled water, treated ultrasonically (2 min, three times at 100 V) to remove the fine particles that electrostatically adhere to the particles with the size required, and then sieved to obtain the size fraction between 0.5 and 1 mm. Pure quartz crystals were prepared the same way and sieved to obtain two size fractions of 0.5 to 1 mm and 1 to 2 mm (2). Biotite and quartz preparations were then sterilized by autoclaving (20 min at 120°C). A previous experiment showed that the dissolution kinetics of both minerals was not modified by autoclaving.
Nutrient solutions. (i) Growth chamber experiment.
Only a deficient nutrient solution was used. The composition of this deficient nutrient solution was adjusted so that it only contained elements that were essential for pine growth but were absent from the biotite composition and in concentrations equivalent to those found in the soils at the experimental forest site at Breuil (1.5 mg liter−1 of sodium, 2 mg liter−1 of phosphorus [10 mg liter−1 NaH2PO4 · 2H2O], 2.3 mg liter−1 of calcium, 1.9 mg liter−1 of sulfur [10 mg liter−1 of CaSO4 · 2H2O], 11.2 mg liter−1 of nitrogen [32 mg liter−1 of NO3NH4]) and trace elements (0.3 mg liter−1 of molybdenum [0.3 mg liter−1 of Mo] and 0.2 mg liter−1 of copper [0.6 mg liter−1 of CuSO4 · 5H2O]).
(ii) Greenhouse experiment.
Two nutrient solutions were used. The composition of the deficient nutrient solution was the same than the one used in the growth chamber experiment. In contrast, the composition of the complete solution was the same that the deficient nutrient one but enriched with potassium, magnesium, iron, manganese, and zinc in concentrations equivalent to those found in the soils at the experimental forest site at Breuil (4.2 mg liter−1 of potassium [8 mg liter−1 of KCl] and 1 mg liter−1 of magnesium [5 mg liter−1 of MgSO4]) and trace elements (0.5 mg liter−1 of iron [2.5 mg liter−1 FeSO4 · 7H2O], 0.2 mg liter−1 of manganese [0.6 mg liter−1 of MnSO4 · H2O], and 0.2 mg liter−1 of zinc [1 mg liter−1 ZnSO4 · 7H2O]).
The pH of the two nutrient solutions was about 6. Given the quantity of nutrient solution required for the pine seedlings during the time of the experiments, the solutions were concentrated 100 times, sterilized (20 min at 120°C), stored at 4°C in 50-ml sterile tubes to limit all risk of contamination, and protected from the light. Each week, the nutrient solutions were replaced by new ones, prepared by mixing the concentrated nutrient solutions with sterile ultrapure water (20 min at 120°C).
Experimental devices and culture conditions. (i) The columns.
The columns were made from sterile inert polypropylene Falcon tubes, 15 cm high and 4 cm in diameter, with a hole drilled at the bottom. They contained a layer of pretreated (by percolation with HCl solution at pH 1 for 1 night and with sterile ultrapure water for 1 day) and sterilized (autoclaved 20 min at 120°C) glass wool (8 mm thick) on which a mixture of 2.8 g of biotite (0.5 to 1 mm diameter), 10 g of quartz (0.5 to 1 mm), and 35 g of quartz (1 to 2 mm) were placed. A preliminary experiment with fluorescein solution showed that this mixture allows a good flow in the whole column. The columns were filled individually under a laminary flow hood. In each column, one pine seedling was planted, except for those of the control treatment without plant (WP), which simulates geochemical processes. Then 1.8 ml of bacterial inoculum with a concentration of 4 × 107 CFU/ml was added to each column, except for the two control treatments, noninoculated plant (P) and without plant (WP; see below), which received 1.8 ml of sterile ultrapure water only.
(ii) Growth chamber experimental device.
The deficient nutrient solution was stored in a 20-liter sterile inert container, which was protected from the light by aluminum foil. Two peristaltic pumps were calibrated to supply the columns with the nutrient solution at a rate of 1 ml per hour via Tygon tubing. The nutrient solution percolating through the columns was collected individually in 100-ml polyethylene tubes placed below each column. The experiment included 9 treatments (with 4 replicates): 1 control without biological material (WP), 1 control with noninoculated plant (P), and 7 treatments with a plant and one bacterial strain [PMB1(7), PML1(4), or PML1(12)] or associations of strains [PMB1(7) plus PMLI(4), PMB1(7) plus PML1(12), PML1(4) plus PML1(12), and PMB1(7) plus PML1(4) plus PML1(12)]. The columns were placed in a growth chamber with 60% humidity, a night temperature of 18°C, a day temperature of 25°C, and a 17-h period of daylight.
(iii) Greenhouse experimental device.
Fifteen milliliters of sterile nutrient solutions was injected manually three times per week (Monday, Wednesday, and Friday) in each column with sterile syringes. The experiment included 4 treatments (with 10 replicates): two control treatments with noninoculated plants, supplied by either a deficient nutrient solution or a complete solution, and two plant treatments inoculated with the bacterial strain PML1(12), supplied by either a complete solution or a deficient nutrient solution. The columns were placed in a greenhouse with 60% humidity, a night temperature of 15°C, a day temperature of 22°C, and a 16-h period of daylight.
Sampling. (i) Growth chamber experiment.
Forty pine seedlings pregrown under the same conditions as those used in the column experiment were sampled at the beginning of the experiment (before the bacterial inoculation) to quantify the initial dry weight biomass and mineral content of the seedlings. For the chemical analyses, the solutions from the four replicates of each treatment were collected throughout the experiment. Four weathering budgets for the drainage solution were thus obtained for each treatment. After 76 days, all of the pine seedlings were sampled to observe root morphology and to quantify their final biomass and mineral content. Four weathering budgets for the plant immobilization were thus obtained for each treatment.
(ii) Greenhouse experiment.
At 102 days after bacterial inoculation, all of the pine seedlings were sampled for analyses: shoot length, fresh and dry weight of the root and aerial part, and length, diameter, and volume of the roots.
Analyses (i) Growth chamber experiment.
The volumes of all of the drainage solutions were measured each week. The potassium and magnesium contents of all solutions sampled were measured by induction-coupled plasma (ICP) emission spectrometer (plasma torch JY180 ULTRACE). A kinetics follow-up of the potassium and magnesium released from the biotite into the drainage solution was made for the treatment without plant to check that the experiment was in a stationary regimen (data not shown).
After the pine seedlings were washed, 0.25 g of oven-dried (65°C) aerial or root parts were ground and pretreated with 5 ml of 30% hydrogen peroxide (H2O2) for one night. Then, 5 ml of perchloric acid (HClO4) was introduced, and the solutions were placed on a warming plate for one night (40°C). These two strong oxidants digest all the organic matter to let and leave behind only the mineral elements. Then the solutions were measured by ICP emission spectrometer.
To observe the weathering surfaces of the biotite, 8 particles adhering to the seedling roots after manual shaking were sampled for each treatment (two particles per replicate). The particles were air dried and glued to a glass slide with varnish. Then the slides were coated with carbon. Images of the biotite surface and a semiquantitative analysis of the constitutive elements of the sample were made simultaneously using a Hitachi S2500 scanning electron microscope (SEM) connected to a Thermonoran microanalysis system.
To observe the root morphology and the root-mineral interface, 8 root pieces (two per replicate) were sampled for each treatment. A low-vacuum (high-pressure) Leo 1450VP SEM coupled to an Oxford microanalysis system was used. It differs from a classical scanning electron microscope by using a controlled pressure function; thus, the sample can be observed in a primary vacuum known as a low vacuum. The low-vacuum mode means that one can work without a preliminary coating and thus without altering the sample. In this way, root architecture and absorbent hairs in contact with the mineral surface could be observed. To semiquantitatively estimate the number of lateral roots and the number of root hairs, we used a binocular microscope Leica MZ 6.
(ii) Greenhouse experiment.
The shoot length (from the leaf insertion base to the terminal bud) was measured for each pine seedling at the end of the experiment. At sampling, the roots were washed very carefully in sterile ultrapure water three times successively to remove the remaining mineral particles. The excess water was removed using absorbent paper, and the fresh weight of the seedlings was determined. Then each root system was photocopied, taking into account the necessity to reduce root overlapping. To measure the different parameters relative to root architecture, the WinRhizo software was used. It allows quantification of root length and total root surface area. Then the plants were dried at 65°C for 5 days to determine the dry weight of the root and aerial parts.
Total weathering budget.
In each column of the growth chamber experiment, the total weathering budget W was calculated as W = (D − N) + I for potassium and magnesium, two elements which were not reprecipitated and not exchanged in the columns, as confirmed by scanning electron microscopy observations. D was the quantity of the element in the drainage solution, N was the quantity of the element in the nutrient solution (0 for potassium and magnesium), and I was the quantity of the element immobilized by the pine seedling during the experiment, with I = If − Ii, where Ii and If were the quantities of the element in the pine seedling at the beginning and end of the experiment, respectively.
Statistical analyses of the results.
The effect of the plant and bacterial inoculations on the weathering budgets, on the growth of the pine seedlings, and on their root architecture were determined using analyses of variance (ANOVA) at the threshold level of a P value of 0.05 and the Bonferroni-Dunn test. The Superanova software was used for all these analyses.
RESULTS
Weathering budget.
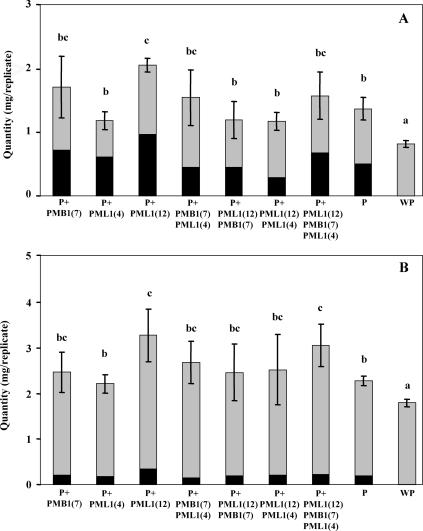
The total amounts of potassium and magnesium mobilized from biotite with noninoculated plants or with plants inoculated by the different bacterial strains were significantly higher than those in the WP treatment (Fig. 1). They were also significantly higher in the treatment inoculated with strain PML1(12) than in the P treatment. In this case, not only the amounts of magnesium leached into the solution but also the one taken up by the plant were significantly higher in the treatment inoculated with strain PML1(12) than in the noninoculated plant treatment, according to a one-factor ANOVA (P = 0.05) and the Bonferroni-Dunn test (comparison to control) (data not shown). In the same way, the amounts of potassium taken up by the plant were significantly higher in the treatment inoculated with strain PML1(12) than in the noninoculated plant treatment, according to a one-factor ANOVA (P = 0.05) and the Bonferroni-Dunn test (comparison to control) (data not shown). In contrast, when strain PML1(12) was coinoculated with strain PMB1(7) or PML1(4), the quantities of potassium and magnesium mobilized from the biotite were not significantly higher than that of the treatment with plant alone (Fig. 1). In the special case of the triple inoculation of strains PMB1(7), PML1(4), and PML1(12), only the amount of magnesium leached into the solution was significantly higher than the one in the treatment with the noninoculated plant, according to a one-factor ANOVA (P = 0.05) and the Bonferroni-Dunn test (comparison to control) (data not shown).
FIG. 1.
Mass balance of potassium (A) and magnesium (B) released into leaching solution (gray) and taken up by plants (black). Each plot is a mean value of results for four replicates. Bars represent standard deviations referring to the values of the weathering budget. For each variable (sum of potassium or magnesium quantities released into leachates and taken up by plants), treatments associated with the same letter are not significantly different according to a one-factor (plant and bacterial treatment) ANOVA (P = 0.05) and the Bonferroni-Dunn test.
Analysis of the biotite weathering processes.
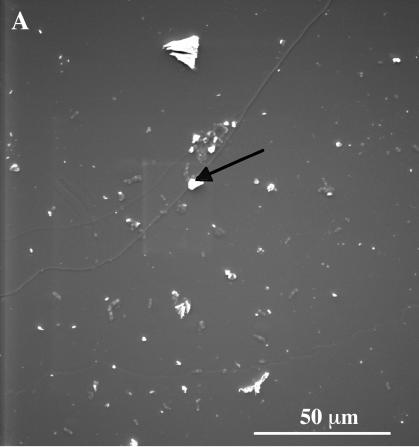
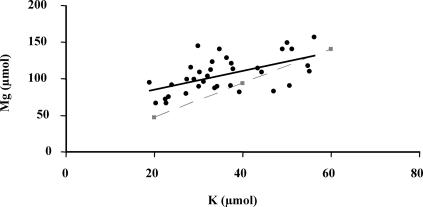
The SEM observations clearly showed that the surface of the biotite particles from the PML1(12)-inoculated plant treatment was more weathered than the one in the treatment without plant (Fig. 2). Bacterial cell accumulations were preferentially observed in carbon-rich areas (probably root exudates). Some carbon precipitates associated with sulfur and phosphorus as well as iron precipitates were also visible (white areas). Conversely, potassium, magnesium, or aluminum precipitates were not observed. The observations showed also that the majority of the biotite particles sampled along the roots were not transformed into vermiculite. This result was confirmed by plotting the total quantity of potassium released from biotite (the quantity of potassium in solution plus the quantity of potassium mobilized by the seedlings) against the total quantity of magnesium released (Fig. 3). Potassium and magnesium were released in a nearly stoichiometric way; there was no preferential release of interlayer potassium responsible for the transformation of biotite into vermiculite.
FIG. 2.
SEM photography of biotite surfaces without plant and bacteria (A) and with plant inoculated with the bacterial strain PML1(12) (B and C). The white and black areas presented with a black arrow correspond, respectively, to iron precipitates and carbon deposits. Panel C corresponds to an enlargement of the square region in panel B. The dotted arrows point to bacteria on the biotite surface.
FIG. 3.
Relation between the magnesium and potassium quantities leached into the solution and taken up by plants, i.e., mobilized in the biotite. The black linear regression curve (y = 1.29x + 59.10; R2 = 0.321) was obtained with the experimental data set. This regression is significant at a P value of <0.001. The dotted curve represents the Mg/K stoichiometry in the Bancroft biotite, which refers to a congruent dissolution process. The area under the dotted curve corresponds to the transformation domain of the biotite into vermiculite.
Growth of pine seedlings. (i) Growth chamber experiment.
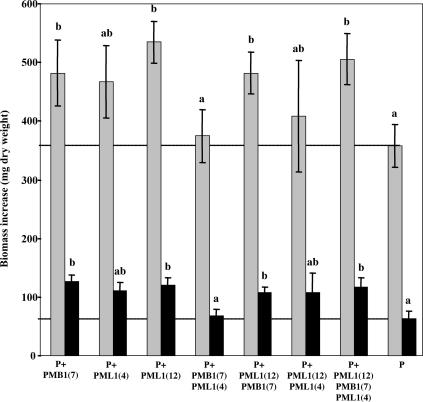
The pine seedlings inoculated with bacterial strains PMB1(7), PML1(12), PMB1(7) plus PML1(12), and PMB1(7) plus PML1(4) plus PML1(12) produced significantly more total and root biomass than the noninoculated plants (Fig. 4). These data were in accordance with low-vacuum SEM and binocular microscopy observations, which showed a higher number of lateral roots and a higher density of root hairs on PML1(12)-inoculated roots than noninoculated plants (Fig. 5 and Table 3).
FIG. 4.
Increase of the root (black) and total plant (gray) biomass during the 76 days of the growth chamber experiment in the different treatments. Each plot is a mean value of results from four replicates. Bars represent standard deviations. For each variable (total plant growth or root growth), treatments associated with the same letter are not significantly different according to a one-factor (bacterial treatment) ANOVA (P = 0.05) and the Bonferroni-Dunn test.
FIG. 5.
Low-vacuum SEM photography of a noninoculated root (A) and a root inoculated with the bacterial strain PML1(12), which presents many root hairs (B).
TABLE 3.
Semiquantitative estimation of number of lateral roots and number of root hairs in the different treatments according to binocular microscopy observations
| Treatment | No. of lateral rootsc | No. of root hairsc |
|---|---|---|
| P + PMB1(7)a | +++ | ++ |
| P + PML1(4) | ++ | + |
| P + PML1(12) | +++ | +++ |
| P + PMB1(7) + PML1(4) | ++ | ++ |
| P + PMB1(7) + PML1(12) | ++ | ++ |
| P + PML1(4) + PML1(12) | ++ | ++ |
| P + PMB1(7) + PML1(4) + PML1(12) | +++ | ++ |
| Pb | ++ | +/− |
Name of the bacterial strain inoculated.
Treatment without bacterial inoculation.
Semiquantitative estimation of the number of lateral roots and the number of root hairs, with four levels: +/−, +, ++, +++.
In contrast to the significant positive effect of some of the bacterial associations, the coinoculation of strain PML1(4) with PMB1(7) significantly reduced the promoting effect of strain PMB1(7) on the growth of pine seedlings.
(ii) Greenhouse experiment.
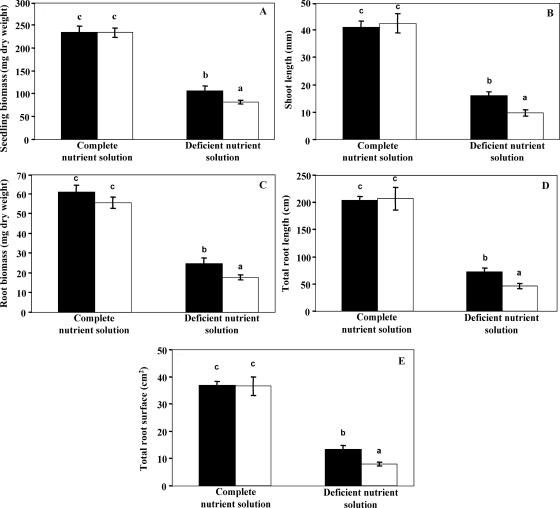
When pine seedlings were supplied by a complete nutrient solution, the inoculation of bacterial strain PML1(12) had no effect on plant growth, according to the five measured parameters: total seedling biomass, shoot length, root biomass, and total length and total surface area of the roots (Fig. 6). In contrast, as observed in the growth chamber experiment, bacterial strain PML1(12) significantly promoted the growth of pine seedlings which were supplied by the deficient nutrient solution: the inoculated plants had a significantly higher seedling biomass (Fig. 6A), shoot length (Fig. 6B), root biomass (Fig. 6C), total length of roots (Fig. 6D), and total surface area of roots (Fig. 6E) than the noninoculated seedlings.
FIG. 6.
Growth of the plants during the 102 days of the greenhouse experiment in the different treatments. (A) Total seedling biomass; (B) shoot length measured above cotyledons; (C) root biomass; (D) total the root length; (E) total root surface area. Black plots correspond to plants inoculated with the bacterial strain PML1(12), and white ones correspond to noninoculated plants. Each plot is a mean value of results from 10 replicates. Bars represent standard deviations. For each variable, treatments associated with the same letter are not significantly different according to a one-factor (bacterial treatment) ANOVA (P = 0.05) and the Bonferroni-Dunn test.
DISCUSSION
In this work, we quantified potassium and magnesium immobilized by pine seedlings or drained out from the columns and the biomass increase of the pine seedlings (i) to quantify the respective contribution of roots and root-associated bacteria to the weathering of biotite and (ii) to assess the effect of the weathering process on seedling growth. To carry out this work, we chose to perform column experiments that only contain a mineral substrate (quartz and biotite) for plant growth and no organic matter to mimic the conditions that exist in the natural forest soil horizons B and C, in which the weathering of minerals is the sole source of potassium and magnesium. To be in accordance with this experimental strategy, all of the bacterial strains we used for this work were isolated from S. citrinum-oak ectomycorrhizas sampled in a forest soil mineral horizon.
Effect of pine roots and bacteria on biotite weathering. (i) Root effect.
All of the results obtained by the different approaches (weathering budget and SEM observations) converge: the pine roots significantly increased biotite weathering by a factor 1.5 in comparison to the treatment without plant. Our results are in accordance with those of Bormann et al. (10), who demonstrated in an experimental forest that red pine (Pinus resinosa) plants improved by factors of 2.4 and 1.8 the quantities of calcium and magnesium released by weathering the primary minerals in a forest soil, respectively. In the same way, our results are in accordance with those of Bakker et al. (4), who measured plagioclase weathering in a laboratory experiment at pH 3 to 4 and 25°C and showed that the release of Si, Mg, Ca, and Al was increased by a factor of 1.8 in the presence of Douglas fir and Scots pine under these acidic conditions.
This effect of plant roots on mineral weathering may result from physical and/or biochemical processes. Indeed, plant roots induce the formation of macropores (18), which play a major role in the preferential flow phenomenon and thus the weathering process (9). Moreover, plant roots fragment soil minerals and thus increase the number of reactive surfaces (8, 43). In addition, through nutrient uptake, the plants modify the nutrient concentrations in the rhizosphere and generate zones of depletion or accumulation of nutrients (5). Plant roots also modify the redox potential and the pH in the rhizosphere (39, 41, 44, 45, 55). For example, the difference between the pH in the rhizosphere soil and in the bulk soil can be up to two pH units (38, 52). This acidification of the rhizospheric soil results from the production of CO2 during respiration, from the release of organic compounds in the root exudates (13, 22, 25, 57), and from the balance of ion charges within the roots which depends on the excretion of one H+ ion for every cation absorbed (20). These modifications of physicochemical conditions in the rhizosphere make the cations from the minerals more accessible to the plant (11, 19, 46). In addition, this phenomenon could also be regulated by nutrient deficiencies because plants that grow in potassium- or phosphorus-deficient conditions are able to modify the composition and quantity of root exudates, thus improving the mobilization of these elements (29, 37).
(ii) Bacterial effect.
The molecular identification of the three bacterial strains we chose for this study revealed that they all belong to the B. glathei species, the occurrence of which in the soil has already been mentioned. The column experiments showed that the weathering rate of biotite varied with the different bacterial strains. These results contrast with those of the in vitro tests, which demonstrated that all three bacterial strains used had previously shown great capacities to mobilize iron, potassium, and phosphorus. Our results thus underline the importance of taking into account biotic and abiotic environments, like the composition of root exudates (42), the presence of ectomycorrhizal fungi, and soil properties (pH, aeration, and physicochemical characteristics), when characterizing the weathering effect of a given bacterial strain because these parameters may influence the expression of the weathering ability of the bacteria. Moreover, our results also suggest that the weathering ability of the bacteria, which involves the production of protons, organic acids, siderophores, and organic ligands (19, 35, 49, 54, 61), depends on the bacterial strain. Finally, the fact that multiple-strain inoculation can be less efficient in mineral weathering than strains inoculated alone suggests for the first time the importance of microbial interactions in the mineral weathering process.
(iii) Plant-bacterium interaction on mineral weathering.
Our results clearly demonstrate a significant positive interaction between pine roots and the bacterial strain B. glathei PML1(12) in mineral weathering. They are consistent with those of Leyval and Berthelin (31) with a beech-Agrobacterium radiobacter model and those of Puente et al. (50) with cactus inoculated or not with different rhizoplane bacteria. In our study, the positive interaction between pine roots and B. glathei PML1(12) could simply result from an additive effect of the weathering abilities of the two partners. Another possibility is that the weathering increase is due to a synergistic effect, which could result from three hypothetical processes: (i) the fragmentation of the mineral caused by root activity increases the direct positive effect of the bacteria on mineral weathering by increasing the reactive surfaces (6); (ii) the root exudates indirectly provide the substrates required for the production of weathering metabolites by the bacteria (19), or (iii) the production of growth phytohormones by the bacteria, in addition to weathering agents, stimulates root development and modifies root physiology (17, 48) and root exudation, which improves mineral weathering and nutrient uptake (16).
Biological processes involved in biotite weathering.
The biotite can undergo two types of weathering mechanisms: dissolution by the destruction of the mineral structure (congruent way) or transformation into vermiculite (incongruent way) by the release of interlayer potassium (53). Our weathering budgets as well as SEM observations and analyses showed that, whatever the bacterial treatments, potassium and magnesium were released in a nearly stoichiometric way: the interlayer potassium was not released faster than the magnesium from the biotite structure. Consequently, biotite had mainly been dissolved by a congruent phenomenon in our experiment. Our results contrast with those from many other studies which showed evidences of incongruent dissolution of biotite under the influence of plant root activity (21) and/or under the influence of rhizospheric microorganisms (33, 47). This discrepancy could result from different experiment durations. In our experiment, the exudation and the uptake by the roots could only play an important role in the weathering process during the last 30 days of the experiment, when root development was large enough to colonize the whole column. This obviously contrasts with the experiments conducted by Hinsinger et al. (21), who put the mineral directly in contact with an already established root mat which readily produced large quantities of weathering substances and was therefore able to take up abundant quantities of potassium. In the same way, Leyval (32) observed a partial vermiculitization of another phyllosilicate (phlogopite) in the rhizosphere of pine seedlings inoculated with Agrobacterium sp. after 1 year of experimentation only.
Effect of bacteria on growth of pine seedlings.
According to the results of the growth chamber experiment, the two mycorrhizosphere bacterial strains PMB1(7) and PML1(12) behave as plant growth-promoting rhizobacteria (28). The beneficial effect of these strains could result from two nonexclusive effects: (i) an indirect effect of the bacteria on plant nutrition via an increased bacterial mobilization of nutrients from the mineral and/or (ii) a direct effect of the bacteria on plant roots by means of phytohormones, like indoleacetic acid, cytokinins, and ethylene, which stimulate the formation of lateral roots and absorbent root hairs (14, 27, 48). Indeed, an increase in the number of lateral roots was observed in the treatments inoculated with strains PMB1(7) and PML1(12) as well as with the association of PMB1(7) plus PML1(4) plus PML1(12). Moreover, for the roots inoculated with strain PML1(12), a large number of absorbing root hairs were also observed.
The greenhouse experiment demonstrated that the beneficial effect of bacterial strain PML1(12) on plant growth only occurred under conditions of nutrient deficiency. Therefore, notwithstanding a possible direct effect of the bacteria on plant growth via phytohormones, the bacteria mainly acted by an indirect way, via mineral weathering, which increased the amount of nutrients available for the plant. Indeed, if the bacteria had only promoted plant growth by a direct way, we would have observed a significant increase in the growth of the plants in the nutrient-rich conditions, too. Our results are in accordance with those of Toro et al. (58), who demonstrated the beneficial effect of two phosphate-solubilizing bacteria (Enterobacter sp. and Bacillus subtilis) on the growth of onion (Allium cepa L.) mycorrhized with Glomus intraradices via the release of P from rock phosphate. They are also in accordance with those of Leyval and Berthelin (31), who grew Pinus sylvestris cultures in lysimetric conditions where the culture substrate contained sand, phlogopite, and rock phosphate. These authors showed that Agrobacterium radiobacter, known to solubilize insoluble phosphates, significantly enhanced beech growth and P, Mg, Al, K, and Fe concentrations in roots and stems. However, the addition of soluble potassium to compensate for the potassium deficiency observed after 1 year of experimentation prevented the authors from measuring the potassium issued from the weathering of phlogopite and consequently from establishing a total weathering budget for potassium.
We confirmed the significant impact of plant roots on mineral weathering and demonstrated that a bacterial isolate, B. glathei PML1(12), significantly improved plant nutrition and promoted plant growth, mainly because of its effect on mineral weathering. We also showed that interactions between different bacterial strains significantly modify the mineral weathering budgets. This is the first step in understanding the mechanisms of sustainability in forest ecosystems on nutrient-poor soils: by their joined contribution to mineral weathering, root and mycorrhizosphere bacteria are efficient enough for mobilizing the nutrients required to maintain tree growth. Further research is now needed to quantify the respective contribution of the ectomycorrhizal fungi and the associated bacteria in the mineral weathering process in relation to tree mineral nutrition.
Acknowledgments
We acknowledge J. Garbaye for critical review of the manuscript; J. Ranger and F. Lapeyrie for helpful advice; G. Nourrisson, C. Hossann, P. Bonnaud, S. Bienaimé, D. Gelhaye, and L. Gelhaye for technical analyses; and J. L. Churin, P. Vion, B. Palin, F. Guinet, B. Simon, A. Normand, M. Cuny, D. Le Thiec, A. Kohler, A. Nys, S. Uroz, J. Jaffrain, and N. Angeli for technical help.
This work was supported by the French Research Ministry (ACI quantitative ecology project) and by the Lorraine Region.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augusto, L., J. Ranger, M. P. Turpault, and P. Bonnaud. 2001. Experimental in situ transformation of vermiculite to study the weathering impact of the tree species on the soil. Eur. J. Soil Sci. 52:81-92. [Google Scholar]
- 3.Badeau, V., E. Dambrine, and C. Walter. 1999. Propriétés des sols forestiers français: résultats du premier inventaire systématique. Etude Gest. Sols 6:165-180. [Google Scholar]
- 4.Bakker, M. R., E. George, M. P. Turpault, J. Zhang, and B. Zeller. 2004. Impact of Douglas-fir and Scots pine seedlings on plagioclase weathering under acidic conditions. Plant Soil 266:247-259. [Google Scholar]
- 5.Barber, S. A. 1995. Soil nutrient bioavailability: a mechanistic approach, 2nd ed. J. B. Wiley & Sons, New York, N.Y.
- 6.Barker, W. W., and J. F. Banfield. 1998. Zones of chemical and physical interaction at inters between microbial communities and minerals. Geomicrobiol. J. 15:223-244. [Google Scholar]
- 7.Bertaux, J., M. Schmid, N. Chemidlin Prevost-Boure, J. L. Churin, A. Hartmann, J. Garbaye, and P. Frey-Klett. 2003. In situ identification of intracellular bacteria related to Paenibacillus spp. in the ectomycorrhizal fungus Laccaria bicolor S238N. Appl. Environ. Microbiol. 69:4243-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthelin, J. 1985. Microbial weathering processes in natural environment, p. 33-59. In A. Lerman and M. Meybeck (ed.), Physical and chemical weathering cycles. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 9.Beven, K., and P. Germann. 1982. Macropores and water flow in soils. Water Resour. Res. 18:1311-1325. [Google Scholar]
- 10.Bormann, T. B., D. Wang, F. H. Bormann, B. Gaboury, R. April, and M. C. Snyder. 1998. Rapid plant-induced weathering in an aggrading experimental system. Biogeochemistry 43:129-155. [Google Scholar]
- 11.Dakora, D. F., and D. A. Phillips. 2002. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35-47. [Google Scholar]
- 12.Dejou, J. 1967. L'altération du granite à deux micas de la Pierre-Qui-Vire. Ann. Agron. 18:145-201. [Google Scholar]
- 13.Drever, J. I. 1994. The effect of land plants on weathering rates of silicate minerals. Geochim. Cosmochim. Acta 58:2325-2332. [Google Scholar]
- 14.Egamberdiyeva, D., and G. Höfflich. 2004. Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a arid region of Uzbekistan. J. Arid Environ. 56:293-301. [Google Scholar]
- 15.Frey-Klett, P., M. Chavatte, M. L. Clausse, S. Courrier, C. Le Roux, J. Raaijmakers, M. G. Martinotti, J. C. Pierrat, and J. Garbaye. 2005. Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol. 165:317-328. [DOI] [PubMed] [Google Scholar]
- 16.Gahoonia, T. S., D. Care, and N. E. Nielsen. 1997. Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant Soil 191:181-188. [Google Scholar]
- 17.Gamalero, E., M. G. Martinotti, A. Trotta, P. Lemanceau, and G. Berta. 2002. Morphological modifications induced by Pseudomonas fluorescens and Glomus mosseae in the root system of tomato differ according to plant growth conditions. New Phytol. 155:293-300. [Google Scholar]
- 18.Gibbs, R. J., and J. B. Reid. 1988. A conceptual model of changes in soil structure under different cropping systems. Adv. Soil Sci. 8:123-149. [Google Scholar]
- 19.Grayston, S. J., D. Vaughan, and D. Jones. 1996. Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl. Soil Ecol. 5:29-56. [Google Scholar]
- 20.Haynes, R. L. 1990. Active ion uptake and maintenance of cation-anion balance: a critical examination of their role in regulating rhizosphere pH. Plant Soil 126:247-264. [Google Scholar]
- 21.Hinsinger, P., B. Jaillard, and J. E. Dufey. 1992. Rapid weathering of a trioctahedral mica by roots of Ryegrass. Soil Sci. Soc. Am. J. 56:977-982. [Google Scholar]
- 22.Hinsinger, P., and R. J. Gilkes. 1997. Dissolution of phosphate rock in the rhizosphere of five plant species grown in an acid, P-fixing mineral substrate. Geoderma 75:231-249. [Google Scholar]
- 23.Hinsinger, P. 1998. How do a plant roots acquire mineral nutrients? Chemical processes involved in the rhizosphere. Adv. Agron. 64:225-265. [Google Scholar]
- 24.Jones, D. L., and P. R. Darrah. 1994. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247-257. [Google Scholar]
- 25.Jones, L. D. 1998. Organics acids in the rhizosphere-a critical review. Plant Soil 205:1849-1859. [Google Scholar]
- 26.Jones, L. D., P. G. Dennis, A. G. Owen, and P. A. W. Van Hees. 2003. Organic acids behavior in soils—misconceptions and knowledge gaps. Plant Soil 248:31-41. [Google Scholar]
- 27.Kapulnik, J., R. Gafni, and J. Okon. 1985. Effect of Azospirillum spp. inoculation on root development and NO3 uptake in wheaten hydrophonic system. Can. J. Bot. 63:627. [Google Scholar]
- 28.Kloepper, J. W., R. Lifshitz, and R. M. Zablotowicz. 1989. Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 7:39-43. [Google Scholar]
- 29.Kraffczyk, I., G. Trolldenier, and H. Beringer. 1984. Soluble root exudates of maize: influence of potassium supply and rhizosphere micro-organisms. Soil Biol. Biochem. 16:315-322. [Google Scholar]
- 30.Landeweert, R., E. Hoffland, R. D. Finlay, T. M. Kuyper, and N. Van Breemen. 2001. Linking plants to rock: ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. 16:248-253. [DOI] [PubMed] [Google Scholar]
- 31.Leyval, C., and J. Berthelin. 1989. Interactions between Laccaria laccata, Agrobacterium radiobacter and beech roots: influence on P, K, Mg and Fe mobilization from minerals and plant growth. Plant Soil 117:103-110. [Google Scholar]
- 32.Leyval, C. 1990. Lysimeters in a greenhouse as an help to study the interactions between micro-organisms, minerals, and forest tree roots, p. 335-346. In A. F. Harrison et al. (ed.), Nutrient cycling in terrestrial ecosystems. Field methods, applications and interpretations. Elsevier Applied Science, London, England.
- 33.Leyval, C., and J. Berthelin. 1991. Weathering of a mica by roots and rhizospheric micro-organisms of pine. Soil Sci. Soc. Am. J. 55:1009-1016. [Google Scholar]
- 34.Leyval, C., and J. Berthelin. 1993. Rhizodeposition and net release of soluble organic compounds by pine and beech seedlings inoculated with rhizobacteria and ectomycorrhizal fungi. Biol. Fertil. Soils 15:259-267. [Google Scholar]
- 35.Liermann, L. J., B. E. Kalinowski, S. L. Brantley, and J. G. Ferry. 2000. Role of bacterial siderophores in dissolution of hornblende. Geochim. Cosmochim. Acta 64:587-602. [Google Scholar]
- 36.Linderman, R. G. 1988. Mycorrhizal interactions with the rhizosphere microflora: the mycorrhizosphere effect. Phytopathology 78:366-371. [Google Scholar]
- 37.Lipton, D., R. W. Blanchar, and D. G. Blevins. 1987. Citrate, malate and succinate in exsudates from P-sufficient and P-stressed Medicago sativa L. seedlings. Plant Physiol. 85:315-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marschner, H., and V. Römheld. 1983. In vivo measurement of root-induced pH changes at the soil-root interface: effect of plant species and nitrogen source. Z. Pflanzenernahr. Bodenk. 111:241-256. [Google Scholar]
- 39.Marschner, H., V. Romheld, W. J. Horst, and P. Martin. 1986. Root-induced changes in the rhizosphere: importance for the mineral nutrition of plants. Z. Pflanzenernahr. Bodenk. 149:441-456. [Google Scholar]
- 40.Marschner, H. 1991. Mechanism of adaptation of plants to acid soils. Plant Soil 134:1-20. [Google Scholar]
- 41.Marschner, H. 1995. Mineral nutrition of higher plants, 2nd ed. Academic Press, London, England.
- 42.Marschner, P., C. H. Yang, R. Lieberei, and D. E. Crowley. 2001. Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol. Biochem. 33:1437-1445. [Google Scholar]
- 43.Mottershead, D. N., B. Bailly, P. Collier, and R. J. Inkpen. 2003. Identification and quantification of weathering by plant roots. Build. Environ. 38:1235-1241. [Google Scholar]
- 44.Muofhe, M. L. 1997. N2 fixation and rhizosphere ecology of Aspalathus linearis ssp. linearis (Rooibos tea). Ph.D. thesis. University of Cape Town, Cape Town, South Africa.
- 45.Nye, P. H. 1981. Changes of pH across the rhizosphere induced by roots. Plant Soil 61:7-26. [Google Scholar]
- 46.Ochs, M., I. Brunner, W. Stumm, and B. Cosovic. 1993. Effects of root exudates and humic substances on weathering kinetics. Water Air Soil Pollut. 68:213-229. [Google Scholar]
- 47.Paris, F., P. Bonnaud, J. Ranger, and F. Lapeyrie. 1995. In vitro weathering of phlogopite by ectomycorrhizal fungi. Plant Soil 177:191-201. [Google Scholar]
- 48.Patten, C. L., and B. R. Glick. 1996. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 42:207-220. [DOI] [PubMed] [Google Scholar]
- 49.Paul, E. A., and F. E. Clark. 1989. Soil microbiology and biochemistry. Academic Press, San Diego, Calif.
- 50.Puente, M. E., Y. Bashan, and V. K. Lebsky. 2004. Microbial populations and activities in the rhizosplane of rock-weathering desert plants. I. Root colonization and weathering of igneous rocks. Plant Biol. 6:629-642. [DOI] [PubMed] [Google Scholar]
- 51.Rambelli, A. 1973. The rhizosphere of mycorrhizae, p. 299-343. In G. C. Marks and T. T. Kozlowski (ed.), Ectomycorrhizae: their ecology and physiology. Academic Press, New York, N.Y.
- 52.Riley, D., and S. A. Barber. 1971. Effect of ammonium and nitrate fertilization on phosphorus uptake as related to root induced pH changes at the root-soil interface. Soil Sci. Soc. Amer. Proc. 35:300-306. [Google Scholar]
- 53.Robert, M., M. K. Razzaghe, M. A. Vicente, and G. Veneau. 1979. Rôle du facteur biochimique dans l'altération des minéraux silicatés. Bull. Ass. fr. Etud. Sol Sci. Sol 1:153-174. [Google Scholar]
- 54.Rogers, J. R., and P. C. Bennett. 2004. Mineral stimulation of subsurface micro-organisms: release of limiting nutrients from silicates. Chem. Geol. 203:91-108. [Google Scholar]
- 55.Römheld, V. 1987. Different strategies for iron acquisition in higher plants. Plant Physiol. 70:231-234. [Google Scholar]
- 56.Rygiewicz, P. T., and C. P. Andersen. 1994. Mycorrhizae alter quality and quantity of carbon allocated below ground. Nature 369:58-60. [Google Scholar]
- 57.Ström, L. 1997. Root exudation of organic acids: importance to nutrient availability and the calcifuge and calcicole behaviour of plants. Oikos 80:459-466. [Google Scholar]
- 58.Toro, M., R. Azcon, and J. M. Barea. 1997. Improvement of arbuscular mycorrhiza development by inoculation of soil with phosphate-solubilizing rhizobacteria to improve rock phosphate bioavailability (32P) and nutrient cycling. Appl. Environ. Microbiol. 63:4408-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Breemen, N., R. Finlay, U. Lundström, A. G. Jongmans, R. Giesler, and M. Olsson. 2000. Mycorrhizal weathering: a true case of mineral plant nutrition. Biogeochemistry 49:53-67. [Google Scholar]
- 60.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welch, S. A., W. W. Barker, and J. F. Banfield. 1999. Microbial extracellular polysaccharides and plagioclase dissolution. Geochim. Cosmochim. Acta 63:1405-1419. [Google Scholar]