Abstract
Fresh-cut apples contaminated with either Listeria monocytogenes or Salmonella enterica serovar Poona, using strains implicated in outbreaks, were treated with one of 17 antagonists originally selected for their ability to inhibit fungal postharvest decay on fruit. While most of the antagonists increased the growth of the food-borne pathogens, four of them, including Gluconobacter asaii (T1-D1), a Candida sp. (T4-E4), Discosphaerina fagi (ST1-C9), and Metschnikowia pulcherrima (T1-E2), proved effective in preventing the growth or survival of food-borne human pathogens on fresh-cut apple tissue. The contaminated apple tissue plugs were stored for up to 7 days at two different temperatures. The four antagonists survived or grew on the apple tissue at 10 or 25°C. These four antagonists reduced the Listeria monocytogenes populations and except for the Candida sp. (T4-E4), also reduced the S. enterica serovar Poona populations. The reduction was higher at 25°C than at 10°C, and the growth of the antagonists, as well as pathogens, increased at the higher temperature.
Nontyphoidal Salmonella species are an important cause of food-borne disease in the United States (37). According to the United States Department of Agriculture (13), in more than half of the cases the bacterium is acquired from meat, poultry, or eggs, with poultry serving as the primary vehicle of transmission. However, fruits and vegetables may also be an important reservoir for Salmonella spp. (2, 8, 51). Salmonella enterica serovar Poona has been implicated in several outbreaks from cantaloupes in the past few years (1).
In the summer of 2004 there was a salmonellosis outbreak due to consumption of contaminated presliced Roma tomatoes in Pennsylvania and the mid-Atlantic states (July 2004), and there were recalls of a number of sprout products due to possible E. coli O157:H7 or Salmonella contamination. All of the recalls and outbreaks are listed on the Food and Drug Administration News website for food recalls, alerts and warnings (http://www.fda.gov/opacom/7alerts.html; http://www.fda.gov/oc/po/firmrecalls/archive.html).
Listeria monocytogenes, a food-borne human pathogen, has been associated with serious food-borne outbreaks and a number of recalls of fresh produce (4, 12). Recently, there were recalls of cut honeydew and cut cantaloupe melons (July 2003), processed and mixed fruits and vegetables (June 2002), and apple slices (March 2001). Some products were prepared in stores such as a bulk salad sold at a store in New York that was recalled due to L. monocytogenes contamination (May 2003).
The native microflora established on food may have inhibitory properties against contaminating food-borne pathogens and therefore, via competition or antibiosis, function as a hurdle to pathogen growth and survival (1, 28, 44). Therefore, it seems promising to find specific organisms among the natural microflora that are responsible for exhibiting these pathogen-inhibitory features. These organisms have the advantage of being part of the natural microbial community already established on the target produce, which may facilitate their colonization of and survival on the produce when applied in appropriate numbers.
Several bacteria, such as pseudomonads, as well as yeasts have been identified and commercialized for the control of postharvest decays caused by fungi on fruits (20). Yeasts can successfully control fungal postharvest decay in the wounded tissue of produce (11, 18, 22, 23, 24, 32, 48, 54). However, there are only a few reports about yeasts as control agents for bacterial human pathogens on produce (9, 19, 34). While some yeasts might exacerbate the problem of food-borne pathogen growth on processed produce, for example by increasing the pH (53), some yeasts and bacteria may prove useful for food safety (9, 19, 29). They may decrease the ability of food-borne pathogens to grow due to a competition for nutrients (6, 33). A combination of bacterial and yeast biocontrol strains may also be effective against pathogenic bacteria (34).
Reported mechanisms for microorganisms that control food-borne pathogens include the reduction of the pH (5), as in the application of lactic acid bacteria or the competition for nutrients and/or space (15, 45, 47). Lactic acid bacteria also produce bacteriocins (14, 27) and they may act as biocontrol agents (40). Yeast species used in our study have been reported to have biocontrol activity against yeasts and fungi. The yeast Metschnikowia pulcherrima is known to inhibit microorganisms such as other yeasts and fungi causing fruit decay by competition for limiting nutrients (21). Hanseniaspora uvarum is a killer yeast and is known for the production of a heat-resistant killer toxin that is active at low pH (38, 56). Others, such as Aureobasidium pullulans (7, 17, 42) and Candida spp. (49, 54). have been used as antagonists to fungal postharvest decays (20).
Gluconobacter strains are gram-negative acetic acid bacteria. While they are nonpathogenic towards humans, they may cause browning of some apples, though ‘Golden Delicious’ seems to be a resistant variety. The bacteria are able to grow at a low pH of 3.5 in highly concentrated sugar solutions and on fruit such as apples, pears and grapes as well as in ciders (10, 16, 52). Gluconobacter species are found and utilized in fermentation processes and production of wine, vinegar, and vitamin C among others (10, 36, 55). To our knowledge, Gluconobacter asaii has not been previously associated with biocontrol activity.
The objective of this research was to determine the inhibitory potential of microorganisms isolated from apple surfaces against the food-borne pathogens L. monocytogenes and S. enterica serovar Poona on fresh-cut apple tissue. We focused mainly on Gluconobacter asaii (T1-D1), a Candida sp. (T4-E4), Discosphaerina fagi (ST1-C9), and Metschnikowia pulcherrima (T1-E2), because these microorganisms showed inhibitory activity in screening tests.
MATERIALS AND METHODS
Fruit.
Golden Delicious apples obtained from the market were cut into wedges. From these, 10-mm-long apple tissue plugs with a radius of 5 mm were taken using a cork borer. The plugs were randomly placed into glass test tubes and treatments were applied.
Antagonists.
All antagonists were originally isolated from apple surfaces, and they were selected for their ability to control fungal postharvest decay on apples according to procedures described previously (21, 26). A preliminary screening procedure was performed on the 17 selected antagonists to select those that were the most effective against L. monocytogenes and S. enterica serovar Poona (Table 1). Apple plugs were coinoculated with the pathogens and the potential antagonist cells from overnight cultures and populations of the pathogens were recovered after 72 h at 10°C for L. monocytogenes and 25°C for S. enterica serovar Poona. The strains were separated into four different categories based on pathogen population levels recovered and consistency of results in two screening tests. In the first screening, the antagonists were placed in different categories if they increased or decreased recovery of the pathogens populations by at least 25%, and in the second screening by a minimum of 2 log units. Of the 17 antagonists studied, 11 are Metschnikowia pulcherrima yeast strains that are less than five nucleotides different from the type strain. Discosphaerina fagi, a fungal strain, differs by 14 nucleotides from Aureobasidium pullulans, a yeast-like fungus.
TABLE 1.
Results of screening various antagonists for their effect on the growth of Salmonella enterica serovar Poona at 25°C and Listeria monocytogenes at 10°C on apple plugs over 3 days
| Antagonist (strain no.) | Pathogen growth on apple plugsa
|
|
|---|---|---|
| Salmonella enterica serovar Poona | Listeria monocytogenes | |
| Metschnikowia pulcherrima (T1-A1) | N | D |
| Metschnikowia pulcherrima (ST2-A10) | I | I |
| Gluconobacter asaii (T1-D1) | N | R |
| Hanseniaspora uvarum (ST4-C14) | I | I |
| Candida sp. (T4-E4) | I | R |
| Metschnikowia pulcherrima (FMB-24H-2) | I | I |
| Metschnikowia pulcherrima (ST1-D9) | I | I |
| Metschnikowia pulcherrima (T4-A2) | I | I |
| Metschnikowia pulcherrima (T1-D2) | I | I |
| Candida sp. (T4-E5) | I | I |
| Metschnikowia pulcherrima (FMB-140H-7A) | I | I |
| Metschnikowia pulcherrima (ST1-D10) | I | D |
| Metschnikowia pulcherrima (ST3-E13) | I | I |
| Discosphaerina fagi (ST1-C9) | R | R |
| Metschnikowia pulcherrima (T1-E2) | R | R |
| Erwinia chrysanthemi (T1-C1) | I | D |
| Metschnikowia pulcherrima (T5-A2) | I | I |
Pathogen populations: R, population reduction in first and second screening; I, population increase; N, not different from control; D, population reduction in first screening, but not in second screening.
The antagonists were grown on nutrient yeast dextrose agar (NYDA) plates overnight at 25°C. The cells were scraped from the agar plates and suspended in a 0.85% NaCl solution, vortexed, centrifuged at 10,000 × g and resuspended in fresh NaCl solution. The cell concentration was adjusted to 105 to 106 CFU/ml using a SmartSpec 3000 spectrophotometer (Bio-Rad Laboratories, Richmond, Calif.) at 420 nm according to standard curves.
Pathogens.
The L. monocytogenes culture, strain LCDC 81-861 serotype 4b, from an outbreak associated with contaminated coleslaw (43), was obtained from Robert Brackett, Department of Food Science and Technology, University of Georgia Agricultural Experiment Station, Griffin, Georgia 30223. The strain was grown on tryptic soy agar (TSA, Becton Dickinson & Co., Sparks, Md.) containing 100 μg per ml of nalidixic acid (NAL, Sigma). It was found to be naturally resistant to NAL. For inoculation of the fruit plugs, L. monocytogenes cultures were grown overnight on TSA plates (BD Diagnostic Systems, Sparks, Md.) with 100 μg of NAL/ml at 37°C.
S. enterica serovar Poona strain 02A3275 was obtained from Sharon Abbott, Calif. State Dept. of Health; Enteric Bacteriology Unit, Microbial Diseases Laboratory, Department of Health Services, Berkeley, CA. The strain was isolated from cantaloupe from a 2002 multistate outbreak and demonstrated the same pulsed-field gel electrophoresis (PFGE) pattern as a strain from a multistate outbreak in 2000. In addition, it was selected in our laboratory for antibiotic resistance to rifampin (100 μg/ml) and streptomycin (50 μg/ml) and was grown on Luria Bertani agar (LBA, Becton Dickinson & Co).
Both L. monocytogenes and S. enterica serovar Poona were scraped from the agar plates, suspended in sterile saline solution (0.85% NaCl [wt/vol]), and centrifuged at 10,000 × g for 15 min. The pellet was resuspended in fresh saline solution and adjusted to a concentration of 108 to 109 CFU/ml at an optical density at 420 nm (OD420) (L. monocytogenes) or OD600 (S. enterica serovar Poona) using a SmartSpec 3000 spectrophotometer (Bio-Rad Laboratories, Richmond, Calif.) and then diluted to concentrations of 104 to 105 CFU/ml unless otherwise indicated. The exact cell concentration of the inocula was determined by spiral plating (spiral plater; DW Scientific; Shipley, West Yorkshire, England) of the bacteria suspension on TSA or LBA medium followed by incubation at 37°C for 1 day.
Application of the treatments.
The antagonist suspensions, each at 105 to 106 CFU/ml, were pipetted (25 μl) onto apple tissue plugs stored in sterile, capped glass tubes. This was followed by contamination with 25 μl of either L. monocytogenes or S. enterica serovar Poona at ∼105 CFU/ml unless otherwise noted. The inoculated plugs were stored at 10 and 25°C for up to 7 days. There were four replicates per treatment and all experiments were repeated at least once.
Recovery of pathogens and antagonists.
The pathogen and antagonist populations were recovered from the apple plugs after storage at 10 or 25°C for 0, 2, 5, and 7 days as described previously (31). Briefly, the apple tissue plugs were each placed into a sterile plastic bag containing 4.5 ml of peptone water and homogenized in a stomacher blender for 120 s at a high speed set at 8 (Bagmixer 100 Minimix; Interscience, Weymouth, Mass.). Aliquots (50 μl) of the homogenized mixtures or dilutions thereof were plated in duplicate on TSA containing 100 μg per ml of NAL for L. monocytogenes, and for S. enterica serovar Poona on LBA containing 100 μg per ml of rifampin (Sigma) or nutrient yeast dextrose agar (NYDA; per liter: 23 g nutrient agar, 10 g dextrose, 5 g yeast extract, pH 6.5; for yeasts) using a spiral plater. The peptone water used to isolate the bacteria also contained 1500 mg/liter of Natamax (Danisco Cultor, New Century, Kans.) to prevent yeast growth. The agar plates were incubated overnight at 37°C. The NYDA plates with the yeasts were incubated overnight at 25°C. Colony counts were determined using an automated plate counter (ProtoCol; Synoptics, Cambridge, United Kingdom), and the data were plotted as CFU per sample. All experiments were repeated.
Statistical analyses.
For the experiments with both L. monocytogenes and S. enterica serovar Poona the CFU data were transformed to log units, log10 (x + 1). One was added to the values to allow the zero values to be used in the analysis. As most values were quite large this should have little to no effect on the analysis results.
Any treatments where all the values were zero or had the same value (no variability) were omitted from the analysis. The data were analyzed using Proc Mixed (41) as the highest-order linear models, although treatments without variability were not included in these models.
The assumptions of the general linear model were tested. When necessary to correct for variance heterogeneity the variance grouping technique was used. When effects were statistically significant, mean comparisons were done with Sidak adjusted P values so that the experimentwise error was 0.05. Separation of least square means is indicated in the tables.
RESULTS AND DISCUSSION
Several of the antagonists that were originally isolated for their biocontrol activity against postharvest fungal decay pathogens were also able to reduce the populations of food-borne pathogens on fresh-cut apples over time. Of the 17 antagonists tested in preliminary experiments against L. monocytogenes and S. enterica serovar Poona on apple tissue plugs, seven antagonists (T1-A1, T1-D1, T4-E4, ST1-D10, ST1-C9, T1-E2, and T1-C1) looked promising in reducing the pathogen populations and were retested (Table 1).
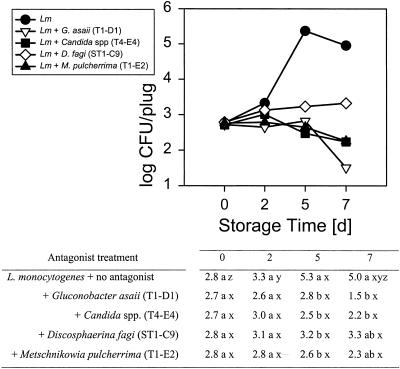
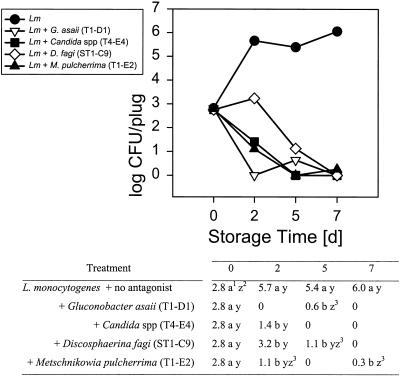
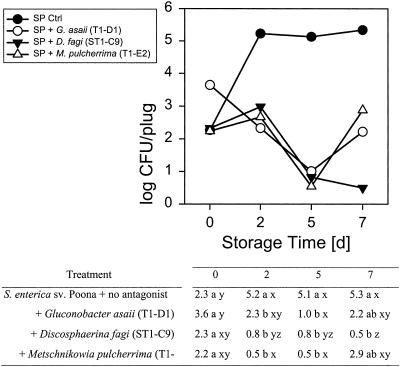
Four of these seven, G. asaii (T1-D1), a Candida sp. (T4-E4), D. fagi (ST1-C9), and M. pulcherrima (T1-E2) had antagonistic activity toward either or both of the food-borne pathogens tested. They inhibited growth or reduced the populations of L. monocytogenes on Golden Delicious apples at 10 and 25°C (Fig. 1 and 2) compared to L. monocytogenes growing alone. At 10°C the reduction was ∼2.1 to 2.8 log units after 5 days of storage (Fig. 1). While the L. monocytogenes populations on apple slices treated with any of the four antagonists did not significantly decrease compared to the initial inoculum at 10°C, they tended to decline in samples treated with G. asaii T1-D1, Candida T4-E4, and M. pulcherrima T1-E2. The reduction was greater at 25°C, at which both the antagonists and the pathogens grew more rapidly, and was already significant after 2 days of storage (Fig. 2). The reduction, compared to L. monocytogenes alone, was as high as 5.7 to 6 log units after 7 days of storage, decreasing the populations to nondetectable levels, which equals a ∼2.5 to 2.8 log reduction of the initial inoculum. The difference in pathogen reduction caused by the four different antagonists was not significant. While, at 25°C, four of the antagonists were active against the gram-positive bacterium L. monocytogenes, only three reduced the gram-negative S. enterica serovar Poona populations at that temperature (Fig. 3) and none were effective at 10°C (data not shown). Thus, at least one of the antagonists had a specific activity against L. monocytogenes, but not against S. enterica serovar Poona.
FIG. 1.
Interaction means of populations of Listeria monocytogenes in the presence of four different antagonists on Golden Delicious apple plugs stored at 10°C over 7 days. In the table, treatment means within columns with different letters (a, b) and time means within rows with different letters (x, y, z) are significantly different at the 0.05 level. Log CFU values were initially analyzed as a three-factor general linear mixed model with treatment and time as the fixed factors and experiment the random block factor. Experiment was not included in the final analysis because it accounted for little variability. Variance grouping was used. Means and mean comparisons are given.
FIG. 2.
Interaction means of populations of Listeria monocytogenes in the presence of four different antagonists on Golden Delicious apple plugs stored at 25°C over 7 days. Table footnotes: 1 and 2, treatment means within columns with different letters (a, b) and time means within rows with different letters (y, z) are significantly different at the 0.05 level; 3, treatment mean not different from zero at P < 0.05. Treatments with at least one nonzero CFU value were analyzed as a one-factor general linear model with Listeria-time coded as treatment. To correct for variance heterogeneity the variance grouping technique was used. The result was statistically significant (F = 1,186,70, P < 0.0001, df = 13). As treatment was statistically significant, mean comparisons are given.
FIG. 3.
Interaction means of populations of Salmonella Poona in the presence of three different antagonists on Golden Delicious apple plugs stored at 25° over 7 days. In the table, treatment means within columns with different letters (a, b) and time means within rows with different letters (x, y, z) are significantly different at the 0.05 level. The treatment × storage time means with all nonzero log CFU values were analyzed as a three-factor general linear mixed model with treatment and storage time as the fixed factors and experiment as the random block factor. As experiment accounted for little variability, about 2% of the residual, all treatment × storage time values were used in a two-factor general linear model for treatment and storage time. To correct for variance heterogeneity the variance grouping technique was used. Means and mean comparisons are given. As the treatment × storage time interaction was statistically significant, mean comparisons are given.
When high inoculum levels of L. monocytogenes were used (∼108 CFU/ml instead of 104 CFU/ml), only G. asaii T1-D1 reduced populations after 7 days at 10°C (Table 2). However, at 25°C, G. asaii T1-D1 and Candida sp. strain T4-E4 reduced these high initial populations of L. monocytogenes to nondetectable levels (up to 5.7 log units reduction) after 7 days, while G. asaii T1-D1, Candida sp. strain T4-E4, and D. fagi ST1-C9 reduced populations by 1.0 to 4.1 log units after 5 days. D. fagi was more effective at 25°C than at 10°C.
TABLE 2.
Interaction means of high population levels of Listeria monocytogenes on apple plugs in the presence of different antagonists and stored at 10 or 25°C over 7 days
| Antagonist | Population (log CFU/plug) after indicated storage time at 10 or 25°Ca
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 10°C
|
25°C
|
|||||||
| 0 | 2 | 5 | 7 | 0 | 2 | 5 | 7 | |
| None | 5.3 a z | 5.8 a yz | 5.7 a y | 5.5 a yz | 5.3 a y | 6.1 a y | 6.1 a y | 5.7 a y |
| Gluconobacter asaii (T1-D1) | 5.3 a yz | 5.4 a y | 5.4 bc yz | 5.0 b z | 5.3 a y | 5.4 a y | 3.2 c z | 0 |
| Candida sp. (T4-E4) | 5.4 a y | 5.5 a y | 5.2 c y | 5.2 ab y | 5.4 a y | 5.7 a y | 2.0 c yb | 0 |
| Discosphaerina fagi (ST1-C9) | 5.3 a yz | 5.6 a yz | 5.8 ab y | 5.2 ab z | 5.3 a y | 5.5 a y | 4.9 b y | 2.1 abb |
| Metschnikowia pulcherrima (T1-E2) | 5.3 a x | 5.5 a yz | 5.5 b y | 5.3 ab yz | 5.3 a y | 5.5 a y | 5.1 ab | 3.9 b z |
Plugs were inoculated with L. monocytogenes at 108 CFU/ml. Means within columns with different letters (a, b, c) are different at the 0.05 significance level. Means within rows with different letters (x, y, z) are different at the 0.05 significance level. To correct variance heterogeneity the variance grouping technique was used. The analysis was done separately for 10 and 25°C. For 10°C, Log CFU values were analyzed as a two-factor general linear model with treatment and storage time as the factors. For 25°C, the 18 treatment × storage time means with at least one nonzero log CFU value were analyzed as a one-factor general linear model with Listeria-storage time coded as the treatment. The result was statistically significant (F = 7.350, P = 0.02, df = 17).
Treatment mean not different from zero at the 0.05 significance level.
S. enterica serovar Poona, which is not a psychrotroph like L. monocytogenes, grew only slightly at 10°C, and the addition of the antagonists did not result in a significant population reduction (data not shown). This may be due to less competition and a slower growth rate at that temperature, since growth and inhibition were greater at 25°C. Three strains, G. asaii T1-D1, D. fagi ST1-C9, and M. pulcherrima T1-E2, inhibited growth of S. enterica serovar Poona at this temperature during 5 days of storage (Fig. 3). D. fagi ST1-C9 was the most effective and reduced populations by up to 4.8 log units after 7 days. While the reduction by G. asaii T1-D1and M. pulcherrima T1-E2 was significant, S. enterica serovar Poona populations increased again between the fifth and the seventh day of storage. This corresponded with a decrease of the antagonist populations (Fig. 4). However, even in that case the populations of S. enterica serovar Poona in the presence of the antagonists were still at least 2 log units below the control. D. fagi tended to reduce S. enterica serovar Poona populations at both 10 and at 25°C over the entire storage period.
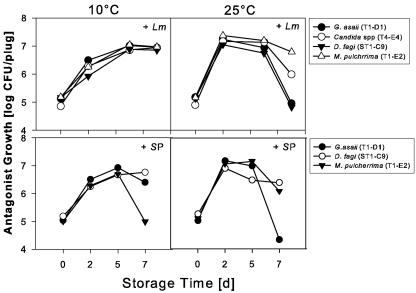
FIG. 4.
Interaction means of populations of antagonists in the presence of either S. enterica serovar Poona or L. monocytogenes from Golden Delicious apple plugs stored at 10 or 25°C over 7 days. The recovered antagonist populations were analyzed separately. As experiment accounted for very little variability, about 1% of the residual, the values were modeled as a two-factor model. To correct for variance heterogeneity the variance grouping technique was used. As time was statistically significant the mean comparisons are indicated with different letters (significant at P < 0.05). There was no difference between the growth of the antagonists. The difference over time was significant and was calculated from the means at 10 and 25°C combined.
Interestingly, all the antagonists were able to grow on apple tissue, and there was no significant difference between their growth in the presence of either pathogen (Fig. 4). The populations of the antagonists G. asaii T1-D1, Candida sp. strain T4-E4, D. fagi ST1-C9, and M. pulcherrima T1-E2 increased during the first 5 days and either stabilized or decreased toward the end of the storage period of 7 days at 10°C in the presence of S. enterica serovar Poona. At 25°C in the presence of S. enterica serovar Poona and L. monocytogenes the populations of all antagonists began to level off after 2 days and decline after 5 days. In general, the presence of either pathogen did not affect antagonist populations, which did not differ from one another at 10 or at 25°C. Based on the treatment variances from the statistical models, it was observed that the treatment variability over time increased and was 10-fold greater at day 7 than at day 0. A visual inspection of the apple plugs did not detect a change in appearance of the apple plugs in comparison to the uninoculated control.
Originally, the antagonists were isolated from apples. Some of them, such as Metschnikovia, Candida, and Gluconobacter strains, can be present in apple cider or other fruit juices (16, 20, 25). Gluconobacter was reported to cause browning in apples, with the exception of Golden Delicious (52). Since we used Golden Delicious in our experiments and did not notice any browning, this bacterium needs to be tested on other apple varieties in order to determine the scope of its applicability. Various Candida spp., e.g., C. sake and C. saitoana, are good colonizers of apple and apple wounds (20, 50). While the Candida strain we used in our research has been used mainly as a biocontrol agent against fungi, it also has biocontrol activity against bacteria. This makes it suitable for postharvest application to control both fungal decays and food-borne pathogens.
Little is known about D. fagi. According to rRNA gene analysis, D. fagi is most closely related to Aureobasidium pullulans, a yeast that has previously been studied for biocontrol purposes (35, 46). In our research we found that D. fagi may be useful for application on fresh-cut apples to control food-borne pathogens such as S. enterica serovar Poona and L. monocytogenes. Other antagonists tested, such as Hanseniaspora uvarum and Erwinia chrysanthemi, that were originally isolated for their antifungal activity, were not active against food-borne pathogens in our experiments. Erwinia chrysanthemi is also a plant pathogen causing bacterial decays on a number of different plants and it may be problematic to use it as a biocontrol agent. H. uvarum is a yeast that is known to produce a killer toxin that is active below pH 5, not sensitive to heat treatment, and lethal to other yeast strains (38, 39, 56). It is also known to inhibit Rhizopus and Botrytis spp. on grapes (3). While this yeast may be useful to control fungal decays on apples in storage, in our experiments it did not show inhibitory activity against either S. enterica serovar Poona or L. monocytogenes.
The inhibitory effect of the antagonists on food-borne pathogens was not instantaneous, and became apparent only after 2 or 5 days of storage. Future experiments will focus on the mechanism of biocontrol as well as the benefits of combining these antagonists with other biocontrol agents. Previously, we reported on reducing the same food-borne pathogens with the application of phage cocktails to fresh-cut fruit (30, 31). Preliminary experiments have shown that there may be a beneficial effect of combining these antagonists with our phage treatment, with the phage treatment having an instantaneous inhibitory effect and the antagonists controlling the pathogen populations over time. This makes them suitable for application to fresh-cut apples as well.
Acknowledgments
We thank Amy Blodgett, Michelle Orton, and Corinne Walters for technical and logistical support. We also thank Carolee Bull and Polly Goldman, ARS, USDA, Salinas, CA, for the identification of the bacterial strains as well as Eleanor Basehoar-Powers and Christie J. Robnett, ARS, USDA, Peoria, IL, for the identification of the yeast strains.
REFERENCES
- 1.Anderson, S. M., L. Verchick, R. Sowadsky, R. Civen, J. C. Mohle-Boetani, S. B. Werner, M. Starr, S. Abbott, M. Gutierrez, M. Palumbo, J. Farrar, P. Shillam, E. Umland, M. Tanuz, M. Sewell, J. Cato, W. Keene, M. Goldoft, J. Hofmann, J. Kobayashi, P. Waller, C. Braden, G. Djomand, M. Reller, and W. Chege. 2002. Multistate outbreaks of Salmonella serotype Poona infections associated with eating cantaloupe from Mexico-United States and Canada, 2000-2002 (Reprinted from Morb. Mortal. Wkly. Rep. 51:1044-1047, 2002). JAMA 288: 2967-2969. [PubMed] [Google Scholar]
- 2.Asplund, K., and E. Nurmi. 1991. The growth of salmonellae in tomatoes. Int. J. Food Microbiol. 13:177-182. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Arie, R., S. Droby, J. Zutkhi, L. Cohen, B. Weiss, P. Sarig, M. Zeidman, A. Daus, and E. Chalutz. 1991. Preharvest and postharvest biological control of rhizopus and botrytis bunch rots of table grapes with antagonistic yeasts. Agric. Res. 100-113.
- 4.Beuchat, L. R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423. [DOI] [PubMed] [Google Scholar]
- 5.Brashears, M. M., and W. A. Durre. 1999. Antagonistic action of Lactobacillus lactis toward Salmonella spp. and Escherichia coli O157: H7 during growth and refrigerated storage. J. Food Prot. 62:1336-1340. [DOI] [PubMed] [Google Scholar]
- 6.Campo, J. D., F. Carlin, and C. Nguyen-the. 2001. Effects of epiphytic Enterobacteriaceae and Pseudomonads on the growth of Listeria monocytogenes in model media. J. Food Prot. 64:721-724. [DOI] [PubMed] [Google Scholar]
- 7.Castoria, R., F. De Curtis, G. Lima, L. Caputo, S. Pacifico, and V. De Cicco. 2001. Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: study on its modes of action. Postharvest Biol. Technol. 22:7-17. [Google Scholar]
- 8.Cook, K. A., T. E. Dobbs, W. G. Hlady, J. G. Wells, T. J. Barrett, N. D. Puhr, G. A. Lancette, D. W. Bodager, B. L. Toth, C. A. Genese, A. K. Highsmith, K. E. Pilot, L. Finelli, and D. L. Swerdlow. 1998. Outbreak of Salmonella serotype Hartford infections associated with unpasteurized orange juice. JAMA 280:1504-1509. [DOI] [PubMed] [Google Scholar]
- 9.Deak, T., and L. Beuchat. 1996. Handbook of food spoilage yeasts. CRC Press, New York, NY.
- 10.Deppenmeier, U., M. Hoffmeister, and C. Prust. 2002. Biochemistry and biotechnological applications of Gluconobacter strains. Appl. Microbiol. Biotechnol. 60:233-242. [DOI] [PubMed] [Google Scholar]
- 11.Droby, S., L. Cohen, A. Daus, B. Weiss, B. Horev, E. Chalutz, H. Katz, M. Keren-Tzur, and A. Shachnai. 1998. Commercial testing of Aspire yeast preparation for the biological control of postharvest decay of citrus. Biol. Control 12:97-101. [Google Scholar]
- 12.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Food Safety and Inspection Service. 1995. Pathogen reduction; hazard analysis and critical control point (HACCP) systems; proposed rule. Fed. Reg. 60:6774-6889. [Google Scholar]
- 14.Ganzle, M. G., S. Weber, and W. P. Hammes. 1999. Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int. J. Food Microbiol. 46:207-217. [DOI] [PubMed] [Google Scholar]
- 15.Geisen, R. 1999. Inhibition of food-related pathogenic bacteria by god-transformed Penicillium nalgiovense strains. J. Food Prot. 62:940-943. [DOI] [PubMed] [Google Scholar]
- 16.Gupta, A., V. K. Singh, G. N. Qazi, and A. Kumar. 2001. Gluconobacter oxydans: Its biotechnological applications. J. Mol. Microbiol. Technol. 3:445-456. [PubMed] [Google Scholar]
- 17.Ippolito, A., A. El Ghaouth, C. L. Wilson, and M. Wisniewski. 2000. Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol. Technol. 19:265-272. [Google Scholar]
- 18.Janisiewicz, W. J. 1987. Postharvest biological control of blue mold on apples. Phytopathology 77:481-485. [Google Scholar]
- 19.Janisiewicz, W. J., W. S. Conway, and B. Leverentz. 1999. Biological control of postharvest decays of apple can prevent growth of Escherichia coli O157:H7 in apple wounds. J. Food Prot. 62:1372-1375. [DOI] [PubMed] [Google Scholar]
- 20.Janisiewicz, W. J., and L. Korsten. 2002. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 40:411-414. [DOI] [PubMed] [Google Scholar]
- 21.Janisiewicz, W. J., T. J. Tworkoski, and C. P. Kurtzman. 2001. Biocontrol potential of Metchnikowia pulcherrima strains against blue mold of apple. Phytopathology 91:1098-1108. [DOI] [PubMed] [Google Scholar]
- 22.Janisiewicz, W. J. 1998. Biocontrol of postharvest diseases of temperate fruits: challenges and opportunities, p. 171-198. In G. J. Boland and D. L. Kuykendall (ed.), Plant-microbe interactions and biological control. Marcel Dekker, Inc., New York, NY.
- 23.Janisiewicz, W. J., and B. Bors. 1995. Development of a microbial community of bacterial and yeast antagonists to control wound-invading postharvest pathogens of fruit. Appl. Environ. Microbiol. 61:3261-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jijakli, M. H., P. Lepoivre, P. Tossut, and P. Honard. 1993. Biological control of Botrytis cinerea and Penicillium sp. on post-harvest apples by two antagonistic yeasts. Med. Fac. Landbouww. Univ. Gent. 58:1349-1358. [Google Scholar]
- 25.Kurtzman, C. P., and S. Droby. 2001. Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst. Appl. Microbiol. 24:395-399. [DOI] [PubMed] [Google Scholar]
- 26.Kurtzman, C. P., and C. J. Robnett. 1998. Identification and phylogeny of ascomycetous yeasts from analysis nuclear large subunit (26S) ribosomal DNA partial sequence. Antonie van Leeuwenhoek 73:331-371. [DOI] [PubMed] [Google Scholar]
- 27.Laukova, A., P. Juris, Z. Vasilkova, and I. Papajova. 2000. Treatment of sanitary-important bacteria by bacteriocin substance V24 in cattle dung water. Lett. Appl. Microbiol. 30:402-405. [DOI] [PubMed] [Google Scholar]
- 28.Leistner, L., and G. Gorris. 1995. Food preservation by hurdle technology. Trends Food Sci. Technol. 6:41-46. [Google Scholar]
- 29.Leverentz, B., W. J. Janisiewicz, and W. S. Conway. 2002. Biological control of minimally processed fruits and vegetables, p. 319-332. In J. S. Novak, G. M. Sapers, and V. K. Juneja (ed.), Microbial safety of minimally processed foods. CRC Press, Boca Raton, Fla.
- 30.Leverentz, B., W. S. Conway, Z. Alavidze, W. J. Janisiewicz, Y. Fuchs, M. J. Camp, E. Chighladze, and A. Sulakvelidze. 2001. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit-a model study. J. Food Prot. 64:1116-1121. [DOI] [PubMed] [Google Scholar]
- 31.Leverentz, B., W. S. Conway, M. J. Camp, W. J. Janisiewicz, T. Abuladze, M. Yang, R. Saftner, and A. Sulakvelidze. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69:4519-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leverentz, B., W. J. Janisiewicz, W. S. Conway, R. A. Saftner, Y. Fuchs, C. E. Sams, and M. Camp. 2000. Combining yeasts or a bacterial biocontrol agent and heat treatment to reduce postharvest decay of ‘Gala’ apples. Postharvest Biol. Technol. 21:87-94. [Google Scholar]
- 33.Liao, C.-H. 1999. Influence of soft rot bacteria on growth of Listeria monocytogenes on potato tuber slices. J. Food Prot. 62:343-348. [DOI] [PubMed] [Google Scholar]
- 34.Liao, C. S., and W. F. Fett. 2001. Analysis of native microflora and selection of strains antagonistic to human pathogens on fresh produce. J. Food Prot. 64:1110-1115. [DOI] [PubMed] [Google Scholar]
- 35.Lumbsch, H. T. and R. Lindemuth. 2001. Major lineages of Dothideomycetes (Ascomycota) inferred from SSU and LSU rDNA sequences. Mycol. Res. 105:901-908. [Google Scholar]
- 36.Macauley, S., B. McNeil, and L. M. Harvey. 2001. The genus Gluconobacter and its applications in biotechnology. Crit. Rev. Biotechnol. 21:1-25. [DOI] [PubMed] [Google Scholar]
- 37.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radler, F., P. Pfeiffer, and M. Dennert. 1985. Killer toxins in new isolates of the yeasts Hanseniaspora uvarum and Pichia kluyveri. FEMS Micro. Biol. Lett. 29:269-272. [Google Scholar]
- 39.Radler, F., M. J. Schmitt, and B. Meyer. 1990. Killer toxin of Hanseniaspora uvarum. Arch. Microbiol. 154:175-178. [DOI] [PubMed] [Google Scholar]
- 40.Reuter, G. 2001. Probiotics—possibilities and limitations of their application in food, animal feed, and in pharmaceutical preparations for man and animals. Berl. Munch. Tierarztl. Wochenschr. 114:410-419. [PubMed] [Google Scholar]
- 41.SAS Institute Inc. 1999. SAS/STAT user's guide, version 8. SAS Institute, Cary, N.C.
- 42.Schena, L., A. Ippolito, T. Zahavi, L. Cohen, F. Nigro, and S. Droby. 1999. Genetic diversity and biocontrol activity of Aureobasidium pullulans isolates against postharvest rots. Postharvest Biol. Technol. 17:189-199. [Google Scholar]
- 43.Schlech, W. F., P. M. Lavigne, R. A. Bortolussi, A. C. Allen, E. V. Haldane, A. J. Wort, A. W. Hightower, S. E. Johnson, S. H. King, E. S. Nicholls, and C. V. Broome. 1983. Epidemic listeriosis—evidence for transmission by food. N. Engl. J. Med. 308:203-206. [DOI] [PubMed] [Google Scholar]
- 44.Schuenzel, M. K., and A. M. Harrison. 2002. Microbial antagonists of foodborne pathogens on fresh, minimally processed vegetables. J. Food Prot. 65:1909-1915. [DOI] [PubMed] [Google Scholar]
- 45.Spadaro, D., A. Garibaldi, and M. L. Gullino. 2004. Control of Penicillium expansum and Botrytis cinerea on apple combining a biocontrol agent with hot water dipping and acibenzolar-S-methyl, baking soda, or ethanol application. Postharvest Biol. Technol. 33:141-151. [Google Scholar]
- 46.Spadaro, D., and M. L. Gullino. 2004. State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 91:185-194. [DOI] [PubMed] [Google Scholar]
- 47.Spadaro, D., R. Vola, S. Piano, and M. L. Gullino. 2002. Mechanisms of action and efficacy of four isolates of the yeast Metschnikowia pulcherrima active against postharvest pathogens on apples. Postharvest Biol. Technol. 24:123-134. [Google Scholar]
- 48.Stack, J. P. 1998. Postharvest biological control: commercial successes and a model for public and private sector cooperation. Seventh International Congress of Plant Pathology, Edinburgh, Scotland.
- 49.Teixido, N., J. Usall, and I. Vinas. 1999. Efficacy of preharvest and postharvest Candida sake biocontrol treatments to prevent blue mould on apples during cold storage. Int. J. Food Microbiol. 50:203-210. [Google Scholar]
- 50.Usall, J., N. Teixido, E. Fons, and I. Vinas. 2000. Biological control of blue mould on apple by a strain of Candida sake under several controlled atmosphere conditions. Int. J. Food Microbiol. 58:83-92. [DOI] [PubMed] [Google Scholar]
- 51.U.S. Food and Drug Administration. 2000. Program information manual. Retail food safety. Food and Drug Administration Program Information Manual. U.S. Food and Drug Administration, Washington, D.C.
- 52.Van Keer, C., P. Vanden Abeele, J. Swings, F. Gossele, and J. De Lay. 1981. Acetic acid bacteria as causal agents of browning and rot of apples and pears. Zentralbl. Bakteriol. Hyg. I Abt. Orig. C 2:197-204. [Google Scholar]
- 53.Wade, W. N., R. Vasdinnyei, T. Deak, and L. R. Beuchat. 2003. Proteolytic yeasts isolated from raw, ripe tomatoes and metabiotic association of Geotrichum candidum with Salmonella. Int. J. Food Microbiol. 86:101-111. [DOI] [PubMed] [Google Scholar]
- 54.Wilson, C. L., and M. E. Wisniewski. 1989. Biological control of postharvest diseases of fruits and vegetables: An emerging technology. Annu. Rev. Phytopathol. 27:425-441. [Google Scholar]
- 55.Yamada, Y., R. Hosono, P. Lisdyanti, Y. Widyastuti, S. Saono, T. Uchimura, and K. Komagata. 1999. Identification of acetic acid bacteria isolated from Indonesian sources, especially of isolates classified in the genus Gluconobacter. J. Gen. Appl. Microbiol. 45:23-28. [DOI] [PubMed] [Google Scholar]
- 56.Zorg, J., S. Kilian, and F. Radler. 1988. Killer toxin producing strains of the yeasts Hanseniaspora uvarum and Pichia kluyveri. Arch. Microbiol. 149:261-267. [Google Scholar]