Abstract
Methyl tert-butyl ether (MTBE), an octane enhancer and a fuel oxygenate in reformulated gasoline, has received increasing public attention after it was detected as a major contaminant of water resources. Although several techniques have been developed to remediate MTBE-contaminated sites, the fate of MTBE is mainly dependent upon natural degradation processes. Compound-specific stable isotope analysis has been proposed as a tool to distinguish the loss of MTBE due to biodegradation from other physical processes. Although MTBE is highly recalcitrant, anaerobic degradation has been demonstrated under different anoxic conditions and may be an important process. To accurately assess in situ MTBE degradation through carbon isotope analysis, carbon isotope fractionation during MTBE degradation by different cultures under different electron-accepting conditions needs to be investigated. In this study, carbon isotope fractionation during MTBE degradation under sulfate-reducing and methanogenic conditions was studied in anaerobic cultures enriched from two different sediments. Significant enrichment of 13C in residual MTBE during anaerobic biotransformation was observed under both sulfate-reducing and methanogenic conditions. The isotopic enrichment factors (ɛ) estimated for each enrichment were almost identical (−13.4 to −14.6
to −14.6 ; r2 = 0.89 to 0.99). A ɛ value of −14.4
; r2 = 0.89 to 0.99). A ɛ value of −14.4 ± 0.7
± 0.7 was obtained from regression analysis (r2 = 0.97, n = 55, 95% confidence interval), when all data from our MTBE-transforming anaerobic cultures were combined. The similar magnitude of carbon isotope fractionation in all enrichments regardless of culture or electron-accepting condition suggests that the terminal electron-accepting process may not significantly affect carbon isotope fractionation during anaerobic MTBE degradation.
was obtained from regression analysis (r2 = 0.97, n = 55, 95% confidence interval), when all data from our MTBE-transforming anaerobic cultures were combined. The similar magnitude of carbon isotope fractionation in all enrichments regardless of culture or electron-accepting condition suggests that the terminal electron-accepting process may not significantly affect carbon isotope fractionation during anaerobic MTBE degradation.
Methyl tert-butyl ether (MTBE) has been used extensively as an octane enhancer and later as a fuel oxygenate in reformulated gasoline, as required by the Clean Air Act Amendments of 1990. The widespread use of MTBE to reduce air pollution has, however, resulted in major contamination of water resources (3, 11, 22, 28-30). There has been increasing interest in the development of effective technologies to remediate MTBE-contaminated sites, for instance, pump-and-treat techniques, biostimulation, and bioaugmentation (7, 9, 15, 31, 33). The full-scale implementation of these techniques is considerably costly and is currently economically applicable for only a few sites with highly sensitive receptors. Therefore, the fate of MTBE in the environment is mainly dependent upon natural remediation processes.
Monitored natural attenuation is being increasingly implemented for a number of environmental pollutants. Natural processes, including dispersion, sorption, dilution, volatilization, and biodegradation, control plume migration and reduce MTBE concentration. Among these processes, biodegradation is the most effective in reducing the mass of contaminant in the environment in a sustainable way. One challenge, however, is to accurately assess the efficiency of the remediation techniques in situ. Generally, the contaminant concentration needs to be recorded to demonstrate losses over time. Other lines of evidence to demonstrate in situ biodegradation may be the detection of degradation intermediates, as well as the depletion of the electron acceptor. In the case of MTBE, the information collected is not always conclusive. tert-Butyl alcohol (TBA), the intermediate of MTBE degradation (7), is also a by-product of MTBE production. Moreover, the biodegradation of relatively more biodegradable gasoline components, such as benzene, toluene, ethylbenzene, and xylene, might lead to the depletion of electron acceptors. Therefore, novel techniques are needed as a tool to assess ongoing in situ MTBE biodegradation and to document in situ MTBE biodegradation in natural attenuation approaches.
Compound-specific stable isotope analysis has been proposed as a potential tool to demonstrate active in situ MTBE degradation. Moreover, with the appropriate isotopic enrichment factor (ɛ), the extent of biodegradation can be estimated by using the following equation:
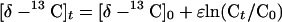 |
(1) |
where [δ-13C] represents the carbon isotope ratios of MTBE, C is the MTBE concentration, and the index value (0 or t) describes the beginning (0) or the reaction time (t). To date, there have been only four studies reporting carbon isotope fractionation studies during anaerobic MTBE degradation (16-17, 27, 35). We have previously demonstrated that anaerobic MTBE transformation to TBA under methanogenic conditions is accompanied with significant enrichment of 13C in the residual MTBE (27). Similar fractionation was also observed when methanogenesis was inhibited. Kolhatkar et al. (16) demonstrated carbon isotope fractionation during anaerobic MTBE degradation at a field site and in laboratory microcosms, although the electron-accepting processes were not identified. Kuder et al. (17) monitored carbon isotope fractionation during anaerobic MTBE by enrichments compared to fractionation in groundwater at nine gasoline spill-sites.
The stable isotope fractionation studies of other environmental contaminants, such as chlorinated solvents (4, 12-13, 25) and petroleum hydrocarbons (1, 14, 18, 20, 24), suggest that a number of factors impact isotope fractionation. These factors include the microorganism, degradation mechanisms, growth conditions, and terminal electron-accepting processes. Anaerobic MTBE degradation has been demonstrated under methanogenic (21, 26-27, 32, 34), sulfate-reducing (26), denitrifying (5-6), manganese(IV)-reducing (6), and iron(III)-reducing (6, 8) conditions. Thus, to accurately assess anaerobic in situ MTBE degradation through carbon isotope analysis, the isotope enrichment factor needs to be determined for different microbial communities and electron-accepting conditions.
In this study, carbon isotope fractionation during MTBE degradation under sulfate-reducing and methanogenic conditions was studied with anaerobic cultures enriched from two different sediments. The isotopic enrichment factor (ɛ) was estimated for each anaerobic condition. Evaluating the influence of cultures and electron-accepting conditions on carbon isotope fractionation contributes crucial information that strengthens the prospect of applying carbon isotope fractionation as a tool to demonstrate in situ MTBE biodegradation.
MATERIALS AND METHODS
Enrichment cultures.
Anaerobic cultures used in this study were enriched from sediment microcosms from two different locations, the Arthur Kill (AK), an intertidal strait between New Jersey and Staten Island, NY, and the Coronado Cays (CC), an estuarine site within the vicinity of the San Diego Bay in California. The Arthur Kill enrichment was previously demonstrated to transform MTBE to TBA under sulfate-reducing conditions (26). The microcosm was originally established using 10% (vol/vol) sediment collected from the Arthur Kill inlet. This sulfate-reducing enrichment has been grown with MTBE as the sole carbon and energy source for >8 years. To an original culture volume of 50 ml, 50 ml sulfate-reducing medium (20 mM sulfate) (26) was added to make a 1:2 dilution. The enrichment was fed twice with 20 mg liter−1 MTBE before the enrichment was split into two serum vials to produce second-generation cultures containing 50 ml enrichment slurry. The propagation was repeated once more. At the end, four serum vials, each containing 50 ml MTBE-degrading enrichment slurry, were obtained. To start the experiment for determining carbon isotope fractionation, all four enrichment cultures were combined, supernatant liquid was discarded, and 100 ml fresh sulfate-reducing medium was added. The sediment slurry was then divided evenly into four serum bottles, and fresh sulfate-reducing medium was added to a final volume of 100 ml.
A second set of enrichments were developed from microcosms established using sediment from CC. MTBE-degrading microorganisms were enriched under two different anoxic conditions, sulfate reduction and methanogenesis, following procedures previously described (26-27). For each enrichment condition, five replicate microcosms were set up, each with 5 g of wet sediment and 5 ml of appropriate medium. To investigate the role of sulfate reduction on carbon isotope fractionation, 20 mM sodium molybdate, a specific inhibitor of sulfate reduction, was added to methanogenic microcosms to suppress sulfate reduction. Two autoclaved controls were prepared for each condition. Live and killed sediments were spiked with MTBE to a final concentration of 20 mg liter−1. After >200 days of incubation, complete loss of MTBE was observed in two of the methanogenic microcosms and two of the sulfate-reducing microcosms. TBA was detected in all four microcosms, indicating biological MTBE transformation. Methane was detected in the headspace of methanogenic microcosms, confirming methanogenesis as the terminal electron acceptor of the community when sulfate reduction was inhibited. For the sulfate-reducing conditions, a reduction of 0.20 mM sulfate was calculated for MTBE transformed (only utilization of the methyl group was assumed), according to Somsamak et al. (26). This amount of sulfate depletion could not be measured accurately from the large sulfate pool (20 mM). To verify sulfate reduction as the terminal electron-accepting process, 5 mM lactate was added to sediment in a separate experiment. The sulfate concentration decreased stoichiometrically with the amount of lactate utilized. No significant loss of sulfate was observed in the presence of sodium molybdate, indicating inhibition of sulfate reduction. The active microcosms were re-fed with 10 mg liter−1 MTBE three more times before fresh medium was added to give a final volume of 100 ml (1:10 dilution). After a lengthy lag period of 75 to 120 days, all active enrichments utilized 20 mg liter−1 MTBE within 20 days (data not shown). The lag period was significantly shorter upon respiking. Before the beginning of the carbon isotope fractionation experiment, the supernatant liquid of all four enrichments was discarded, and fresh medium was replenished to a final volume of 100 ml.
Experimental setup.
To examine the carbon isotope fractionation during anaerobic MTBE degradation, eight batch experiments were set up. Four serum bottles, each containing 100 ml of sulfate-reducing AK enrichment, were prepared as described above. Sodium molybdate (20 mM), a specific inhibitor of sulfate reduction, was added to two sulfate-reducing AK cultures. The two remaining cultures were kept under conditions promoting sulfate reduction. From the CC enrichment, two of the 100-ml cultures were prepared for each methanogenic and sulfate-reducing enrichment. Each culture had been enriched individually from separate CC sediment microcosms and had never been combined. Methanogenic and sulfate-reducing media were used as abiotic controls. Anaerobic MTBE stock solution was added to all live enrichments and abiotic controls to a final concentration of 25 to 30 mg liter−1. All vials were shaken for 12 h and allowed to settle on the bench top for 30 min, and then liquid sample was taken for analysis of MTBE and TBA concentrations at day 0. For carbon isotope composition analysis, 7-ml samples were taken and added to 15-ml serum vials containing 0.6 g NaCl. The serum vials were precapped with gray Teflon-lined butyl rubber septa and crimped with aluminum seals. The samples were adjusted to pH 1 by the addition of 3N HCl. The cultures and abiotic controls were incubated in the dark, unshaken, at 37°C. The headspace MTBE concentrations of the cultures were monitored over time. After approximately 50% of MTBE utilization was observed, liquid samples were taken at selected time points for immediate MTBE and TBA analysis and for later carbon isotope composition analysis. These samples were stored at −20°C until analysis.
Analytical methods.
The concentration of MTBE was determined by a static headspace method. A 100-μl headspace sample was analyzed for MTBE with a Hewlett-Packard 5890 gas chromatograph (GC) equipped with a DB1 column (0.53 mm by 30 m; J&W Scientific, Folsom, CA) and a flame ionization detector with He as the carrier gas. The GC column temperature was first held at 35°C for 3 min, increased to 120°C at a rate of 5°C min−1, and then held for 1 min. In addition, the concentrations of MTBE and TBA were confirmed by direct injection of 1 μl aqueous sample using the same instrument and temperature program. TBA was identified by comparison of its retention times to an authentic standard. For quantification, external aqueous standards of 3.0, 9.0, 15.0, and 30.0 mg liter−1 for each compound were used. Detection limits were 0.5 mg liter−1 for MTBE and 1.0 mg liter−1 for TBA, respectively.
Stable isotope analyses were conducted at the Stable Isotope Laboratory of the UFZ Centre for Environmental Research, Leipzig-Halle, Germany. The system consisted of a GC (6890 series; Agilent Technology) coupled with a combustion interface (ThermoFinnigan GC-combustion III; ThermoFinnigan, Bremen, Germany) and a Finnigan MAT 252 isotope ratio mass spectrometer (ThermoFinnigan, Bremen, Germany). The organic substances in the CG effluent were oxidized to CO2 on a CuO-Ni-Pt catalyst held at 960°C. A Poraplot Q column (0.32 mm by 25 m; Chrompack, The Netherlands) was used for separation. Helium at a flow rate of 1.5 ml/min was used as carrier gas. The GC temperature program was held at 150°C for 15 min, increased to 220°C at a rate of 3°C min−1, and then held for 10 min isothermally. Samples were injected in split mode with a split ratio 1:1 into a hot injector held at 220°C. Headspace injection volumes ranged from 0.2 to 1 ml, based on the concentration of MTBE determined previously. Each sample was analyzed at least in triplicate.
The direct headspace method had a detection limit of approximately 4 mg liter−1 for MTBE. The carbon isotopic compositions (R) are reported as δ notation in parts per thousand (indicated as per mille values) enrichments or depletions relative to the Vienna Pee Dee Belemnite standard of the International Atomic Energy Agency (2). δ values of carbon were calculated as follows:
|
|
(2) |
where Rsample and Rstandard represent 13C/12C ratios of the sample and the Vienna Pee Dee Belemnite standard. The direct headspace analysis of standard MTBE had a mean isotope composition of −30.6 ± 0.5
± 0.5 (for the results of eight individual measurements).
(for the results of eight individual measurements).
RESULTS
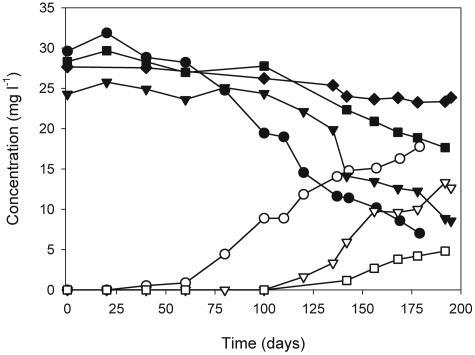
The anaerobic cultures investigated in this study were enriched from two different estuarine sediments, AK and CC. The sulfate-reducing AK enrichment has been maintained with MTBE as the sole carbon source for >8 years. In this study, the sulfate-reducing AK enrichment was used to set up duplicate cultures under conditions promoting either sulfate reduction or depressing sulfate reduction with the specific inhibitor of sulfate reduction, molybdate. MTBE and TBA concentrations in sulfate-reducing and molybdate-inhibited AK cultures and abiotic controls are shown in Fig. 1. In sulfate-reducing cultures, the MTBE concentration gradually decreased over 200 days. From an initial concentration of 29.6 mg liter−1 and 24.3 mg liter−1, 73% and 68% of MTBE utilization were achieved in replicate 1 and replicate 2 after 179 days and 195 days of incubation, respectively. Stoichiometric amounts of TBA accumulated. In the presence of molybdate, the MTBE utilization rate decreased drastically. TBA was first detected after incubation for >140 days. Therefore, the enrichments incubated with molybdate were excluded from carbon isotope fractionation analysis. At the end of the experiment, 13% of MTBE was lost in abiotic controls, but TBA was not detected.
FIG. 1.
MTBE (solid symbols) and TBA (open symbols) concentrations during anaerobic MTBE biodegradation by sulfate-reducing Arthur Kill enrichment (replicate 1, circles; replicate 2, triangles), sulfate-reducing Arthur Kill enrichment with molybdate (squares), and abiotic controls (diamonds). The data for enrichment with molybdate and abiotic controls are the average of duplicates.
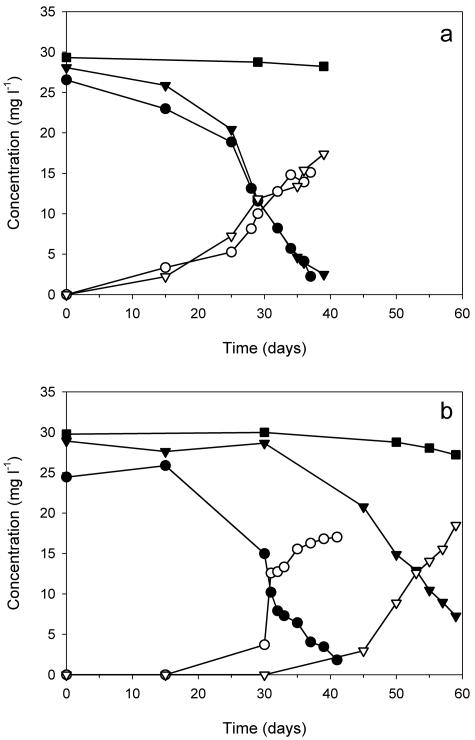
Experiments with CC enrichments were conducted with two methanogenic and two sulfate-reducing cultures. All four cultures were enriched individually from different CC microcosms. Figure 2a shows the MTBE and TBA concentration profiles for the two sulfate-reducing CC enrichments. Initial concentrations of MTBE were 26.6 mg liter−1 and 28.1 mg liter−1. After a lag period of 15 to 20 days, the enrichments utilized >90% of MTBE fed within 40 days. At the end of the experiment, TBA concentrations accounted for 74% and 81% of MTBE utilized. One of the two methanogenic CC enrichments utilized MTBE from an initial concentration of 24.5 mg liter−1 to 1.8 mg liter−1 within 40 days (Fig. 2b). MTBE degradation in the other methanogenic CC enrichment was observed after 30 days of incubation. From an initial concentration of 28.9 mg liter−1, 75% MTBE utilization was achieved within 60 days of incubation. Stoichiometric amounts of TBA (91% and 104%) accumulated. MTBE loss in abiotic controls for both experiments was <10% over 60 days.
FIG. 2.
MTBE (solid symbols) and TBA (open symbols) concentrations during anaerobic MTBE biodegradation by sulfate-reducing (a) and methanogenic (b) Coronado Cay enrichment cultures (enrichment 1, circles; enrichment 2, triangles; abiotic control, squares). The data points of abiotic controls are the averages of duplicate cultures.
All live culture samples with MTBE concentrations of >4 mg liter−1 were analyzed for carbon isotope composition. The [δ-13C] of MTBE used as reference compound was −30.6 ± 0.5
± 0.5 and the mean [δ-13C] of all samples (enrichments and abiotic controls) collected on day 0 was −28.6
and the mean [δ-13C] of all samples (enrichments and abiotic controls) collected on day 0 was −28.6 ± 0.2
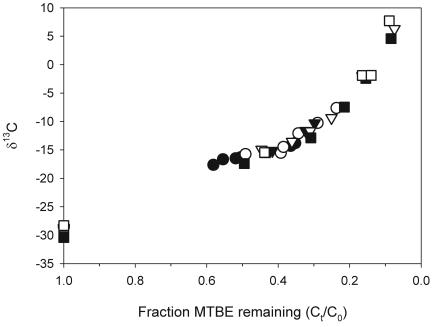
± 0.2 (for 15 samples). The isotopic values of residual MTBE at different stages of MTBE degradation were found to be enriched in 13C in all cultures (Fig. 3). A highly similar magnitude of fractionation was observed regardless of the source of enrichment culture or the electron-accepting condition. An enrichment of >12
(for 15 samples). The isotopic values of residual MTBE at different stages of MTBE degradation were found to be enriched in 13C in all cultures (Fig. 3). A highly similar magnitude of fractionation was observed regardless of the source of enrichment culture or the electron-accepting condition. An enrichment of >12 of δ13C values was observed at 50% of MTBE degradation. The enrichment in the residual fraction was >30
of δ13C values was observed at 50% of MTBE degradation. The enrichment in the residual fraction was >30 when >90% of MTBE was degraded. The mean [δ-13C] values of MTBE in abiotic control vials collected on day 40 and day 60 (−28.8
when >90% of MTBE was degraded. The mean [δ-13C] values of MTBE in abiotic control vials collected on day 40 and day 60 (−28.8 ± 0.2
± 0.2 and −28.3
and −28.3 ± 0.6
± 0.6 , respectively) were comparable to the mean initial value (−28.6
, respectively) were comparable to the mean initial value (−28.6 ± 0.2
± 0.2 ). The mean of [δ-13C] of abiotic control sample collected on day 190 (−27.1
). The mean of [δ-13C] of abiotic control sample collected on day 190 (−27.1 ± 0.4
± 0.4 ) was slightly less negative. Because the differences in [δ-13C] values of MTBE in abiotic controls at the beginning and the end of the experiment were minimal, the isotope ratios were not analyzed for other abiotic controls collected during the course of experiment.
) was slightly less negative. Because the differences in [δ-13C] values of MTBE in abiotic controls at the beginning and the end of the experiment were minimal, the isotope ratios were not analyzed for other abiotic controls collected during the course of experiment.
FIG. 3.
[δ-13C] value of residual MTBE versus the fraction of MTBE remaining in the sulfate-reducing Arthur Kill (circles) and methanogenic (triangles) and sulfate-reducing (squares) CC cultures. Duplicate enrichments are represented by solid and open symbols. The uncertainty of [δ-13C] measurement is 0.4 (standard deviation, 1σ).
(standard deviation, 1σ).
Calculation of the isotopic fractionation factor (ɛ) was based on the Rayleigh equation for a closed system (19, 23):
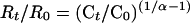 |
(3) |
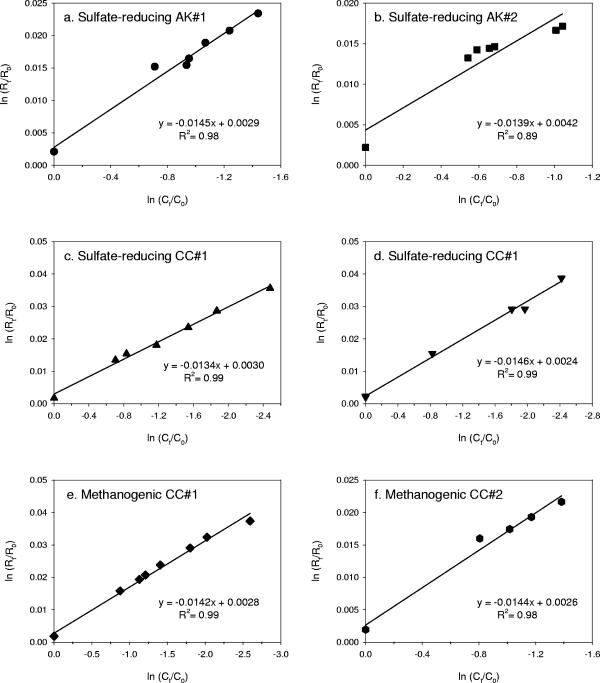
where R is the isotope ratio, C is the concentration, and the index (0 and t) describes the incubation time at the beginning (0) and during the reaction time of experiment (t). Isotope ratios (Rt/R0) were determined from the equation Rt/R0 = (δt/1,000 + 1)/(δ0/1,000 + 1). When ln Rt/R0 versus ln Ct/C0 was plotted, the isotopic enrichment factor (ɛ) could be determined from the slope of the curve (b), with b = 1/α −1 and ɛ = 1,000 × b (Fig. 4). Linear regression was used to estimate the slope of each data set.
FIG. 4.
Double logarithmic plots according to Rayleigh equation of the isotopic composition versus the residual concentration of substrate: sulfate-reducing AK duplicate enrichments (a and b), sulfate-reducing CC enrichments (c and d), and methanogenic CC enrichments (e and f).
The isotopic enrichment factor (ɛ) of each enrichment is listed in Table 1. Τhe ɛ values varied from −13.4 to −14.6
to −14.6 . The relatively good correlation between concentration and isotope composition indicated by r2 values of 0.89 to 0.99 suggested that carbon isotope fractionation during anaerobic MTBE degradation can be modeled as a Rayleigh process.
. The relatively good correlation between concentration and isotope composition indicated by r2 values of 0.89 to 0.99 suggested that carbon isotope fractionation during anaerobic MTBE degradation can be modeled as a Rayleigh process.
TABLE 1.
Isotopic enrichment factors (ɛ) for anaerobic biodegradation of MTBE
| Source | Anaerobic condition(s) | ɛ ( |
R2 | n | Source or reference |
|---|---|---|---|---|---|
| Arthur Kill | Sulfate reducing; duplicates | −14.5 ± 2.5 | 0.98 | 7 | This work |
| −13.9 ± 5.6 | 0.89 | 7 | |||
| Coronado Cays | Sulfate reducing; two enrichments | −13.7 ± 1.5 | 0.99 | 7 | This work |
| −14.4 ± 3.6 | 0.99 | 5 | |||
| Coronado Cays | Methanogenic; two enrichments | −14.0 ± 1.5 | 0.99 | 8 | This work |
| −14.4 ± 1.5 | 0.98 | 6 | |||
| Arthur Kill | Methanogenic | −15.6 ± 4.1 | 0.97 | 6 | 27 |
| With inhibitor of methanogenesis | −14.6 ± 5.2 | 0.86 | 9 | 27 | |
| All data for AK and CC | −14.4 ± 0.7b | 0.97 | 55 | ||
| Anaerobic laboratory microcosms | −9.16 ± 5.0 | 0.728 | 16 | ||
| Anaerobic field | −8.10 ± 0.9 | 0.946 | 16 | ||
| Anaerobic laboratory enrichmenta | −13.0 ± 1.1 | 17 |
The enrichment cultures were derived from microcosms previously reported by Kolhatkar et al. (16).
The value represents ɛ (per mille); n is the number of samples. The standard deviation is given with a confidence interval of ±95%.
DISCUSSION
Our current study has demonstrated significant enrichment of 13C in residual MTBE fractions during anaerobic biotransformation under both sulfate-reducing and methanogenic conditions, strongly indicating that carbon isotope fractionation has the potential to be used as a tool to demonstrate in situ anaerobic MTBE degradation. The carbon isotope ratios ([δ-13C]) in relation to the fraction of MTBE remaining (Ct/C0) can be applied to approximate the extent of in situ MTBE degradation once the carbon isotope ratios of MTBE from contaminated sites are available. At 50% of anaerobic MTBE degradation, the residual MTBE fraction may become enriched in about 15 . Assuming a typical isotope composition of about −30
. Assuming a typical isotope composition of about −30 for MTBE, the detection of positive [δ-13C] from environmental samples should undoubtedly demonstrate that >90% of the original amount of MTBE has been degraded (Ct/C0, <0.1). Since the carbon isotopic fractionation is conducted on residual MTBE, it does not provide any information about biodegradation of TBA, which is also reportedly persistent in the environment.
for MTBE, the detection of positive [δ-13C] from environmental samples should undoubtedly demonstrate that >90% of the original amount of MTBE has been degraded (Ct/C0, <0.1). Since the carbon isotopic fractionation is conducted on residual MTBE, it does not provide any information about biodegradation of TBA, which is also reportedly persistent in the environment.
As shown in Table 1, the isotopic enrichment factors (ɛ) estimated for the sulfate-reducing AK enrichment and the methanogenic and sulfate-reducing CC enrichments are almost identical. The ɛ values obtained in this study are also similar to ones previously estimated from methanogenic AK cultures and under conditions when methanogenesis was inhibited (27). These ɛ values are also similar to the values estimated by Kolhatkar et al. (16) and Kuder et al. (17), taking the uncertainty of previously reported isotope enrichment factors into account.
The evaluation of the isotope fractionation of enrichment cultures using various electron acceptors is very important for a reasonable application of isotope fractionation factors in field studies. To apply a representative fractionation factor (α or ɛ), it is important to understand the specific isotope fractionation associated with a degradation pathway. The carbon isotope fractionation technique can be applied to calculate the extent of in situ MTBE degradation by using equation 1. In this case, an appropriate ɛ value for the environmental conditions of the contaminated site is crucial in obtaining an accurate estimation. The similar magnitude of carbon isotope fractionation in all enrichments regardless of culture type or electron-accepting condition suggests that environmental condition may not significantly affect carbon isotope fractionation during anaerobic MTBE degradation. When all data from the current study and from our previous experiment (27) are combined, an ɛ value with 95% confidence intervals of −14.4 ± 0.7
± 0.7 is obtained from regression analysis (r2 = 0.97; 55 samples). This ɛ value reflects the strong correlation and low variation. Since the ɛ of −14.4
is obtained from regression analysis (r2 = 0.97; 55 samples). This ɛ value reflects the strong correlation and low variation. Since the ɛ of −14.4 ± 0.7
± 0.7 determined in our studies is more negative than that reported by Kolhatkar et al. (16) and demonstrates a greater degree of isotope fractionation, a higher ɛ value is more sensitive to trace in situ biodegradation and less likely to overestimate the extent of in situ MTBE degradation. One should note that if the initial step of anaerobic MTBE degradation were governed by different mechanisms, this would be reflected in significantly different magnitude of carbon isotope fractionation and thus compromise the estimation. Our results, however, suggest that this is not the case.
determined in our studies is more negative than that reported by Kolhatkar et al. (16) and demonstrates a greater degree of isotope fractionation, a higher ɛ value is more sensitive to trace in situ biodegradation and less likely to overestimate the extent of in situ MTBE degradation. One should note that if the initial step of anaerobic MTBE degradation were governed by different mechanisms, this would be reflected in significantly different magnitude of carbon isotope fractionation and thus compromise the estimation. Our results, however, suggest that this is not the case.
Unlike other major environmental contaminants, there is very limited information available for anaerobic MTBE degradation. To date, none of the anaerobic MTBE-degrading cultures have been microbially characterized, and the mechanisms of anaerobic MTBE degradation have yet to be elucidated. Attempts to isolate the MTBE-utilizing organisms in pure culture and to study the microbial community using 16S rRNA gene analysis are under way. Nonetheless, the information currently available suggests that the initial step of degradation is similar among studied MTBE-degrading cultures. For instance, all enrichments produce TBA as an intermediate or end product of degradation, suggesting that cleavage of ether linkage is the initial step of MTBE degradation. Both the methanogenic and sulfidogenic enrichments continued to utilize MTBE even when the electron-accepting process of the community was inhibited, although retardation of overall utilization rate occurred. The finding suggests that MTBE degradation is not directly coupled to sulfate reduction or methanogenesis. It is thus possible that the same microorganisms are responsible for MTBE degradation in both methanogenic and sulfate-reducing communities. The possible hypothesis is that MTBE-degrading microorganisms cleave the ether linkage and produce a C-1 compound or acetate through acetogenic pathways (10), which consequently serve as a carbon source for the overall methanogenic or sulfate-reducing communities. Therefore, these MTBE-utilizing microorganisms could function in various types of environments and electron-accepting conditions. As the initial step of MTBE degradation was mechanistically independent from the terminal electron-accepting process as shown by the inhibitor studies, the overall terminal electron-accepting process and associated microbial community did not significantly influence carbon isotope fractionation. According to our current knowledge, isotope fractionation is dependent to a large extent on the biochemistry of the initial step in the degradation pathway. However, it is clear that also other factors, in addition to the biochemical mechanism, can effect isotope fractionation; it will be important to study more anaerobic cultures for a more complete view.
Further studies of MTBE-degrading microorganisms, including microbial community structure and function and biochemical reaction mechanisms of MTBE-degrading pure culture, are essential to understanding anaerobic MTBE degradation. Our results provide undeniable evidence of strong and almost identical carbon isotope fractionation during anaerobic MTBE degradation among different microbial communities, demonstrating the usefulness of this technique in demonstrating and estimating the extent of in situ MTBE degradation. Isotope fractionation factors of MTBE can be used to evaluate MTBE contaminated sites and contribute to the development of appropriate remediation measures.
Acknowledgments
We are grateful to Anko Fischer, Ursula Günther, and Matthias Gehre for support in the isotope laboratory of the UFZ.
P.S. was a recipient of a Royal Thai Government Graduate Fellowship. This work was supported in part by the New Jersey Water Resources Research Institute. Work at the UFZ was supported by the German Federal Ministry of Education and Research (grants 02WN0348 and 02WT0041).
REFERENCES
- 1.Ahad, J. M. E., B. Sherwood Lollar, E. A. Edwards, G. F. Slater, and B. E. Sleep. 2000. Carbon isotope fractionation during anaerobic biodegradation of toluene: implication for intrinsic biodegradation. Environ. Sci. Technol. 34:892-896. [Google Scholar]
- 2.Anonymous. 1995. Reference and intercomparison materials for stable isotopes of light elements. IAEA-TECDOC-825. International Atomic Energy Agency, Vienna, Austria.
- 3.Ayoutte, J. D., D. M. Argue, and F. J. McGarry. 2005. Methyl tert-butyl ether occurrence and related factors in public and private wells in southeast New Hampshire. Environ. Sci. Technol. 39:9-16. [DOI] [PubMed] [Google Scholar]
- 4.Bloom, Y., R. Aravena, D. Hunkeler, E. Edwards, and S. K. Frape. 2000. Carbon isotope fractionation during microbial dechlorination of trichloroethene, cis-1,2-dichloroethene, and vinyl chloride: implications for assessment of natural attenuation. Environ. Sci. Technol. 34:2768-2772. [Google Scholar]
- 5.Bradley, P. M., F. H. Chapelle, and J. E. Landmeyer. 2001. Methyl t-butyl ether mineralization in surface water sediment microcosms under denitrifying conditions. Appl. Environ. Microbiol. 67:1975-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley, P. M., J. E. Landmeyer, and F. H. Chapelle. 2002. TBA biodegradation in surface-water sediments under aerobic and anaerobic conditions. Environ. Sci. Technol. 36:4087-4090. [DOI] [PubMed] [Google Scholar]
- 7.Fayolle, F., J. P. Vandecasteele, and F. Monot. 2001. Microbial degradation and fate in the environment of methyl tert-butyl ether and related fuel oxygenates. Appl. Microbiol. Biotechnol. 56:339-349.0. [DOI] [PubMed] [Google Scholar]
- 8.Finneran, K. T., and D. R. Lovley. 2001. Anaerobic degradation of methyl tert-butryl ether (MTBE) and tert-butyl alcohol (TBA). Environ. Sci. Technol. 35:1785-1790. [DOI] [PubMed] [Google Scholar]
- 9.Fiorenza, S., and H. S. Rifai. 2003. Review of MTBE biodegradation and bioremediation. Bioremediation J. 7:1-35. [Google Scholar]
- 10.Frazer, A. C. 1994. O-Demethylation and other transformations of aromatic compounds by acetogenic bacteria, p. 445-483. In H. L. Drake (ed.), Acetogenesis. Chapman & Hall, New York, N.Y.
- 11.Heald, P. C., S. G. Schladow, J. E. Reuter, and B. C. Allen. 2005. Modeling MTBE and BTEX in lakes and reservoirs used for recreational boating. Environ. Sci. Technol. 39:1111-1118. [DOI] [PubMed] [Google Scholar]
- 12.Hunkeler, D., R. Aravena, and B. J. Butler. 1999. Monitoring microbial dechlorination of tetrachloroethene (PCE) in groundwater using compound-specific stable isotope ratios: Microcosm and field studies. Environ. Sci. Technol. 33:2733-2738. [Google Scholar]
- 13.Hunkeler, D., and R. Aravena. 2000. Evidence of substantial carbon isotope fractionation among substrate, inorganic carbon, and biomass during aerobic mineralization of 1,2-dichloroethane by Xanthrobacter autotrophicus. Appl. Environ. Microbiol. 66:4870-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunkeler, D., B. J. Butler, R. Aravena, and J. F. Barker. 2001. Monitoring biodegradation of methyl tert-butyl ether (MTBE) using compound-specific carbon isotope analysis. Environ. Sci. Technol. 35:676-681. [DOI] [PubMed] [Google Scholar]
- 15.Kolhatkar, R., J. T. Wilson, and L. E. Dunlap. 2000. Evaluating natural biodegradation of MTBE at multiple UST sites, p. 2-49. In Petroleum hydrocarbons and organic chemicals in ground water: prevention, detection, and remediation. National Ground Water Association, Westerville, OH.
- 16.Kolhatkar, R., T. Kuder, P. Philp, J. Allen, and J. T. Wilson. 2002. Use of compound-specific stable carbon isotope analyses to demonstrate anaerobic biodegradation of MTBE in groundwater at a gasoline release site. Environ. Sci. Technol. 36:5139-5146. [DOI] [PubMed] [Google Scholar]
- 17.Kuder, T., J. T. Wilson, P. Kaiser, R. Kolhatkar, P. Philp, and J. Allen. 2005. Enrichment of stable carbon and hydrogen isotopes during anaerobic biodegradation of MTBE: microcosm and field evidence. Environ. Sci. Technol. 39:213-220. [DOI] [PubMed] [Google Scholar]
- 18.Mancini, S. A., A. C. Ulrich, G. Lacrampe-Couloume, B. Sleep, E. A. Edwards, and B. Sherwood Lollar. 2003. Carbon and hydrogen isotopic fractionation during anaerobic biodegradation of benzene. Appl. Environ. Microbiol. 69:191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariotti, A., J. C. Germon, P. Hubert, P. Kaiser, T. Letolle, A. Tardieux, and P. Tardieux. 1981. Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes. Plant Soil 62:413-430. [Google Scholar]
- 20.Morasch, B., H. H. Richnow, B. Schink, and R. U. Meckenstock. 2001. Stable hydrogen and carbon isotope fractionation during microbial toluene degradation: mechanistic and environmental aspects. Appl. Environ. Microbiol. 67:4842-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mormile, M. R., S. Liu, and J. M. Suflita. 1994. Anaerobic biodegradation of gasoline oxygenates: extrapolation of information to multiple sites and redox conditions. Environ. Sci. Technol. 28:1727-1732. [DOI] [PubMed] [Google Scholar]
- 22.Pankow, J. F., N. R. Thomson, R. L. Johnson, A. L. Baehr, and J. S. Zogorski. 1997. The urban atmosphere as non-point source for the transport of MTBE and other volatile organic compounds (VOCs) to shallow groundwater. Environ. Sci. Technol. 31:2821-2828. [Google Scholar]
- 23.Rayleigh, J. W. S. 1896. Theoretical considerations respecting the separation of gases by diffusion and similar processes. Philos. Mag. 42:493-498. [Google Scholar]
- 24.Richnow, H. H., E. Annweiler, W. Michaelis, and R. U. Meckenstock. 2003. Microbial in situ degradation of aromatic hydrocarbons in a contaminated aquifer monitored by carbon isotope fractionation. J. Contam. Hydrol. 65:101-120. [DOI] [PubMed] [Google Scholar]
- 25.Slater, G. F., B. Sherwood Lollar, B. E. Sleep, and E. A. Edwards. 2001. Variability in carbon isotopic fractionation during biodegradation of chlorinated ethenes: implication for field application. Environ. Sci. Technol. 35:901-907. [DOI] [PubMed] [Google Scholar]
- 26.Somsamak, P., R. M. Cowan, and M. M. Häggblom. 2001. Anaerobic biotransformation of fuel oxygenates under sulfate-reducing conditions. FEMS Microbiol. Ecol. 37:259-264. [Google Scholar]
- 27.Somsamak, P., H. H. Richnow, and M. M. Häggblom. 2005. Carbon isotopic fractionation during anaerobic biotransformation of methyl tert-butyl ether (MTBE) and tert-amyl methyl ether (TAME). Environ. Sci. Technol. 39:103-109. [DOI] [PubMed] [Google Scholar]
- 28.Squillace, P. J., J. S. Zogorski, W. G. Wilber, and C. V. Price. 1996. Preliminary assessment of the occurrence and possible sources of MTBE in groundwater in the United States, 1993-1994. Environ. Sci. Technol. 30:1721-1730. [Google Scholar]
- 29.Squillace, P. J., J. Pankow, N. E. Korte, and J. S. Zogorski. 1997. Review of the behavior and fate of methyl tert-butyl ether. Environ. Toxicol. Chem. 16:1836-1844. [Google Scholar]
- 30.Squillace, P. J., M. J. Moran, W. W. Lapham, C. V. Price, R. M. Clawges, and J. S. Zogorski. 1999. Volatile organic compounds in untreated ambient groundwater of the United States, 1985-1995. Environ. Sci. Technol. 33:4176-4187. [Google Scholar]
- 31.Stocking, A. J., R. A. Deeb, A. E. Flores, W. Stringfellow, J. Talley, R. Brownnell, and M. C. Kavanaugh. 2000. Bioremediation of MTBE: a review from a practical perspective. Biodegradation 11:187-201. [DOI] [PubMed] [Google Scholar]
- 32.Suflita, J. M., and M. R. Mormile. 1993. Anaerobic biodegradation of known and potential gasoline oxygenates in the terrestrial subsurface. Environ. Sci. Technol. 27:976-978. [Google Scholar]
- 33.U.S. Environmental Protection Agency. 2004. Technologies for treating MTBE and other fuel oxygenates. U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response, Office of Superfund Remediation and Technology Innovation, Washington, D.C.
- 34.Wilson, J. T., J. Soo Cho, B. H. Wilson, and J. A. Vardy. 2000. Natural attenuation of MTBE in the subsurface under methanogenic conditions. U.S. Environmental Protection Agency, Office of Research and Development, Washington, D.C.
- 35.Zwank, L., M. Berg, M. Elsner, T. C. Schmidt, R. P. Schwarzenbach, and S. B. Haderlein. 2005. New evaluation scheme for two-dimensional isotope analysis to decipher biodegradation processes: application to groundwater contamination by MTBE. Environ. Sci. Technol. 39:1018-1029. [DOI] [PubMed] [Google Scholar]