Abstract
A 1.5-kb region immediately downstream of the styABCD operon involved in styrene degradation in Pseudomonas putida CA-3 has been cloned. Sequence analysis revealed a 1,296-bp open reading frame, designated styE, and BLAST P database comparisons of the deduced StyE amino acid sequence revealed 33 to 98% identity with several membrane-associated ATPase-dependent kinase proteins involved in the active transport of aromatic hydrocarbons across bacterial membranes and also with FadL, an outer membrane protein necessary for the uptake of long-chain fatty acids in Escherichia coli. Transcription of styE is styrene dependent, and the gene is cotranscribed with the styABCD structural genes. StyE appears to be membrane associated, with a corresponding 45.9-kDa band being identified following sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of membrane preparations from styrene-grown cells. P. putida CA-3 cells in which the styE gene had been interrupted were no longer capable of growth on styrene. In contrast, overexpression of styE in P. putida CA-3 resulted in a 4.2-fold increase in styrene monooxygenase activity compared with wild-type cells grown on styrene, with a concomitant 8-fold increase in styA mRNA transcript levels. Experiments with the classic, ATPase inhibitor vanadate revealed that growth of wild-type cells on styrene was inhibited at a concentration of 1 mM, while 1.75 mM was required to achieve a similar effect in the StyE overexpression strain. Growth of either strain on citrate was not inhibited in the presence of up to 7 mM vanadate. These findings suggest a role for StyE in the active transport of styrene in Pseudomonas putida CA-3 and identify styrene transport as a potentially limiting factor with respect to mRNA transcript levels and associated enzymatic activity of the styrene degradative pathway.
Styrene, the simplest of the alkenylbenzenes, is used extensively in the petrochemical industry both as a solvent in polymer processing and as a starting material in the production of many synthetic polymers. Gaseous and effluent emissions from these industries release significant quantities of styrene into the environment, which is a cause for some concern due not only to the malodorous properties of the compound but also to the toxic and potentially carcinogenic nature of styrene (11, 18). Consequently, much interest has focused on the elucidation of different microbial catabolic pathways involved in styrene degradation, with a view to potentially developing whole-cell-based bioremediation strategies (2, 17). In this respect, the styrene-degradative pathways in a number of different bacterial strains have been studied at both the biochemical and genetic levels (22), including Pseudomonas fluorescens ST (17), Pseudomonas sp. strain Y2 (31, 32), Pseudomonas sp. strain VLB120 (27), Xanthobacter strain 124X (9), Xanthobacter strain S5 (10), and Pseudomonas putida CA-3 (20, 23).
Styrene degradation in P. putida CA-3 involves two distinct catabolic pathways. The styABCD operon, which is regulated at the transcriptional level by the StySR two-component sensory apparatus, encodes the enzymes necessary for the initial, stepwise conversion of styrene to phenylacetic acid (PAA) (24). This intermediate is subsequently activated to phenylacetyl (PA) coenzyme A (CoA), the sole substrate of the PA-CoA catabolic operon found in a wide variety of microorganisms capable of PAA catabolism (16, 26). Enzymatic manipulations within the pathway reduce the aromatic compound to acetyl-CoA, which is subsequently shuttled to the tricarboxylic acid cycle (25).
Despite recent advances in our understanding of microbial styrene catabolism, little is currently known about the mechanism by which styrene enters the cell, i.e., whether passive diffusion occurs or whether the compound is actively transported across the outer membrane. In an attempt to address this issue, we cloned a 1.5-kb region downstream of the styD gene in P. putida CA-3 and identified a 1,296-bp open reading frame (styE), the product of which displayed significant similarity at the deduced amino acid level with other membrane proteins that are known to be involved in the active uptake of aromatic hydrocarbons (8, 12, 13, 33) and also with the fatty acid transporter, FadL, a reported ABC transporter (4, 6). Here, we describe the functional characterization of this membrane-associated StyE protein and demonstrate its likely involvement in the facilitated uptake of styrene in P. putida CA-3.
MATERIALS AND METHODS
Bacterial strains, plasmids, culture media, and growth.
Pseudomonas putida CA-3 cultures were grown in 100 ml of minimal salts (MS) medium in 1-liter Erlenmeyer flasks, incubated at 30°C, and aerated via continuous agitation in an orbital shaker at 120 rpm. MS medium contained 7.0 g K2HPO4, 3.0 g KH2PO4, 1.0 g (NH4)2SO4, and 2 ml of 10% (wt/vol) MgSO4 (added postautoclaving) per liter of demineralized water. Carbon sources were added as follows (wt/vol): 0.1% PAA, 0.1% succinate, and 0.1% citrate. Growth on styrene required the addition of 70 μl of liquid styrene to a test tube fixed centrally to the bottom of a baffled 1-liter Erlenmeyer flask. Cell growth was monitored by measuring optical density at 540 nm with a Beckman DU640 spectrophotometer. Cells were harvested at mid-log phase (optical density at 540 nm, 0.6) for both whole-cell enzyme assays and RNA isolations. When necessary, the following antibiotic concentrations were added to the different media: ampicillin (100 μg/ml), kanamycin (50 μg/ml), and gentamicin (20 μg/ml). Escherichia coli strains were grown on Luria-Burtani (LB) medium at 37°C. E. coli DH5α (Invitrogen) and XL-1 Blue (Stratagene) were used for routine transformation and maintenance of plasmids. TOPO pCR 2.1 (Invitrogen) was used for cloning of PCR products (according to the manufacturer's instructions).
Cloning and sequencing of the styE gene.
A 1.5-kb region downstream from the styD gene was cloned using amplified flanking-region PCR. The method used was a modification of that previously described (29) and included the following steps. (i) An initial round of PCR using a 5′ biotinylated gene specific primer, StyD-BIO, and a degenerate flanking primer, primer A (Table 1), was carried out. (ii) Purification of biotinylated (i.e., gene-specific) PCR products involved physicochemical separation with streptavidin-coated magnetic beads (DYNAL). (iii) The purified, biotinylated amplicons served as the template for a second PCR with an internal styD gene-specific primer (StyDF) and a nondegenerate oligonucleotide, primer B, identical to the 5′ region of primer A (Table 1). PCR products from this PCR were then separated by electrophoresis on a 1% agarose gel, purified (QIAGEN Gel Extraction), and cloned into a pCR 2.1TOPO vector (Invitrogen). Transformants in E. coli were analyzed by PCR using the internal primers StyDF and StyDR (Table 1). Sequencing reactions were performed on positive transformants using Big Dye Terminator cycle sequencing and analyzed on 5.75% and 4.75% Long Ranger gels for ABI 377 (Lark Technologies, Inc.). Sequence data were assembled (accession number AY450871) and processed using DNASTAR (Madison, WI) software.
TABLE 1.
Primer sequences and annealing temperatures
| Name | Primer sequence (5′-3′) | Annealing temp (°C) |
|---|---|---|
| StyD-BIO | CTGGAACTGGGTGGCAAGAGC | 68 |
| StyDF | TCCAAGAAACACCACGAGAATG | 64 |
| StyDR | ACGCCCGAGTCCTTGAAC | 58 |
| StyDF(1074) | TGCTTCGGCACATCCGTAATG | 62 |
| Primer A | CAGTTCAAGCTTGTCCAGGAATTCNNNNNNNCGCGGT | 78 |
| Primer B | CAGTTCAAGCTTGTCCAGGAATTC | 72 |
| StyEF | CAACAGATGTTCTGCGCCTCGa | 70 |
| StyER | TCAGAAATTATGGGTATACGAb | 56 |
| StyABF | CAACAGCTTAGTCAGCAGCAACCCACAACAa | 84 |
| StyABR | TCAGAAAGATTTTGTTCGTTGCACACTTAAb | 80 |
| CitF | AACTTCCTGCACATGATGTT | 56 |
| CitR | GCGAAGATCACGGTGAACAT | 60 |
| StyAF | CATCTTGGCCTCTTCCTC | 60 |
| StyAR | GATTTCCAGGTCGCTACC | 60 |
| GentF | CTGTCCAACCCGGCT | 40 |
| GentR | CTGCGGGGCG | 36 |
The sequences underlined indicate an XbaI restriction site.
The sequences underlined indicate an HindIII restriction site.
RT-PCR Analysis.
Total RNA was isolated (QIAGEN RNeasy Mini kit) from cells grown on styrene, PAA, and succinate, respectively; and 1 μg was reverse transcribed (RT) in a reaction containing 100 ng random hexamer primers (Boehringer), 1 mM deoxynucleoside triphosphates, 10.5 mM dithiothreitol, 1× RT buffer, 50 U of Expand reverse transcriptase (Roche), and 40 U of RNase inhibitor (Roche) as previously described (24). The final reaction volume was adjusted to 20 μl using diethyl pyrocarbonate-treated water. Reverse transcription was performed at 30°C for 10 min, followed by 45 min at 45°C, prior to incubation on ice. Two microliters of the cDNA generated was then employed in subsequent PCR amplifications, carried out with a PTC200 DNA thermal cycler (MJ Research) using the primer pairs StyDF-StyDR and StyDF(1074)-StyER (Table 1). Primers specific for the constitutively expressed citrate synthase gene in P. putida (CitF and CitR) (Table 1) were used as a positive control. In each case, the number of cycles used in the PCR was varied to avoid reaching a point at which bands representing different conditions would have equal intensities, due to a plateau effect. Amplified RT-PCR products were partially sequenced confirm their identity.
Inactivation of styE in P. putida CA-3 by homologous recombination. (i) Construction of the styE-targeted pKnockout-G derivative pKGmE1.
The use of pKnockout vectors for rapid and efficient inactivation of genes in Pseudomonas aeruginosa has been previously reported (34). In this study, the full-length styE gene was PCR amplified from CA-3 genomic DNA as described above and subjected to a double restriction digest with BamHI and SalII (New England Biolabs) at 37°C for 1 h in the presence of 1× NEB buffer 4. The suicide vector pKnockout-G (ColE1ori mob+; lacking tra), containing the complementary restriction enzyme sites in the polylinker region was similarly digested, and both samples were subjected to electrophoresis and visualization, following ethidium bromide staining on a 1% agarose gel. The resulting 813-bp, 5′- and 3′-truncated internal styE fragment and the ∼6-kb linearized pKnockout-G vector were purified using Qiaquick gel purification columns (QIAGEN) and an overnight ligation (insert:vector ratio, 3:1), performed at 16°C using T4 DNA ligase (Promega) in accordance with the manufacturer's instructions. Subsequently, Top10F′ chemically competent Escherichia coli DH5α (Invitrogen, Calif..) was transformed with 2 μl of the ligation mixture and plated out on LB agar with isopropyl-β-d-thiogalactopyranoside, X-galactosidase, and 20-μg/ml gentamicin. Blue-white screening was employed to identify ligation of the 813-bp styE fragment into the polylinker region of pKnockout-G. Several white colonies (10 in total) were selected for overnight growth at 37°C in 10 ml liquid LB medium (Gm20); 2-ml aliquots of each were subjected to plasmid purification (QIAGEN Miniprep kit), BamHI/SalII digestion, and visualization in an ethidium bromide-stained 1% agarose gel to confirm the presence of the insert. This process generated the styE-targeted pKnockout derivative pKGmE1.
(ii) Conjugation and screening for styE homologous recombination with pKGmE1.
Conjugal transfer of the mobilizable pKGmE1 suicide vector into P. putida CA-3 involved triparental mating in a 50-μl volume spotted onto the surface of an LB agar plate overnight at 30°C. The pRK2013 helper strain provided the transfer function (tra genes) for pKGmE1. Following incubation, the mixture was lightly scraped from the plate surface with a sterile pipette tip and washed twice, each time in 1 ml minimal salts medium lacking any carbon source. Aliquots (each, 50 μl) were subsequently spread plated onto minimal salts agar containing 15 mM citrate and Gm20 to remove the non-citrate-utilizing E. coli donor and helper strains. Colonies capable of growth on citrate in the presence of Gm20 were subsequently screened for altered indole-to-indigo conversion phenotypes, as previously reported in the assessment of styrene monooxygenase (SMO) activity (21), following replica plating onto minimal salts agar containing 10 mM glucose (which does not impose catabolite repression on the sty operon in CA-3) and the gratuitous inducer 3 mM indole. Two colonies completely incapable of indole-to-indigo conversion were identified (A6 and D9). Ten-milliliter minimal salts-citrate broth overnight cultures of A6, D9, and the parent strain were washed twice in MS broth lacking any carbon source prior to inoculation into minimal salts broth with styrene as the sole carbon source. Growth at 30°C was monitored over a 24-h period. Genomic DNA was isolated from 10 ml LB broth cultures of A6, D9, and the wild-type CA-3 strain grown overnight at 30°C (1). Analysis of the respective genomic DNA samples for an intact styE gene was performed via PCR screening using the styEF-styER primers and amplification conditions described above. Screening for the 5′- and 3′-truncated gene duplications arising from homologous recombination was performed by Southern blot analysis with the [α-32P]dATP-labeled (30 μCi) 813-bp styE internal fragment.
Homologous overexpression of StyE and StyAB in P. putida CA-3.
Full-length styE gene primers StyEF and StyER (Table 1) were designed, which allowed the mispriming incorporation of XbaI and HindIII restriction enzyme sites at the 5′ and 3′ ends of the gene, respectively, permitting styE to be cloned as an XbaI/HindIII fragment into the lacZ expression vector pBBR1MCS (15) to form the pStyEF expression vector. styE was also cloned into pBBR1MCS in the reverse orientation to generate pStyER, which acted as a negative control. StyABF and StyABR primers (Table 1) were employed to generate the full-length styAB genes. Again, these primers allowed the mispriming incorporation of XbaI and HindIII restriction enzyme sites at the 5′ and 3′ ends of the gene, respectively, which allowed styAB to be cloned as an XbaI/HindIII fragment into pBBR1MCS to form the pStyAB expression vector. pRK2013 was employed as a helper for the mobilization of plasmids pStyEF, pStyER, and pStyAB into Pseudomonas putida CA-3 via triparental mating (15). P. putida CA-3 transconjugants resulting from triparental mating were selected on MS citrate solid medium supplemented with gentamicin. The presence of the plasmid was subsequently verified by PCR using the primers GentF and GentR (Table 1).
Extraction of outer membrane fraction proteins.
To isolate the membrane fraction, the cultures used were grown as described above, but samples were harvested by centrifugation, washed with a 100 mM potassium phosphate buffer (pH 7.0), and resuspended in the same buffer. Lysozyme (5 mg/ml) was added to the cell suspension, and the resulting suspension was incubated at 30°C for 30 min. Cells were ruptured using a Mini-BeadBeater-8 (BIOSPEC Products) (30 s at force 6). MgSO4 and DNase were added to a final volume of 10 mM and 0.1 mg/ml, respectively, and the cell suspension was incubated at 30°C for a further 15 min. K-EDTA was added to a final concentration of 15 mM and large-cell debris was removed by centrifugation at 14,600 × g for 5 min at 4°C. After this step, the supernatant was removed and centrifuged at 4°C for 1 h at 60,000 × g in a Beckman L-60 ultracentrifuge. The membrane preparations were then washed with 50 mM potassium phosphate buffer and collected by centrifugation for 1 h at 60,000 × g. The resulting pellet was resuspended in 50 mM potassium phosphate buffer, and the protein concentration was determined by the Bio-Rad assay (5). Five micrograms of each protein sample was used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a Mini-Protean II system (Bio-Rad, California) according to the manufacturer's instructions. Gels were run for 2 h at 30 mA through a 5% acrylamide stacking gel and a 10% separating gel. Prestained protein marker weight standards (New England Biolabs) were used for molecular mass estimation. Gels were stained with Coomassie brilliant blue and destained in methanol/acetic acid/water (7:3:7).
Styrene monooxygenase assay.
To quantify styrene monooxygenase activity, indole-to-indigo conversion was assayed as previously described (21). Assays were performed in triplicate to facilitate standard deviation calculations.
Quantification of gene transcript levels.
Real-time PCR analysis of styrene monooxygenase styA gene transcript levels was performed with the LightCycler (Roche Diagnostics, Germany) using cDNA templates generated from styrene-grown wild-type P. putida CA-3, the pStyE overexpression strain, and P. putida CA-3 harboring the negative control vector pStyER. Primers StyAF and StyAR (Table 1) were used with the LightCycler FastStart DNA Master SYBER Green I kit (Roche) in precooled centrifuge adapters per the manufacturer's instructions. The final concentrations of Mg2+ and primers were 2.5 mM and 50 pmol, respectively. Citrate synthase gene transcript levels were also monitored as a control with the primer pair CitF-CitR (Table 1). Thermal cycling was as follows: initial denaturation of 95°C for 10 min; 40 cycles, each consisting of denaturation (10 s at 95°C), annealing (10 s at 55°C), and extension (12 s at 72°C); and melting curve analysis (65 to 95°C at 0.2°C/s). Products were visualized on a 1% agarose gel (660 bp for styA and 540 bp for citrate synthase, respectively). P. putida CA-3 genomic DNA acted as a template in the generation of styA and citrate synthase amplification standards. The appropriate band was excised and purified (QIAGEN; gel extraction). Serial dilutions of each gene were then prepared with LightCycler-grade water, where the styA dilution series ranged from 1 × 1018 to 1 × 107 copies and the citrate synthase ranged from 2.5 × 1016 to 2.5 × 106 copies.
The LightCycler software generated a standard curve by plotting crossing cycle number versus logarithms of the given concentration for each standard. A regression curve was plotted between these points and used to calculate the copy number of the styA and citrate synthase genes in each sample.
Addition of the ATPase inhibitor vanadate.
Vanadate (Sigma) a classic p-type ATPase inhibitor (14, 30), was added to the culture medium of P. putida CA-3 wild-type cells and those containing the pStyE expression vector, cultured on MS medium with styrene as the sole carbon source, at vanadate concentrations of 0.25 mM to 2.00 mM. As a control, P. putida CA-3 cells were also grown on MS citrate medium in the presence of vanadate (0.25 to 1.75 mM). In addition to the growth inhibition studies, vanadate was also added to washed-cell suspensions of styrene-grown wild-type, StyE, and StyAB overexpression strains prior to carrying out the styrene monooxygenase assay. The inclusion of the StyAB overexpression strain was aimed at highlighting differences between increased monooxygenase activity and any potential increase in substrate availability as a result of StyE overexpression.
RESULTS AND DISCUSSION
Cloning and sequence analysis of the styE gene in P. putida CA-3.
Amplified flanking-region PCR was used to clone a 1.5-kb region immediately downstream of the styABCD operon involved in styrene degradation in P. putida CA-3. The region was found to contain a 1,296-bp open reading frame designated styE, encoding a 434-amino-acid peptide with an estimated molecular mass of 45.9 kDa. BLAST P database comparisons of the deduced StyE amino acid sequence revealed significant identity (ranging from 33 to 98%) with proposed transport proteins previously reported in a number of hydrocarbon-degrading bacteria, namely, StyE from Pseudomonas sp. strain Y2 (32), CumH from Pseudomonas fluorescens IP01 (8), XylN from Pseudomonas putida PAW1 (13), TbuX from Ralstonia picketti PK01 (12), and TodX from Pseudomonas putida F1 (33), together with FadL from E. coli, which is involved in the transport of long-chain fatty acids across the outer membrane (Table 2) (4, 6). These proteins contained a high number of hydrophobic amino acids, XylN and StyE in particular, which are composed of >30% hydrophobic amino acids and few cysteinyl residues, properties common among membrane proteins. XylN is an outer membrane protein involved in the transport of m-xylene and its analogues across the outer membrane of Pseudomonas putida F1 (13). Hydropathy analysis of XylN and StyE using DNASTAR software indicates the comparable location of hydrophobic regions within both proteins (19). Further analysis of StyE using both PSORTb (version 2.0; http://psort.nibb.ac.jp) (7) and SignalP (version 3; Center for Biological Sequence Analysis [http://www.cbs.dtu.dk]) (3) predicted a signal sequence at the N-terminal region with a proposed cleavage site between position 9 and 10. Based on these initial observations, it was decided to further investigate the potential role of StyE as a membrane-associated transport protein involved in styrene metabolism in P. putida CA-3.
TABLE 2.
Other membrane-associated proteins sharing homology with StyE
| Protein | Organism | % Identification | Accession no. | Proposed function |
|---|---|---|---|---|
| StyE | Pseudomonas sp. strain Y2 | 98.1 | CAA04004 | Putative active transporter |
| CumH | P. fluorescens IP01 | 51.4 | BAA12149 | Transport of cumene across the cell membrane |
| XylN | P. putida PAW1 | 46.5 | BAA09665 | Transport of xylene across the cell membrane |
| TbuX | Ralstonia pickettii PK01 | 45.1 | AAF03168 | Transport of toluene across the cell membrane |
| TodX | P. putida F1 | 38.4 | AAC43318 | Transport of toluene across the cell membrane |
| FadL | E. coli | 33.3 | CDD11775 | Transport of fatty acids across the cell membrane |
Transcriptional analysis of the styE gene.
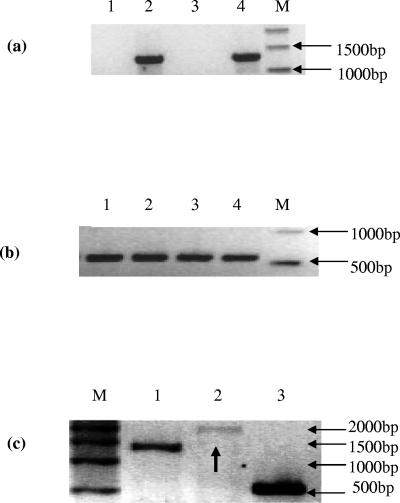
RT-PCR analysis of total RNA from P. putida CA-3 cells cultured on styrene, phenylacetic acid, and succinate, respectively, revealed that transcription of styE only occurs in the presence of styrene, (Fig. 1a, lane 2). We have previously shown styrene-dependent, polycistronic expression of the styABCD operon genes (24) and therefore investigated cotranscription of styE as part of the sty catabolic operon. RT-PCR analysis of total RNA from styrene-grown P. putida CA-3 cells with the StyDF-StyER primer pair generated a 1,730-bp product, indicating read-through transcription between styD and styE (Fig. 1c, lane 2). This finding was in marked contrast to the sty catabolon in Pseudomonas species Y2, where a putative transcriptional termination sequence was reported to exist between styD and styE (32). A similar termination sequence also appears to exist between styD and styE in P. putida CA-3, but our ability to detect styE mRNA transcripts in styrene-grown cells indicated that it may not be functional. Indeed, a similar putative transcriptional termination sequence also appears to exist between styB and styC, despite cotranscription of these genes being previously reported in both Pseudomonas species Y2 and P. putida CA-3 (24, 32).
FIG. 1.
(a) RT-PCR analysis of styE mRNA expression in P. putida CA-3 grown on various carbon sources. Lanes: 1, succinate; 2, styrene; 3, phenylacetic acid; 4, P. putida CA-3 genomic DNA as positive control; M, molecular weight markers. (b) RT-PCR analysis of citrate synthase mRNA expression in P. putida CA-3. Lanes: 1, succinate; 2, styrene; 3, phenylacetic acid as a sole carbon source; 4, P. putida CA-3 genomic DNA as a positive control; M, molecular weight markers. (c) RT-PCR analysis of total RNA from a styrene-grown culture of P. putida CA-3, assessing operonic expression of styE. Lanes: 1, 1,508-bp product obtained with styDF/styDR; 2, black arrow indicating 1,730-bp product obtained with styDF(1074)/styER; 3, 540-bp product obtained with CitF/CitR; M, molecular weight markers.
Inactivation of styE by homologous recombination with pKGmE1.
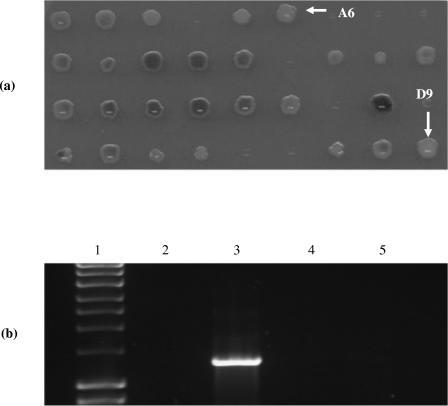
The pKnockout-G suicide vector derivative, pKGmE1, carried an 813-bp, 5′- and 3′-truncated internal fragment of the wild-type styE gene. Conjugal transfer into P. putida CA-3 generated 43 recombinant colonies capable of growth on MS citrate agar in the presence of 20-μg/ml gentamicin. The recombinant colonies were replica plated onto MS glucose agar plates containing 3 mM indole to monitor for altered indole-to-indigo conversion phenotypes. Two well-defined colonies (A6 and D9) (Fig. 2a) incapable of indole-to-indigo conversion were selected for further analysis. Subsequent PCR screening of genomic DNA from A6 and D9, using the styE full-length gene primers, failed to amplify the anticipated 1,296-bp gene product (Fig. 2b). This result was consistent with disruption of styE via insertion of the 6-kb pKGmE1 vector by homologous recombination in these strains, which was confirmed by Southern blot analysis of EcoRI genomic digests from cultures of the styE mutants A6 and D9 and the P. putida CA-3 wild type (data not shown). The effects of styE disruption on styrene utilization were also investigated, and it was observed that the styE mutant strains A6 and D9 were no longer capable of utilizing styrene as a sole carbon source, compared with the wild-type P. putida CA-3 (data not shown). These findings indicate that the styE gene product is essential for styrene degradation in P. putida CA-3.
FIG. 2.
(a). Screening of pKGmE1 recombinant colonies for altered indole-to-indigo conversion phenotypes on minimal salts glucose with 3 mM indole. White arrows indicate colonies A6 and D9. (b) PCR screening of genomic DNA for the intact 1,296-bp styE gene. Lanes: 1, Hyperladder I (Promega); 2, negative control; 3, P. putida CA-3 genomic DNA; 4, A6; 5, D9.
The effects of StyE overexpression on upper pathway transcriptional activation in Pseudomonas putida CA-3.
The effect of StyE overexpression on the rate of styrene degradation in P. putida CA-3 was assessed via SMO-dependent indigo formation, which has previously been shown to correlate directly with styrene utilization in this strain (21, 23). P. putida CA-3 cells harboring pStyEF demonstrated a 4.26-fold maximal increase in SMO activity following growth on styrene, compared to wild-type P. putida CA-3 cells (see Table 4, 0 mM vanadate) or cells harboring the negative control vector pStyER (data not shown) grown under similar conditions. This increased SMO activity was mirrored at the transcriptional level by real-time quantitative PCR revealing an eightfold maximal increase in transcriptional activity of the sty operon, as represented by styA transcript levels, with little variation observed for mRNA transcript levels from the constitutively expressed citrate synthase gene (Table 3). In each case, transcript copy numbers were calculated from standard curves where regression coefficients of 0.98 and 0.96 were obtained for styA and citrate synthase, respectively. Thus, it appears that overexpression of StyE stimulates enhanced transcriptional activation of the upper pathway styABCD genes by increasing styrene dependent activation of these genes in P. putida CA-3 cells. One potential way in which this may be achieved is by increasing the levels of styrene entering the cell. To test this hypothesis, we further investigated the potential of StyE to act as a membrane-associated transport protein.
TABLE 4.
Effects of increasing vanadate concentrations on SMO activity from washed-cell suspensions of mid-log phase styrene-grown cultures
| Vanadate [mM] | Styrene monooxygenase activity (nmol/min/mg cell [dry wt])
|
||
|---|---|---|---|
| Wild-type CA-3 | pStyAB | pStyEF | |
| 0 | 2.67 ± 0.27 | 3.86 ± 0.66 | 11.66 ± 0.98 |
| 0.5 | 2.54 ± 0.13 | 2.24 ± 0.52 | 4.48 ± 0.35 |
| 0.75 | 2.6 ± 0.12 | 1.94 ± 0.91 | 3.84 ± 0.61 |
| 1.25 | 0.51 ± 0.05 | 0.38 ± 0.14 | 1.73 ± 0.13 |
| 1.75 | 0.32 ± 0.03 | 0.28 ± 0.01 | 0.27 ± 0.03 |
TABLE 3.
Transcript copy numbers quantified by real-time PCR
| Strain | Transcript level
|
||
|---|---|---|---|
| styA | citS | styA/citS | |
| Pseudomonas putida CA-3 (wild type) | 4.076 × 106 | 13.700 × 1015 | 1:3.36 × 109 |
| Pseudomonas putida CA-3 (StyEF) | 3.233 × 107 | 11.130 × 1015 | 1:3.44 × 108 |
| Pseudomonas putida CA-3 (StyER) | 6.492 × 106 | 11.090 × 1015 | 1:1.71 × 109 |
Localization of the styE gene product in the outer membrane of P. putida CA-3.
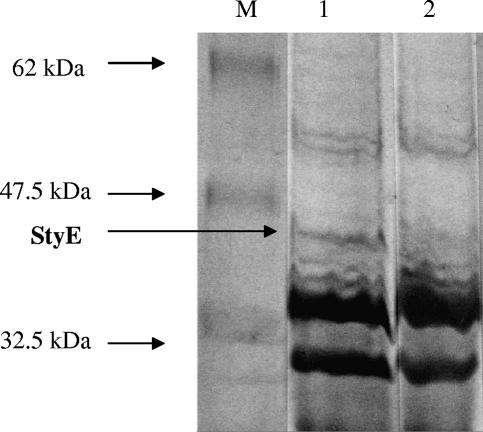
In an attempt to identify the localization of StyE, we performed SDS-PAGE analysis of outer membrane protein preparations from citrate-grown P. putida CA-3 wild-type and StyE-overexpressing strains. A 45.9-kDa band, corresponding to StyE, was identified in the membrane protein fraction of the overexpression strain only (Fig. 3, lane 1). The membrane protein fraction from the wild-type strain did not contain a corresponding 45.9-kDa band (Fig. 3, lane 2), which was consistent with earlier RT-PCR data suggesting that styE transcription in the wild-type strain is induced only in the presence of styrene.
FIG. 3.
SDS-PAGE analysis of outer membrane protein fraction of Pseudomonas putida CA-3 cells harboring pStyEF (lane 1) and Pseudomonas putida CA-3 wild-type cells (lane 2). M, protein markers (New England Biolabs); 62 kDa, glutamic dehydrogenase (bovine liver); 47.5 kDa, aldolase (rabbit muscle); 32.5 kDa, triosephosphate isomerase (E. coli). StyE (∼46 kDa) is indicated by a black arrow.
The potential role of StyE in ATPase-dependent transport of styrene.
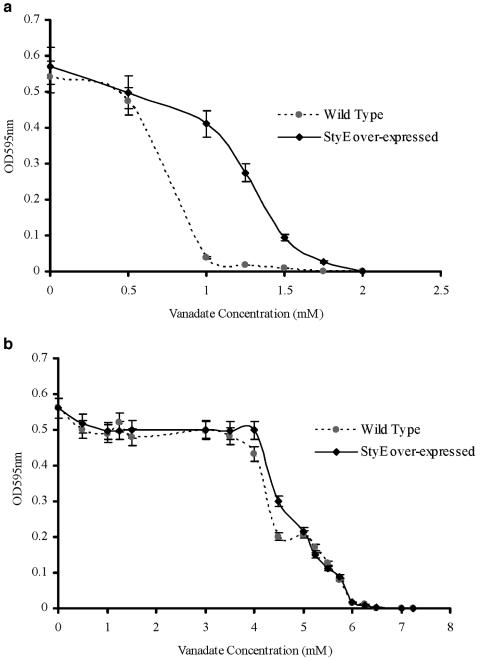
Attempts were made to inhibit any potentially active styrene transport by including a classic p-type ATPase inhibitor, vanadate, in the culture medium (28). We observed that the growth of the wild-type P. putida CA-3 on styrene as the sole carbon source was inhibited in the presence of 1 mM vanadate, while higher levels of vanadate (1.75 mM) were required to markedly affect cell growth in P. putida cells overexpressing styE (Fig. 4a). To assess whether these observations related specifically to styrene utilization or occurred simply as a result of general physiological stress imposed by the compound, citrate-grown cultures were also exposed to vanadate. Under these conditions, the MIC for both the wild-type cells and those overexpressing styE was determined to be 7.00 mM vanadate (Fig. 4b), with the growth rate and effect of vanadate addition on each culture being comparable. These findings suggest that vanadate-induced repression in P. putida cells relates specifically to styrene utilization and also appears to directly affect StyE activity, as overexpressing strains require greater vanadate concentrations than wild-type cells to cause growth inhibition on styrene.
FIG. 4.
Determination of the MIC of vanadate on P. putida CA-3 wild-type and StyE-overexpressing strains. (a) Effects of vanadate on cells growing on minimal salts medium with styrene alone. (b) Effects of vanadate when the cultures were fed citrate as the sole carbon source. ⧫, styE-overexpressing strain; ▪, CA-3 wild-type strain.
To further characterize the inhibitory effects of vanadate on styrene metabolism in P. putida CA-3, we performed experiments using cultures grown on styrene, thereby ensuring transcription of the styABCD operon. Vanadate concentrations ranging from 0 to 1.75 mM were added to washed-cell suspensions from these cultures immediately prior to performance of the SMO assay. In addition to the wild-type P. putida CA-3- and StyE-overexpressing strains, we also analyzed a P. putida CA-3 strain overexpressing the styAB (styrene monooxygenase) genes. In particular, we wished to determine how vanadate affected the strains overexpressing styE and styAB, where it was observed that while both exhibited increased styAB levels relative to the wild-type strain; higher levels of styE occurred only in the pStyEF culture. In contrast to our earlier observation of inhibition of growth on styrene, analysis of the data in Table 4 revealed that SMO activity in wild-type cells grown on styrene was largely unaffected at vanadate concentrations of up to 0.75 mM, (despite 1 mM inhibiting growth cultures) and still persisted at a basal level at 1.75 mM (Table 4, column “Wild-type CA-3”). At 0 mM vanadate, cells overexpressing styAB exhibited approximately 1.5-fold-higher levels of SMO activity than the wild type, consistent with higher levels of available enzyme. However, upon the addition of 0.5 mM vanadate, SMO activity in the culture fell back to levels comparable to those of the wild type; this trend continued throughout the subsequent additions of vanadate. Therefore, despite the presence of increased intracellular levels of functional styrene monooxygenase enzyme, the pStyAB culture was unable to transform indole to indigo above wild-type conversion rates in the presence of vanadate, suggesting a lack of substrate availability. As we have shown, earlier overexpression of styE resulted in an increase in styABCD mRNA transcript levels (Table 3). However, Table 4, column “pStyEF,” reveals a very different profile from that presented by the pStyAB overexpression strain. The addition of 0.5 mM vanadate to washed-cell suspensions of styrene-grown pStyE cultures caused a significant reduction in SMO activity, although conversion rates were still almost twofold higher than both the wild-type and StyAB overexpression strains. Indeed, this relative trend continued for all subsequent vanadate additions until 1.75 mM was added preassay. The significantly different SMO activity profiles of the pStyAB and pStyEF cultures were interesting, revealing that while both cultures overexpress styAB, it is the availability of intracellular substrate which is the limiting factor; this limitation is overcome by overexpression of styE. However, it should be noted that at the final 1.75 mM vanadate concentration, all cultures demonstrated a comparative basal level of SMO activity, which would suggest that very low levels of passive diffusion of the styrene substrate may occur.
In conclusion, the data presented here indicate the likely involvement of StyE, a membrane-associated protein, in the facilitated uptake of styrene in P. putida CA-3. The styE gene is cotranscribed with the styABCD genes and is thus under the control of the two-component StySR regulatory system. Thus, it appears that batch culture growth of wild-type P. putida CA-3 cells with styrene as the sole carbon source is suboptimal with respect to extracellular substrate concentration. We hypothesize that this condition reflects a somewhat limited capacity for the facilitated uptake of substrate due to stringent, coordinated regulation of the StyE transport protein, together with the styABCD degradative genes. While further kinetic and regulatory characterization of this system is currently under way, it is clear to us at this stage that StyE may represent a valuable target for recombinant technologies aimed at improving whole-cell-based styrene bioremediation-bioconversion strategies.
Acknowledgments
This project was partially funded under the PRTLI program for Irish third-level institutions, administered by the Higher Education Authority.
REFERENCES
- 1.Ausubel, F. M., J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
- 2.Beltrametti, F., A. M. Marconi, G. Bestetti, C. Colombo, E. Galli, M. Ruzzi, and E. Zennaro. 1997. Sequencing and functional analysis of styrene catabolism genes from Pseudomonas fluorescens ST. Appl. Environ. Microbiol. 63:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 4.Black, P. N. 1991. Primary sequence of Escherichia coli fadL gene encoding an outer membrane protein required for long chain fatty acid transport. J. Bacteriol. 173:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.DiRusso, C. C., and P. N. Black. 2004. Bacterial long chain fatty acid transport: gateway to a fatty acid-responsive signalling system. J. Biol. Chem. 279:49563-49566. [DOI] [PubMed] [Google Scholar]
- 7.Gardy, J. L., M. R. Laird, F. Chen, S. Rey, C. J. Walsh, M. Ester, and F. S. L. Brinkman. 2005. PSORTb v.2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21:617-623. [DOI] [PubMed] [Google Scholar]
- 8.Habe, H., K. Kasuga, H. Norjiri, H. Yamane, and T. Omori. 1996. Analysis of cumene (isopropylbenzene) degradation genes from Pseudomonas fluorescens IP01. Appl. Environ. Microbiol. 62:4471-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartmans, S., J. P. Smits, M. J. van der Werf, F. Volkering, and J. A. M. de Bont. 1989. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl. Environ. Microbiol. 55:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmans, S., M. J. van der Werf, and J. A. M. de Bont. 1990. Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl. Environ. Microbiol. 56:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson, L. M., and G. Speit. 2005. Review of the genotoxicity of styrene in humans. Mutat. Res. 589:158-191. [DOI] [PubMed] [Google Scholar]
- 12.Kahng, H.-Y., A. M. Byrne, R. H. Olsen, and J. J. Kukor. 2000. Characterization and role of tbuX in utilization of toluene by Ralstonia pickettii PKO1. J. Bacteriol. 182:1232-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasai, Y., J. Inoue, and S. Harayama. 2001. The TOL plasmid pWW0 xylN gene product from Pseudomonas putida is involved in m-xylene uptake. J. Bacteriol. 183:6662-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko, Y. H., M. Bianchet, L. M. Amzel, and P. L. Pedersen. 1997. Novel insights into the chemical mechanism of ATP synthase. Evidence that in the transition state the γ-phosphate of ATP is near the conserved alanine within the P-loop of the β-subunit. J. Biol. Chem. 272:18875-18881. [DOI] [PubMed] [Google Scholar]
- 15.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Roberston, M. A. Farris, R. M. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad host range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 16.Leungo, M., J. L. Garcia, and E. R. Olivera. 2001. The phenylacetyl-CoA catabolon: a complex catabolic unit with broad biotechnological applications. Mol. Microbiol. 39:1434-1442. [DOI] [PubMed] [Google Scholar]
- 17.Marconi, A. M., F. Beltrametti, G. Bestetti, F. Solinas, M. Ruzzi, E. Galli, and E. Zenarro. 1996. Cloning and characterization of styrene catabolism genes from Pseudomonas fluorescens ST. Appl. Environ Microbiol. 62:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marczynski, B., M. Peel, X. Baur. 2000. New aspects in genotoxic risk assessment of styrene exposure—a working hypothesis. Med. Hypotheses 54:619-623. [DOI] [PubMed] [Google Scholar]
- 19.Mooney, A. 2005. The genetics of styrene uptake and transcriptional activation of the sty catabolon in Pseudomonas putida CA-3. Ph.D. thesis. National University of Ireland, Cork, Ireland.
- 20.O'Connor, K. E., C. M. Buckley, S. Hartmans, and A. D. W. Dobson. 1995. Possible regulatory role for non-aromatic carbon sources in styrene degradation by Pseudomonas putida CA-3. Appl. Environ. Microbiol. 61:544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Connor, K. E., A. D. W. Dobson, and S. Hartmans. 1997. Indigo formation by microorganisms expressing styrene monooxygenase activity. Appl. Environ. Microbiol. 63:4287-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Leary, N. D., K. E. O' Connor, and A. D. W. Dobson. 2002. Biochemistry, genetics and physiology of microbial styrene degradation. FEMS Microbiol. Rev. 26:403-417. [DOI] [PubMed] [Google Scholar]
- 23.O'Leary, N. D., W. A. Duetz, A. D. W. Dobson, and K. E. O'Connor. 2002. Induction and repression of the sty operon in Pseudomonas putida CA-3 during growth on phenylacetic acid under organic and inorganic nutrient limiting continuous culture conditions. FEMS Microbiol. Letts. 208:263-268. [DOI] [PubMed] [Google Scholar]
- 24.O'Leary, N. D., K. E. O'Connor, W. Duetz, and A. D. W. Dobson. 2001. Transcriptional regulation of styrene degradation in Pseudomonas putida CA-3. Microbiology 147:973-979. [DOI] [PubMed] [Google Scholar]
- 25.O'Leary, N. D., K. E. O'Connor, P. Ward, M. Goff, and A. D. W. Dobson. 2005. Genetic characterization of accumulation of polyhydroxyalkonate from styrene in Pseudomonas putida CA-3. Appl. Environ. Microbiol. 71:4380-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivera, E. R., B. Minambres, B. Garcia, C. Muniz, M. A. Moreno, A. Ferrandez, E. Diaz, J. L. Gracia, and J. M. Leungo. 1998. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc. Natl. Acad. Sci. USA 95:6419-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panke, S., V. de Lorenzo, A. Kaiser, B. Witholt, and M. G. Wubbolts. 1999. Engineering of a stable whole-cell biocatalyst capable of (S)-styrene oxide formation for continuous two-liquid-phase applications. Appl. Environ. Microbiol. 65:5619-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezza, R. J., M. A. Villarreal, G. G. Montich, and C. E. Argarana. 2002. Vanadate inhibits the ATPase activity and DNA binding capability of bacterial MutS. A structural model for the vanadate-MutS interaction at the Walker A motif. Nucleic Acid Res. 30:4700-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soden, D. M., and A. D. W. Dobson. 2003. The use of amplified flanking region-PCR in the isolation of laccase promoter sequences from the edible fungus Pleurotus sajor-caju. J. Appl. Microbiol. 95:553-562. [DOI] [PubMed] [Google Scholar]
- 30.Tang, W. Y., and I. R. Gibbons. 1987. Photosensitized cleavage of dynein heavy chain. Cleavage at the V2 site by irradiation at 365nm in the presence of oligovanadate. J. Biol. Chem. 262:17728-17734. [PubMed] [Google Scholar]
- 31.Utkin, I. B., M. M. Yakimov, L. N. Mateeva, E. I. Koziyak, I. S. Rogozhin, Z. G. Solomon, and A. M. Bezborodov. 1991. Degradation of styrene and ethylbenzene by Pseudomonas species Y2. FEMS Microbiol. Lett. 77:237-242. [Google Scholar]
- 32.Velasco, A., S. Alsonso, J. L. Garcia, J. Perera, and E. Diaz. 1998. Genetic and functional analysis of the styrene catabolic cluster of Pseudomonas sp. strain Y2. J. Bacteriol. 180:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, Y., M. Rawlings, D. T. Gibson, D. Labbe, H. Bergeron, R. Brousseau, and P. C. K. Lau. 1995. Identification of a membrane protein and a truncated LysR-type regulator associated with the toluene degradation pathway in Pseudomonas putida F1. Mol. Gen. Genet. 246:570-579. [DOI] [PubMed] [Google Scholar]
- 34.Windgassen, M., A. Urban, and K.-E. Jaeger. 2000. Rapid gene inactivation in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 193:201-205. [DOI] [PubMed] [Google Scholar]