Abstract
The water channel protein PvTIP3;1 (α-TIP) is a member of the major intrinsic protein (MIP) membrane channel family. We overexpressed this eukaryotic aquaporin in the methylotrophic yeast Pichia pastoris, and immunogold labeling of cellular cryosections showed that the protein accumulated in the plasma membrane, as well as vacuolar and other intracellular membranes. We then developed an in vivo functional assay for water channel activity that measures the change in optical absorbance of spheroplasts following an osmotic shock. Spheroplasts of wild-type P. pastoris displayed a linear relationship between absorbance and osmotic shock level. However, spheroplasts of P. pastoris expressing PvTIP3;1 showed a break in this linear relationship corresponding to hypo-osmotically induced lysis. It is the difference between control and transformed spheroplasts under conditions of hypo-osmotic shock that forms the basis of our aquaporin activity assay. The aquaporin inhibitor mercury chloride blocked water channel activity but had no effect on wild-type yeast. Osmotically shocked yeast cells were affected only slightly by expression of the Escherichia coli glycerol channel GlpF, which belongs to the MIP family but is a weak water channel. The important role that aquaporins play in human physiology has led to a growing interest in their potential as drug targets for treatment of hypertension and congestive heart failure, as well as other fluid overload states. The simplicity of this assay that is specific for water channel activity should enable rapid screening for compounds that modulate water channel activity.
The first water channel gene was cloned serendipitously during the characterization of human blood group antigens and was found to encode a putative channel in the red blood cell plasma membrane. When the protein was expressed in Xenopus laevis oocytes, its function as a water channel was suggested by a concomitant increase in the swelling rate of osmotically shocked cells (41). This demonstration of water channel activity ended decades of speculation that proteins were responsible for the high water permeability observed in certain biological membranes.
Over the last decade many organisms have been shown to possess a class of protein channels, termed “aquaporins,” which are specialized to facilitate the transcellular movement of water. Aquaporins are members of the major intrinsic protein (MIP) superfamily found in all organisms, from archaebacteria to animals (27). The family progenitor is thought to have arisen from the tandem duplication of a three-transmembrane spanning domain protein with an Asn-Pro-Ala consensus sequence duplicated between the two halves of the protein (45). Generally, aquaporins facilitate the movement of water across cell membranes in response to osmotic gradients, functioning in cellular and organismal osmoregulation and solute transport (5, 32).
MIP family proteins are small (∼28 kDa) and are usually found as homotetramers. Recent high-resolution structures determined by electron crystallography (11, 37, 46) and X-ray crystallography (52) show that each subunit within the tetramer contains a water channel formed by a bundle composed of six transmembrane α-helices. Selectivity for water is accomplished by a filter that excludes larger molecules and a hydrophobic entrance to the pore that blocks the passage of hydrated ions (12). Electrostatic interactions between highly conserved asparagine residues in the Asn-Pro-Ala consensus sequence and water molecules in the pore disrupt the hydrogen bonding pattern that would be formed by the chain of water molecules, thereby preventing the conduction of protons (37). The unusual combination of a hydrophobic pore and a small number of solute binding sites was proposed to facilitate water transport (52).
PvTIP3;1 (formerly called α-TIP) is a plant aquaporin found in bean seed vacuole membranes (33). This protein is a model for studying water channel function since it is strictly selective for water and impermeable to ions and small nonpolar solutes such as glycerol and urea (33). In addition, the aquaporin activity of PvTIP3;1 is directly modulated by phosphorylation (33). To investigate the water channel properties of PvTIP3;1, we developed an overexpression system in Pichia pastoris and devised an in vivo assay of aquaporin function. We also expressed the Escherichia coli glycerol channel GlpF that served as a negative control for aquaporin function. The simplicity of our assay should enable rapid screening for compounds that modulate water channel activity.
MATERIALS AND METHODS
Materials.
Unless otherwise noted, all chemicals were obtained from Sigma-Aldrich and were either ACS reagent or SigmaUltra grade. Protein A-gold was obtained from George Posthuma (University Medical Center, Utrecht, The Netherlands). Sorbitol solutions were checked before use to verify that their pH was between 6 and 7.
Construction and overexpression of TIP3;1-G3-H6 in Pichia pastoris.
The gene encoding TIP3;1-G3-H6 was constructed, transformed into P. pastoris strain KM71H, and overexpressed as previously described (8). During a time course of overexpression, aliquots (10 ml) of the induced culture were taken at various times and mixed with 1 ml glycerol, and the cells were pelleted by centrifugation for 5 min at 1,500 × g. The cell pellets were immediately frozen and stored at −80°C.
Construction and overexpression of GlpF in Pichia pastoris.
The sequence flanking the E. coli GlpF glycerol channel cDNA (23) was modified by PCR to facilitate cloning and protein purification. Two oligonucleotide primers were constructed for this purpose. The forward-strand primer was 5′-AATTC GAAAA TGAGT CAAAC ATCAA CC-3′, which incorporated a BstBI restriction site before the glpF start codon. The reverse-strand primer was 5′-TGTTC TAGAT TACAG CGAAG CTTTT TG-3′, which introduced an XbaI restriction site following the GlpF stop codon. Transformation of P. pastoris strain KM71H with the modified GlpF gene and expression of GlpF in P. pastoris was carried out as described above for TIP3;1-G3-H6.
Pichia osmotic shock assay.
Fifty OD600 units of induced cells were isolated by centrifugation for 5 min at 1,500 × g and 4°C. The cell pellet was resuspended in 10 ml of BMMY medium supplemented with 1.0 M sorbitol. This suspension was then incubated at 30°C for 1 h with vigorous shaking. Lytic enzyme was added in threefold excess of what was previously shown to spheroplast P. pastoris (15). The solution of yeast lytic enzyme (ICN Biomedicals) was prepared by mixing the dry powder in BMMY medium supplemented with 1.0 M sorbitol. Yeast spheroplasts were generated by adding 1.0 ml of yeast lytic enzyme solution (3,000 U of lytic activity/ml) to the cell suspension, followed by incubation at 30°C for 1 h with gentle mixing. An aliquot (100 ml) of spheroplasts was then transferred immediately to a spectrophotometer cuvette (1.0-cm path length). Cells were osmotically shocked by a tenfold dilution with sorbitol at 1.8, 1.4, 1.0, 0.50, or 0.25 M in water. In some cases, pure water was used as a diluent. Optical absorbance (λ = 600 nm) was then measured with a Pharmacia Ultrospec 2000 spectrophotometer ∼10 s following the addition of diluent. In some assays 6 to 8 mg mercury chloride (Fluka) was added to the spheroplast preparation 10 min prior to the osmotic shock (producing a 2 to 3 mM solution of Hg2+). Alternatively, mercury chloride was added as a 1.0 M solution in dimethyl sulfoxide to produce a 3 mM solution of Hg2+. In these cases the same volume of dimethyl sulfoxide was added to the control experiments. Osmotic shock responses were measured for wild-type P. pastoris strain KM71H and KM71H transformed with either TIP3;1-G3-H6 or GlpF. Experiments were repeated three to eight times and used four separate preparations of P. pastoris cell cultures. To compensate for slight variations in cell density and growth rates between preparations, before the average osmotic shock response curve was determined for each treatment group, each individual curve was shifted in optical density so that the absorbance measured at an osmotic shock of 0 M sorbitol equaled the average absorbance of the treatment group.
Yeast cell counting.
Aliquots of yeast lytic enzyme-treated P. pastoris were diluted 10-fold either with water or with an aqueous solution of 1.0 M sorbitol. For some experiments the diluents contained 0.2% trypan blue to improve contrast. Ten microliters of each cell suspension was applied to a hemacytometer, and cells were viewed with a Nikon Eclipse TE300 inverted microscope using a 10× objective lens. Cells were counted manually, or digital images (1024 by 768 pixels) were recorded using a Sony DFW-X700 color CCD camera. Photoshop software (Adobe Systems) was used in automated batch mode to generate grayscale images, which were converted to binary images using a 30 to 50% grayscale cutoff. The same grayscale cutoff value was used for each image captured during a single microscope session. Cell counting was performed manually or by the Analyze Particles routine in the NIH-ImageJ software package (http://rsb.info.nih.gov/ij/). Cell counts were obtained from three separate preparations of P. pastoris.
Isolation of Pichia membrane-enriched fractions.
Ten-milliliter cultures of wild-type or transformed P. pastoris were induced for 42 h, and the optical absorbance at 600 nm was measured to estimate the final cell density of each culture. Cells were harvested by centrifugation for 5 min at 1,000 × g and 4°C. Pelleted yeast were resuspended in 50 ml cold water, and cells were isolated by centrifugation for 10 min at 1,000 × g at 4°C. Cell pellets were frozen and stored at −20°C.
Frozen yeast cells were thawed and resuspended in 300 μl lysis buffer (50 mM triethanolamine [pH 7.5], 10% glycerol [vol/vol], 5 mM EDTA, 5 mM EGTA, 5 mM benzamidine). Aliquots (300 μl) were then mixed with 200 μl of 0.5-mm-diameter acid-washed glass beads and 6 μl of yeast protease inhibitor cocktail in 1.5-ml plastic microcentrifuge tubes. Yeasts were disrupted by vortex mixing the tubes for 15 min at 4°C, and the resulting cellular material was isolated by centrifugation for 15 min at 16,000 × g at 4°C. The pellet and glass beads were resuspended in 400 μl lysis buffer and 8 μl yeast protease inhibitor cocktail. These suspensions were vortex mixed for 15 min at 4°C, and the cellular material was isolated by centrifugation for 15 min at 16,000 × g at 4°C. Lipid membranes were solubilized by resuspending the cell debris in 400 μl 25 mM Tris-HCl (pH 6.8) with 3% sodium dodecyl sulfate (SDS) and gently mixing the suspension for 45 min at 23°C.
SDS-PAGE chromatography and Western immunoblotting.
Cell pellets were thawed, resuspended in SDS sample buffer (2% SDS, 50 mM Tris-HCl [pH 7.5], 10% glycerol, 100 mM dithiothreitol), and then incubated at 70°C for 20 min. SDS-polyacrylamide gel electrophoresis (PAGE) chromatography was performed using a 12% acrylamide Tris-glycine gel (49). Solubilized P. pastoris membrane samples were mixed with NuPAGE lithium dodecylsufate (LDS) sample buffer (Invitrogen) and NuPAGE sample-reducing agent (Invitrogen) and then incubated at 37°C for 30 min. LDS-PAGE chromatography was performed using a 4-to-12% acrylamide gradient NuPAGE Bis-Tris gel (Invitrogen) with Precision Plus (Bio-Rad) prestained protein molecular weight standards.
Gel-separated proteins were then electroblotted to a nitrocellulose membrane. Western immunoblotting (49) was performed with either PvTIP3;1 polyclonal antibodies (22) or GlpF polyclonal antibodies (28). Goat anti-rabbit immunoglobulin G antisera coupled to horseradish peroxidase (Bio-Rad) was diluted 1,000-fold and used to label the primary antibodies, and visual detection of the bound antibodies was accomplished using a colorimetric assay (Bio-Rad). For some experiments, goat anti-rabbit immunoglobulin G antisera coupled to horseradish peroxidase (Bio-Rad) was diluted 50,000-fold and then used to label the primary antibodies. Visual detection of the bound antibodies was accomplished using a chemiluminescent assay (Pierce). Digitized images of the immunoblot results were corrected for background and contrast using Photoshop (Adobe Systems).
Cryosectioning and immunogold labeling.
Cell fixation, freezing, cryosectioning, and immunogold labeling were performed as previously described (44), with modifications. One OD600 unit of induced P. pastoris culture was diluted to 1 ml with BMMY medium supplemented with 1.0 M sorbitol and then incubated at 23°C for 1.5 h while tumbling at 20 rpm. An equal volume of fixative solution (4% paraformaldehyde and 0.025% glutaraldehyde in 50 mM sodium phosphate [pH 7.4]) was added for 10 min. Cells were pelleted by centrifugation for 10 min at 100 × g, the supernatant was removed and fresh fixative was added. The fixed cells were then resuspended in 7.5% gelatin in phosphate-buffered saline (PBS) for 10 min and repelleted by centrifugation for 1 min at 10,000 × g. The solidified gelatin-embedded pellets were removed, chopped into ∼1-mm blocks, and incubated overnight in 2.3 M sucrose in PBS; each cryoprotected block was placed atop specimen pins and frozen in liquid nitrogen.
The frozen blocks of P. pastoris were sectioned on a Reichert Ultracut FC4E freezing ultramicrotome (Leica, Deerfield, IL), mounted on Parlodion-coated nickel grids (200 mesh), and immediately inverted onto droplets of 1% bovine serum albumin in PBS. Sections were subsequently incubated in primary antisera (nonspecific immunolabeling was reduced by preincubating the PvTIP3;1 antisera with fixed wild-type yeast), washed several times, and then incubated in protein A-gold (10 nm). Following multiple PBS washes and fixation in 1% glutaraldehyde, each grid was subjected to H2O washes, and the sections were contrasted in uranyl oxalate at pH 7 and then concomitantly embedded and stained in a solution of 0.3% uranyl acetate and 1.8% methyl cellulose (pH 4). Images were recorded at a magnification of ×13,000 on Kodak SO163 film using a Philips CM100 electron microscope (Philips/FEI, Hillsboro, OR) at 100 kV.
RESULTS
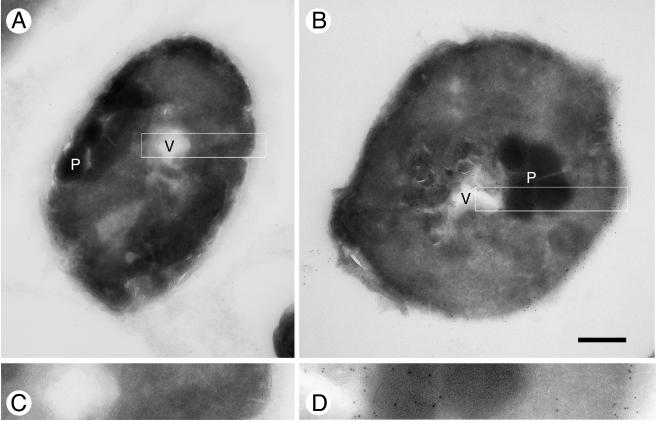
The PvTIP3;1 gene was subcloned into the pPICZ Pichia expression vector (20) and modified to include a carboxy-terminal extension of three glycine and six histidine residues to facilitate purification by immobilized-metal affinity chromatography. The final gene construct (TIP3;1-G3-H6) was linearized and integrated into the yeast genome by homologous recombination, and recombinant yeast were selected by antibiotic screening. Since gene expression was under the control of the AOX1 alcohol oxidase promoter, protein production was induced by switching to methanol as the sole carbon source in the growth medium. Cells were harvested 25 to 45 h after induction. Immunocytochemistry on ultrathin cryosections of aquaporin-expressing P. pastoris showed that the expressed TIP3;1-G3-H6 was located in the plasma membrane, as well as in vacuolar and other intracellular membranes (Fig. 1). Curiously, the growth rate of yeast expressing the recombinant protein was ∼35% of the wild-type rate (data not shown).
FIG. 1.
Immunogold labeling of TIP3;1-G3-H6 in cryosections of wild-type KM71H P. pastoris and aquaporin-expressing KM71H P. pastoris. (A) Section of wild-type KM71H P. pastoris. (B) Section of KM71H P. pastoris expressing the TIP3;1-G3-H6 aquaporin. (C) Enlargement of boxed region from panel A. (D) Enlargement (×2.33) of boxed region from panel B. Abbreviations: V, vacuole; P, peroxisome. Bar, 0.5 μm (A and B).
In order to verify that the overexpressed protein was functional and therefore properly folded, we developed an osmotic shock assay to measure aquaporin-mediated osmotic water permeability. We reasoned that the rapidity of osmotically induced swelling in aquaporin-expressing yeast would cause the cells to lyse more readily than wild-type cells. In order to test this prediction, the optical absorbances of osmotically shocked TIP3;1-G3-H6-expressing and wild-type P. pastoris cultures were measured and compared (Fig. 2). To allow cells to change volume freely, P. pastoris cultures were treated with β-1,3-glucanase to produce spheroplasts lacking a cell wall. Sorbitol was used as an osmotic protectant.
FIG. 2.
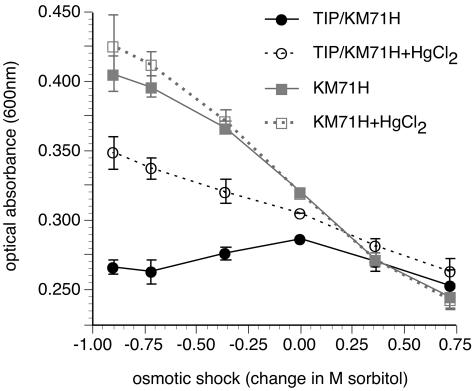
Pichia in vivo water permeability assay. Comparison of absorbance with osmotic shock gradient (change in M sorbitol) for TIP3;1-G3-H6-expressing yeast and the control, parent yeast strain KM71H. Where indicated, the assay was performed in the presence of mercury ions (2 to 3 mM HgCl2 with a 10-min preincubation prior to the osmotic shock). For this assay the osmotic shock gradient is defined as the molarity of sorbitol in the cell suspension prior to the osmotic shock, subtracted by the molarity of sorbitol in the suspension following the osmotic shock. Data are means ± standard deviations for the population (three to eight replicates). The response of each treatment group was normalized to the group average optical absorbance following an osmotic shock of 0 M sorbitol. Optical absorbance was measured ∼10 s following the osmotic shock.
When subjected to an osmotic shock, wild-type yeast cultures exhibited a change in optical absorbance inversely proportional to the change in external osmolarity (Fig. 2). For P. pastoris cultures expressing the TIP3;1-G3-H6 aquaporin, this linear correlation broke down under hypotonic conditions. Compared to wild-type yeast, there was an overall decrease in optical absorbance with hypo-osmotic shocks (Fig. 2). Differences in the osmotic shock response between wild-type and recombinant yeast cells were concomitant with cellular accumulation of TIP3;1-G3-H6 (Fig. 3). When the cell wall was not enzymatically degraded prior to the osmotic shock, wild-type and aquaporin-expressing yeasts behaved identically (data not shown). Significantly, addition of the aquaporin inhibitor mercury chloride to 3 mM did not affect the osmotic sensitivity of wild-type P. pastoris but restored the linear relationship for TIP3;1-G3-H6 expressing yeast (Fig. 2). Concentrations of mercury chloride down to 0.2 mM also produced this effect, to a lesser extent (data not shown).
FIG. 3.
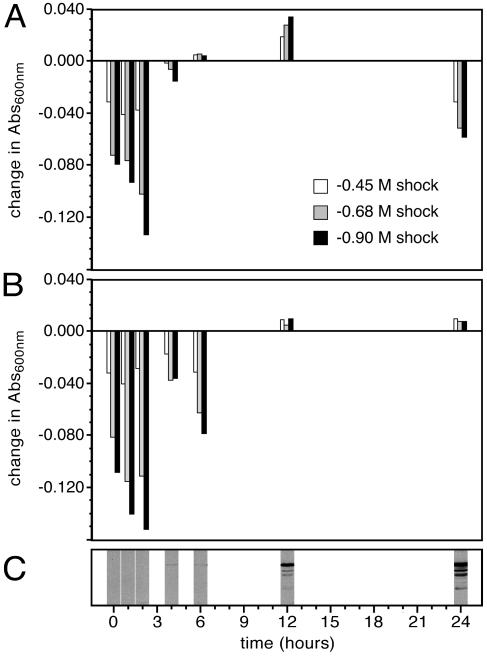
Time course of the hypo-osmotic shock response in P. pastoris expressing TIP3;1-G3-H6 and wild-type yeast. Differences in the optical absorbance between recombinant (A) and wild-type (B) yeast spheroplasts resulting from hypo-osmotic shock correspond to the start of TIP3;1-G3-H6 aquaporin expression, shown by SDS-PAGE and Western immunoblotting of yeast spheroplasts using PvTIP3;1 antisera (C). The primary immunolabeled protein has a molecular weight (MW) of ∼25,000, which agrees well with the predicted MW of 28,200 for the TIP3;1-G3-H6 monomer and is typical of PvTIP3;1 during SDS-PAGE (22, 23). Optical absorbance was measured ∼10 s following the osmotic shock. In performing the assay, there is a lapse of 2 h between culture sampling and the application of the osmotic shock.
We assume that the optical absorbance of a P. pastoris spheroplast suspension exposed to a hypo-osmotic shock will either not increase or show a decrease if the cells lyse as a result of the shock. Following a hypotonic shock (1.0 to 0.1 M sorbitol), the optical absorbance (Fig. 2) and the cell count (Table 1) of untransformed P. pastoris increased by 26% and 20%, respectively. However, for TIP3;1-G3-H6 expressing P. pastoris, the optical absorbance and cell count decreased by 8.5% and 13%, respectively. By comparison, when either untransformed or recombinant yeasts were hypo-osmotically shocked and then plated on solid growth medium, the number of yeast colonies was proportional to the cell count (data not shown).
TABLE 1.
Cell counts of aquaporin-expressing P. pastoris cultures decrease significantly following hypotonic shock
| Trial | KM71H
|
KM71H TIP3;1-G3-H6
|
||||
|---|---|---|---|---|---|---|
| Cell counta
|
% Change | Cell counta
|
% Change | |||
| 0 | −0.90 | 0 | −0.90 | |||
| 1 | 110 (4) | 113 (4) | 3 | 78 (4) | 74 (4) | −5 |
| 2 | 139 (3) | 173 (3) | 24 | 141 (3) | 127 (3) | −10 |
| 3 | 176 (3) | 210 (3) | 19 | 148 (3) | 146 (3) | −1 |
| 4 | 253 (3) | 232 (3) | −8 | 179 (3) | 185 (3) | 3 |
| 5 | 111 (4) | 107 (4) | −4 | 59 (6) | 47 (6) | −20 |
| 6 | 44 (4) | 41 (4) | −7 | 172 (3) | 37 (3) | −78 |
| 7 | 345 (1) | 539 (1) | 56 | 123 (1) | 93 (1) | −24 |
| 8 | 340 (2) | 467 (2) | 37 | 274 (2) | 252 (2) | −8 |
| 9 | 302 (1) | 395 (1) | 31 | 219 (1) | 220 (1) | 0 |
| 10 | 312 (1) | 391 (1) | 25 | 236 (1) | 234 (1) | −1 |
| 11 | 272 (2) | 381 (2) | 40 | 226 (2) | 226 (2) | 0 |
| Mean | 20 | −13 | ||||
0 and −0.90, change in sorbitol concentration (molar). Numbers in parentheses indicate numbers of replicates. The difference in cell count change upon hypotonic shock is highly significant (P < 0.003; paired Student's t test).
Untransformed P. pastoris showed a slight increase in optical absorbance and cell number following a hypotonic shock (Table 1). This effect may be caused by minor cell aggregation prior to the shock, or following the shock, either by separation of daughter cells from parent yeast or by the production of cellular ghosts, released organelles and resealed vesicles. When cells were not treated with yeast lytic enzyme prior to the osmotic shock, no difference was observed between transformed and untransformed cells (data not shown), indicating that lyticase activity is required for this assay to distinguish between aquaporin-expressing and control P. pastoris cells.
Changes in optical absorbance that occurred with osmotic shock required removal of the cell wall with yeast lytic enzyme. The effects of yeast cell wall-digesting enzymes on P. pastoris are not well understood, and yeast species can vary widely in their sensitivity to yeast lytic enzyme (24). Furthermore, Saccharomyces cerevisiae spheroplasts prepared by lyticase treatment exhibited a resistance to lysis that was dependent on growth conditions (2, 24) and could show an increase in optical absorbance during lysis (26). Consequently, in order to reduce variability in yeast lytic enzyme sensitivity and protoplasting efficiency, the same strain of P. pastoris and the same growth medium were used in all experiments.
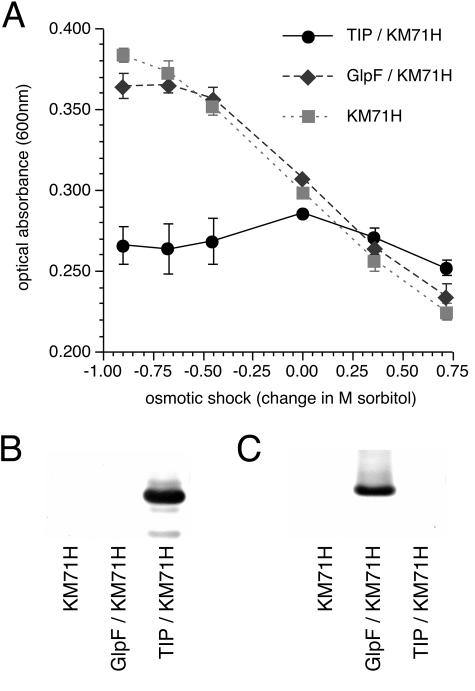
It is possible that in vivo overexpression of an integral membrane protein could disrupt the integrity or fluidity of the plasma membrane and cause anomalous responses to an extracellular osmotic shock. This was tested by generating recombinant P. pastoris that overexpressed the homologous (11) E. coli glycerol channel GlpF, which is a weak water channel (35). The behavior of GlpF-expressing yeast in response to a hypo-osmotic shock was similar to that of wild-type P. pastoris (Fig. 4). Shifts in the range of optical absorbance between the experiments shown in Fig. 2 and 4 represented differences in averaged cell density of the yeast populations used for each set of experiments.
FIG. 4.
Comparison of aquaporin-expressing yeast and glycerol channel-expressing yeast using the Pichia in vivo water permeability assay. (A) Relationship of optical absorbance with osmotic shock gradient (change in sorbitol concentration) for TIP3;1-G3-H6-expressing yeast, GlpF-expressing yeast, and the parent yeast strain KM71H. Data are means ± standard deviations for the population (four replicates). The response of each treatment group was normalized to the group average optical absorbance following an osmotic shock of 0 M sorbitol. Optical absorbance was measured ∼10 s following the osmotic shock. LDS-PAGE and Western immunoblotting, using GlpF antisera (B) and PvTIP3;1 antisera (C), of membrane-enriched fractions from P. pastoris strain KM71H, P. pastoris expressing GlpF, and P. pastoris expressing TIP3;1-G3-H6 is also shown.
Immunoblot analysis showed that TIP3;1-G3-H6 accumulated over time as a protein of ∼25 kDa (Fig. 3C). The protein was first detected ∼4 h after induction, which corresponded to significant differences in the hypo-osmotic shock behavior between wild-type and recombinant yeast spheroplasts (Fig. 3A and B). After 24 h of induction, aquaporin-expressing P. pastoris showed a −14 to −28% decrease in optical absorbance (Fig. 3B), whereas wild-type yeast showed a slight increase in optical absorbance. This result parallels what is observed in our osmotic shock assay in which absorbance is measured 24 to 40 h after induction (Fig. 2 and Fig. 4A). It was noted that the osmotic shock response of recombinant yeast was not proportional to the amount of aquaporin present. This observation can be explained by the fact that the water channel activity of PvTIP3;1 is regulated by phosphorylation as well as abundance (34). Consequently, the P. pastoris water permeability assay will not show a linear correlation between abundance and activity. Rather, TIP3;1-G3-H6 aquaporin activity will fluctuate according to the level of yeast kinase activity, which is highly dependent on cellular metabolic state and which will vary during the course of incubation (53). A fraction of the protein expressed in P. pastoris is appropriately phosphorylated (8) and hence active, which would account for the observed water channel activity.
DISCUSSION
Abundance of aquaporins in plants.
Plants possess a surprising variety of aquaporins and other MIP family proteins. To date, over a hundred isoforms have been identified; curiously, 38 have been found in the Arabidopsis thaliana genome (43), while only 13 have been identified in the human genome (21). The relative abundance of these proteins in plants is thought to be due to the greater number of selectively filtered compounds and the wider variety of subcellular membrane targets and regulatory mechanisms (43, 50).
We sought to develop a versatile expression system in which a variety of aquaporins could be expressed and assayed. For this purpose PvTIP3;1 was particularly useful since it is a strict aquaporin, being selective for water and impermeable to ions and small nonpolar solutes such as glycerol and urea (33, 34). The protein accumulates in membranes of protein storage vacuoles during embryo maturation and disappears rapidly after germination (22, 36). It is thought to play important roles during embryo desiccation (47) and seed germination (33). Whether the primary function of PvTIP3;1 is during seed development or after germination is unclear. Nevertheless, it is thought to have an important and evolutionarily conserved function, since it shares immunogenic epitopes and sequence identity with seed membrane proteins in a wide variety of plant species (16, 17, 19, 22, 40).
Pichia pastoris for expression and functional analysis of aquaporins.
The methylotrophic yeast Pichia pastoris is finding increasing use as a eukaryotic protein expression system (7) and was therefore chosen for heterologous production of the PvTIP3;1 aquaporin. Yeast is a simple and well-characterized eukaryotic organism that can perform many posttranslational modifications. In addition, membrane proteins overexpressed in yeast tend to not form insoluble inclusion bodies. These advantages over an Escherichia coli expression system have led to an increasing use of yeast for eukaryotic membrane protein production (13, 14), especially the methylotrophic yeast Pichia pastoris. Examples of membrane protein expression in P. pastoris include the renal peptide transporter PEPT2 (9), bovine opsin (1), the plant plasma membrane aquaporin PM28a (25), and the mammalian voltage-dependent K+ channel Kv1.2 (31). Immunogold labeling of ultrathin P. pastoris cryosections showed that TIP3;1-G3-H6 was targeted to the plasma membrane, as well as vacuolar and other intracellular membranes (Fig. 1).
In vivo assay of aquaporin function.
Traditional assays for determining membrane protein water channel activity require either the use of a stopped-flow light-scattering spectrophotometer (54) or the availability of an X. laevis oocyte expression system (41). The materiel and expertise required to perform these assays are not widely available. Furthermore, the complexity of these methods and the time required per sample preclude their use in high-throughput applications.
The method that we developed relies on the change in optical absorbance of a spheroplast suspension following an osmotic shock to determine whether or not the expressed aquaporin is functional. For our purposes we define “osmotic shock” as a sudden and significant change in the external osmolarity of the yeast culture. Theory and experimental evidence indicate that an increase in the average cell volume of a culture, as would result from a hypo-osmotic shock, will increase its optical absorption (30). Cellular water flux can therefore be measured by a standard spectrophotometer. In addition to its simplicity, a further advantage of a non-light-scattering photometric technique is that the measurements are relatively insensitive to population heterogeneity and multiple scattering events (30).
Osmotic shock experiments were performed using spheroplast suspensions from either P. pastoris strain KM71H or recombinant KM71H expressing TIP3;1-G3-H6 (Fig. 2). Untransformed yeast exhibit an inverse linear relationship between absorbance and osmotic shock level. P. pastoris expressing the TIP3;1-G3-H6 water channel exhibits a break in this linear relationship under hypotonic conditions and shows an overall decrease in optical absorbance with increasing hypo-osmotic shock. It is the difference between control and transformed spheroplasts under conditions of hypo-osmotic shock that forms the basis of our aquaporin activity assay.
Differences in the magnitude of osmotic shock responses between wild-type and TIP3;1-G3-H6 transformed P. pastoris can be noted as differences in the slope of the osmotic shock versus optical absorbance plots (Fig. 2 and 4) under hyperosmotic conditions. The hyperosmotic shock response of TIP3;1-G3-H6-expressing P. pastoris is slightly less than that of untransformed yeast (Fig. 2 and Fig. 4A), which could indicate that spheroplasts from the recombinant yeast swell to a lesser extent than the wild type. These differences may be due to variations in cellular osmolyte levels or in the elastic properties of the cell membranes; alternatively, the experimental conditions may cause TIP3;1-G3-H6 to form a channel for osmotically active compounds. However, previous results have indicated that PvTIP3;1 is strictly a water channel (33). A test of the fidelity of the assay was to perform experiments in the presence of the aquaporin inhibitor mercury chloride, which did not affect wild-type P. pastoris but restored the linear relationship for TIP3;1-G3-H6-expressing yeast (Fig. 2).
Yeast expressing the structurally homologous GlpF bacterial glycerol channel behaved the same as wild-type P. pastoris (Fig. 4). Previous studies have shown that GlpF is functional when expressed in S. cerevisiae (28). Water permeability of GlpF was shown to be negligible when assayed in an X. laevis oocyte expression system (10, 28) but was significant—only four- to sevenfold less than that of the bacterial aquaporin AqpZ—when the purified protein was reconstituted into proteoliposomes and assayed using stopped-flow spectrophotometry (4). With GlpF-expressing P. pastoris there appears to be a slight deviation in the linear relationship between osmotic shock and absorbance at higher values of hypo-osmotic shock (Fig. 4), which may reflect the water permeability enabled by GlpF. The lack of a significant osmotic shock response with GlpF-expressing P. pastoris suggests that our assay is specific for water channel activity and that the response observed with aquaporin-expressing yeast is not an artifact of membrane protein overexpression.
With our in vivo assay of water channel activity, there is a correlation between optical absorbance and cell volume. Wild-type and GlpF-expressing P. pastoris spheroplasts displayed a ∼15% increase in absorbance resulting from a 0.5 M drop in external osmolarity. This result is in agreement with the previously noted ∼15% volume increase in S. cerevisiae spheroplasts given a 0.5 M decrease in external sorbitol (18).
However, for P. pastoris expressing TIP3;1-G3-H6, optical absorbance decreased with decreasing osmolarity, which may be the result of cell lysis not observed with wild-type yeast. To test this possibility, yeast cells were counted following a hypotonic shock and compared to the quantity measured after a sham, isotonic shock. Hypotonic shock caused cell number to increase in wild-type yeast and to decrease in TIP3;1-G3-H6-expressing yeast, leading to a highly significant 33% difference in yeast cell number following the osmotic shock (Table 1), which suggests that control spheroplasts lyse to a lesser extent than aquaporin-expressing spheroplasts. We therefore propose that the reduction in absorbance under hypo-osmotic conditions results from the disintegration of spheroplasts due to an aquaporin-mediated influx of water and rapid cellular expansion that leads to rupture.
Potential applications for drug discovery.
Completely apart from their role in plant-water relations, aquaporins are also exceedingly important in human physiology. Consequently, there is growing interest in their potential as drug targets (3, 29). At least seven aquaporin isoforms are present in the kidney, where they act to facilitate osmotically driven water reabsorption (38). Mercury and other heavy metal sulfhydryl reagents are the only known aquaporin inhibitors (39, 42), and their toxicity precludes their use. Safe and effective kidney and lung aquaporin inhibitors would represent a novel class of reagents for the treatment of hypertension and congestive heart failure, as well as other fluid overload states.
Our cell-based light absorbance test of aquaporin activity is more biologically relevant than a solution-based in vitro assay because the activity of a lead compound to its target is measured in vivo (51). The simplicity of measuring absorbance for drug activity suggests that this in vivo assay should be readily adaptable for high-throughput drug screening methods.
Acknowledgments
M.J.D. was supported by an NIH NRSA fellowship (EY 06906). M.Y. was supported by grants from the NIH, NHLBI (R01 HL48908), and NIGMS (R01 GM65399). During this work, M.Y. was a recipient of a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund.
We are grateful to Jean-François Hubert for providing antisera to GlpF and to Alexandrine Froger and Catherine Derivera for providing helpful advice. We thank Anchi Cheng and Barbie Ganser-Pornillos for their critical reading of the manuscript, and we appreciate the useful comments made by the anonymous reviewers.
REFERENCES
- 1.Abdulaev, N. G., M. P. Popp, W. C. Smith, and K. D. Ridge. 1997. Functional expression of bovine opsin in the methylotrophic yeast Pichia pastoris. Protein Expr. Purif. 10:61-69. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Uscanga, B., and J. M. François. 2003. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 37:268-274. [DOI] [PubMed] [Google Scholar]
- 3.Beitz, E., and J. E. Schultz. 1999. The mammalian aquaporin water channel family: a promising new drug target. Curr. Med. Chem. 6:457-467. [PubMed] [Google Scholar]
- 4.Borgnia, M. J., and P. Agre. 2001. Reconstitution and functional comparison of purified GlpF and AqpZ, the glycerol and water channels from Escherichia coli. Proc. Natl. Acad. Sci. USA 98:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chrispeels, M. J., and P. Agre. 1994. Aquaporins: water channel proteins of plant and animal cells. Trends Biochem. Sci. 19:421-425. [DOI] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Cregg, J. M., J. L. Cereghino, J. Shi, and D. R. Higgins. 2000. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 16:23-52. [DOI] [PubMed] [Google Scholar]
- 8.Daniels, M. J., and M. Yeager. 2005. Phosphorylation of aquaporin PvTIP3;1 defined by mass spectrometry and molecular modeling. Biochemistry 44:14443-14454. [DOI] [PubMed] [Google Scholar]
- 9.Döring, F., T. Michel, A. Rösel, M. Nickolaus, and H. Daniel. 1998. Expression of the mammalian renal peptide transporter PEPT2 in the yeast Pichia pastoris and applications of the yeast system for functional analysis. Mol. Membrane Biol. 15:79-88. [DOI] [PubMed] [Google Scholar]
- 10.Duchesne, L., I. Pellerin, C. Delamarche, S. Deschamps, V. Lagree, A. Froger, G. Bonnec, D. Thomas, and J. F. Hubert. 2002. Role of C-terminal domain and transmembrane helices 5 and 6 in function and quaternary structure of major intrinsic proteins: analysis of aquaporin/glycerol facilitator chimeric proteins. J. Biol. Chem. 277:20598-20604. [DOI] [PubMed] [Google Scholar]
- 11.Fu, D., A. Libson, L. J. W. Miercke, C. Weitzman, P. Nollert, J. Krucinski, and R. M. Stroud. 2000. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290:481-486. [DOI] [PubMed] [Google Scholar]
- 12.Fujiyoshi, Y., K. Mitsuoka, B. L. de Groot, A. Philippsen, H. Grubmüller, P. Agre, and A. Engel. 2002. Structure and function of water channels. Curr. Opin. Struct. Biol. 12:509-515. [DOI] [PubMed] [Google Scholar]
- 13.Grisshammer, R., and C. Tate. 2003. Preface: overexpression of integral membrane proteins. Biochim. Biophys. Acta 1610:1. [Google Scholar]
- 14.Grisshammer, R., and C. G. Tate. 1995. Overexpression of integral membrane proteins for structural studies. Q. Rev. Biophys. 28:315-422. [DOI] [PubMed] [Google Scholar]
- 15.Han, C.-H., M. Werder, W. von Gustedt, and H. Hauser. 2003. Heterologous expression of human scavenger receptor class B type I (SR-BI) in Pichia pastoris, p. 49-63. In B. S. Selinsky (ed.), Membrane protein protocols: expression, purification, and characterization, vol. 228. Humana Press, Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 16.Harvengt, P., A. Vlerick, B. Fuks, R. Wattiez, J.-M. Ruysschaert, and F. Homble. 2000. Lentil seed aquaporins form a hetero-oligomer which is phosphorylated by a Mg2+-dependent and Ca2+-regulated kinase. Biochem. J. 352:183-190. [PMC free article] [PubMed] [Google Scholar]
- 17.Höfte, H., L. Hubbard, J. Reizer, D. Ludevid, E. M. Herman, and M. J. Chrispeels. 1992. Vegetative and seed-specific forms of tonoplast intrinsic protein in the vacuolar membrane of Arabidopsis thaliana. Plant Physiol. 99:561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossack, J. A., V. J. Sharpe, and A. H. Rose. 1977. Stability of the plasma membrane in Saccharomyces cerevisiae enriched with phosphatidylcholine or phosphatidylethanolamine. J. Bacteriol. 129:1144-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue, K., Y. Takeuchi, M. Nishimura, and I. Hara-Nishimura. 1995. Characterization of two integral membrane proteins located in the protein bodies of pumpkin seeds. Plant Mol. Biol. 28:1089-1101. [DOI] [PubMed] [Google Scholar]
- 20.Invitrogen. 2000. Pichia expression kit: a manual of methods for expression of recombinant proteins in Pichia pastoris, L ed. Invitrogen Corporation, Carlsbad, Calif.
- 21.Ishibashi, K., T. Morinaga, M. Kuwahara, S. Sasaki, and M. Imai. 2002. Cloning and identification of a new member of water channel (AQP10) as an aquaglyceroporin. Biochim. Biophys. Acta 1576:335-340. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, K. D., E. M. Herman, and M. J. Chrispeels. 1989. An abundant, highly conserved tonoplast protein in seeds. Plant Physiol. 91:1006-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, K. D., H. Höfte, and M. J. Chrispeels. 1990. An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GlpF). Plant Cell 2:525-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko, T., K. Kitamura, and Y. Yamamoto. 1973. Susceptibilities of yeasts to yeast cell wall lytic enzyme of Arthrobacter luteus. Agric. Biol. Chem. 37:2295-2302. [Google Scholar]
- 25.Karlsson, M., D. Fotiadis, S. Sjövall, I. Johansson, K. Hedfalk, A. Engel, and P. Kjellbom. 2003. Reconstitution of water channel function of an aquaporin overexpressed and purified from Pichia pastoris. FEBS Lett. 537:68-72. [DOI] [PubMed] [Google Scholar]
- 26.Kovac, L., E. Bohmerova, and O. Necas. 1987. The plasma membrane of yeast protoplasts exposed to hypotonicity becomes porous but does not disintegrate in the presence of protons or polyvalent cations. Biochim. Biophys. Acta 899:265-275. [DOI] [PubMed] [Google Scholar]
- 27.Kozono, D., X. Ding, I. Iwasaki, X. Meng, Y. Kamagata, P. Agre, and Y. Kitagawa. 2003. Functional expression and characterization of an archaeal aquaporin, AqpM, from Methanothermobacter marburgensis. J. Biol. Chem. 278:10649-10656. [DOI] [PubMed] [Google Scholar]
- 28.Lagree, V., A. Froger, S. Deschamps, I. Pellerin, C. Delamarche, G. Bonnec, J. Gouranton, D. Thomas, and J. F. Hubert. 1998. Oligomerization state of water channels and glycerol facilitators. Involvement of loop E. J. Biol. Chem. 273:33949-33953. [DOI] [PubMed] [Google Scholar]
- 29.Laski, M. E., and T. A. Pressley. 1999. Aquaporin mediated water flux as a target for diuretic development. Sem. Nephrol. 19:533-550. [PubMed] [Google Scholar]
- 30.Latimer, P. 1979. Light scattering vs. microscopy for measuring average cell size and shape. Biophys. J. 27:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long, S. B., E. B. Campbell, and R. MacKinnon. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309:897-903. [DOI] [PubMed] [Google Scholar]
- 32.Maurel, C. 1997. Aquaporins and water permeability of plant membranes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:399-429. [DOI] [PubMed] [Google Scholar]
- 33.Maurel, C., M. Chrispeels, C. Lurin, F. Tacnet, D. Geelen, P. Ripoche, and J. Guern. 1997. Function and regulation of seed aquaporins. J. Exp. Bot. 48:421-430. [DOI] [PubMed] [Google Scholar]
- 34.Maurel, C., R. T. Kado, J. Guern, and M. J. Chrispeels. 1995. Phosphorylation regulates the water channel activity of the seed-specific aquaporin α-TIP. EMBO J. 14:3028-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurel, C., J. Reizer, J. I. Schroeder, M. J. Chrispeels, and M. H. Saier. 1994. Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J. Biol. Chem. 269:11869-11872. [PubMed] [Google Scholar]
- 36.Melroy, D. L., and E. M. Herman. 1991. TIP, an integral membrane protein of the protein-storage vacuoles of the soybean cotyledon undergoes developmentally regulated membrane accumulation and removal. Planta 184:113-122. [DOI] [PubMed] [Google Scholar]
- 37.Murata, K., K. Mitsuoka, T. Hirai, T. Walz, P. Agre, J. B. Heymann, A. Engel, and Y. Fujiyoshi. 2000. Structural determinants of water permeation through aquaporin-1. Nature 407:599-605. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen, S., J. Frøkiaer, D. Marples, T.-H. Kwon, P. Agre, and M. A. Knepper. 2002. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 82:205-244. [DOI] [PubMed] [Google Scholar]
- 39.Niemietz, C. M., and S. D. Tyerman. 2002. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 531:443-447. [DOI] [PubMed] [Google Scholar]
- 40.Oliviusson, P., and I. Hakman. 1995. A tonoplast intrinsic protein (TIP) is present in seeds, roots, and somatic embryos of Norway spruce (Picea abies). Physiol. Plant. 95:288-295. [Google Scholar]
- 41.Preston, G. M., T. P. Carroll, W. B. Guggino, and P. Agre. 1992. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385-387. [DOI] [PubMed] [Google Scholar]
- 42.Preston, G. M., J. S. Jung, W. B. Guggino, and P. Agre. 1993. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J. Biol. Chem. 268:17-20. [PubMed] [Google Scholar]
- 43.Quigley, F., J. M. Rosenberg, Y. Shachar-Hill, and H. J. Bohnert. 2001. From genome to function: the Arabidopsis aquaporins. Genome Biol. 3:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raposo, G., M. J. Kleijmeer, G. Posthuma, J. W. Slot, and H. J. Geuze. 1996. Immunogold labeling of ultrathin cryosections: application in immunology, p. 1-11. In L. A. Herzenberg, D. M. Weir, L. A. Herzenberg, and C. Blackwell (ed.), Weir's handbook of experimental immunology, vol. 4. Blackwell Science, Oxford, United Kingdom. [Google Scholar]
- 45.Reizer, J., A. Reizer, and M. H. Saier. 1993. The MIP family of integral membrane channel proteins: sequence comparisons, evolutionary relationships, reconstructed pathway of evolution, and proposed functional differentiation of the two repeated halves of the proteins. Crit. Rev. Biochem. Mol. Biol. 28:235-257. [DOI] [PubMed] [Google Scholar]
- 46.Ren, G., V. S. Reddy, A. Cheng, P. Melnyk, and A. K. Mitra. 2001. Visualization of a water-selective pore by electron crystallography in vitreous ice. Proc. Natl. Acad. Sci. USA 98:1398-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson, D. G., and G. Hinz. 1997. Vacuole biogenesis and protein transport to the plant vacuole: a comparison with the yeast vacuole and the mammalian lysosome. Protoplasma 197:1-25. [Google Scholar]
- 48.Reference deleted.
- 49.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 50.Santoni, V., P. Gerbeau, H. Javot, and C. Maurel. 2000. The high diversity of aquaporins reveals novel facets of plant membrane functions. Curr. Opin. Plant Biol. 3:476-481. [DOI] [PubMed] [Google Scholar]
- 51.Silverman, L., R. Campbell, and J. R. Broach. 1998. New assay technologies for high-throughput screening. Curr. Opin. Chem. Biol. 2:397-403. [DOI] [PubMed] [Google Scholar]
- 52.Sui, H., B.-G. Han, J. K. Lee, P. Walian, and B. K. Jap. 2001. Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872-877. [DOI] [PubMed] [Google Scholar]
- 53.Thevelein, J. M. 1994. Signal transduction in yeast. Yeast 10:1753-1790. [DOI] [PubMed] [Google Scholar]
- 54.Zeidel, M. L., S. V. Ambudkar, B. L. Smith, and P. Agre. 1992. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry 31:7436-7440. [DOI] [PubMed] [Google Scholar]