Abstract
Water delivered by dental unit water systems (DUWS) in general dental practices can harbor high numbers of bacteria, including opportunistic pathogens. Biofilms on tubing within DUWS provide a reservoir for microorganisms and should be controlled. This study compared disinfection products for their ability to meet the American Dental Association's guideline of <200 CFU · ml−1 for DUWS water. Alpron, BioBlue, Dentosept, Oxygenal, Sanosil, Sterilex Ultra, and Ster4Spray were tested in DUWS (n = 134) in Denmark, Germany, Greece, Ireland, The Netherlands, Spain, and the United Kingdom. Weekly water samples were tested for total viable counts (TVCs) on yeast extract agar, and, where possible, the effects of products on established biofilm (TVCs) were measured. A 4- to 5-week baseline measurement period was followed by 6 to 8 weeks of disinfection (intermittent or continuous product application). DUWS water TVCs before disinfection ranged from 0 to 5.41 log CFU · ml−1. Disinfectants achieved reductions in the median water TVC ranging from 0.69 (Ster4Spray) to 3.11 (Dentosept) log CFU · ml−1, although occasional high values (up to 4.88 log CFU · ml−1) occurred with all products. Before treatment, 64% of all baseline samples exceeded American Dental Association guidelines, compared to only 17% following commencement of treatment; where tested, biofilm TVCs were reduced to below detectable levels. The antimicrobial efficacies of products varied (e.g., 91% of water samples from DUWS treated with Dentosept or Oxygenal met American Dental Association guidelines, compared to 60% of those treated with Ster4Spray). Overall, the continuously applied products performed better than those applied intermittently. The most effective products were Dentosept and Oxygenal, although Dentosept gave the most consistent and sustained antimicrobial effect over time.
Dental unit water systems (DUWS) are used to irrigate the oral cavity during dental treatment. Water delivered from these devices is not sterile and has been shown to contain high numbers of bacteria (9, 38, 52). Biofilms accumulating on the inner surface of the tubing can be responsible for high levels of contamination of water delivered by DUWS (21, 38, 51). A number of surveys have reported that the majority of DUWS are supplied by tap water (54). European Union (EU) guidelines recommend that tap water should be delivered at <100 CFU · ml−1 (2); however, once the water enters the DUWS the number of bacteria can increase, with numbers as high as 1.6 × 108 CFU · ml−1 having been recovered in the outflow (12). Such high numbers can result from numerous factors, including ambient temperatures, stagnation, and the presence of biofilm (30). In the United States, the American Dental Association (ADA) and the Centers for Disease Control and Prevention have proposed a guideline for DUWS water of ≤200 CFU · ml−1 (equivalent to that required for dialysis water) (1). Currently, dentists across the world have little or no evidence-based guidelines to control bacterial numbers in DUWS.
Typically, patients in the EU visit general dental practices (GDPs) every 6 months, with over 20 million visits per year in 1998 in one large EU country alone (5). During almost every visit, the patient and the dental health care staff are exposed to the water from DUWS. These medical devices have the potential to harbor opportunistic or frank pathogens, and Legionella pneumophila, Mycobacterium spp., Pseudomonas aeruginosa, and Candida spp. have been recovered from DUWS (4, 7, 28, 34, 46, 56). Exposure of dental personnel to such pathogens can be inferred from the finding that dentists have significantly higher antibody titers to L. pneumophila than individuals in other, equivalent employment sectors (10, 11, 24, 35), and asthma may be associated with occupational exposure to endotoxin in aerosols from contaminated DUWS (29). In addition, P. aeruginosa isolated from a DUWS was found to be responsible for the hospitalization of two patients following a visit to a dental surgery (19). The presence of pathogens has further implications when one considers that failure of the three-in-one hand piece antiretraction valve (32, 37) could result in microorganisms being transferred among patients (cross-infection).
A wide range of disinfectant products are now being developed for use in DUWS, and these have been evaluated using a variety of approaches (39, 48, 49), although rarely have these products been compared in a general dental practice setting. The aim of this study was therefore to evaluate and compare the levels of control achievable by the application of different disinfectants to DUWS in GDPs.
MATERIALS AND METHODS
Selection of GDPs.
GDPs in seven European countries (Denmark, Germany, Greece, Ireland, Spain, The Netherlands, and the United Kingdom) were contacted by mail and invited to participate in the study. Representative dental practices that (i) were willing to participate in trials of disinfectants, (ii) would allow sampling to evaluate the efficacy of the product, and (iii) would also comment on their ease of use were selected from each country. The selected practices used units supplied by bottled water, mains water, or header tank systems. All the samples were collected and processed in the same time frame and according to the same protocols in all countries.
Modification of DUWS.
In the case of mains water systems, dental units were modified to facilitate the addition of a disinfectant to the water used in the DUWS by fitting an externally mounted purge system (Micrylium Laboratories, Inc., Phoenix, AZ). This was carried out by a qualified dental unit installer following the manufacturer's instructions. The system consisted of a 700-ml bottle and two switches; one to toggle between municipal and bottle water supplies and the other to turn the air purge on or off. Using this system, the municipal water supply could be bypassed and the bottle used to add a disinfectant intermittently to the DUWS, e.g., overnight or during the weekend, after which time the municipal water supply could be reconnected for use during treatment. Alternatively, if a disinfectant was to be used continuously, it was added to the bottle supply, which was subsequently used throughout patient treatment sessions. Air purging of the waterlines, if recommended by the disinfectant's manufacturer, could also be carried out using this system.
Sampling of DUWS.
A test period consisted of 4 to 5 weeks of baseline value measurement followed by 6 to 8 weeks of disinfection. This period was always preceded by a nondisinfecting period of at least 14 days to allow biofilm formation (48) and washout of residual product when multiple products were tested serially in one DUWS. In order to measure DUWS contamination, samples (100 ml) were taken weekly for 4 to 5 weeks before and for 6 to 8 weeks after disinfection. Samples were collected at approximately mid-morning from the distal outlets of the three-in-one syringes of all DUWS. Where possible, a 1-centimeter length of the waterline tubing supplying the three-in-one syringe was cut out for biofilm analysis (48). One tubing sample was taken during pretreatment baseline measurement, and one was taken after the disinfection period was finished. All the staff performing the disinfecting and sampling procedures were instructed to use the same methodology.
Water samples were processed as described previously (45). Approximately 100 ml of water sample was passed through a sterile nozzle into a sterile water bottle containing 0.1 g sodium thiosulfate to remove any residual disinfectant (Abinghurst Ltd., Northampton, United Kingdom). Samples were returned to the laboratory in a cool box (4 to 8°C) within 3 h and then filtered through 100-ml capacity, 0.2-μm analytical test filter funnels (Techware, Poole, United Kingdom) in order to recover the waterborne microorganisms. The membrane was removed from the funnel with sterile forceps and placed in a screw cap sterile container (Elkay Products Inc., Shrewsbury, MA). Microorganisms were washed from the membrane by vortexing the container for 1 min in 10 ml of sterile phosphate-buffered saline (PBS).
TVCs and detection of Pseudomonas aeruginosa in water samples.
Total viable counts (TVCs) and counts of Pseudomonas aeruginosa (as a marker of the presence of opportunistic pathogens) were carried out as described previously (45), using decimal dilutions of the water samples (in sterile PBS) on (i) yeast extract agar for environmental microorganisms (3), incubated at 37°C for up to 3 days, and (ii) CFC supplemented with SR103 for Pseudomonas spp. (26), incubated at 37°C for up to 48 h. Colonies were counted on day 3, and the TVC was calculated as CFU · milliliter−1. Colonies on the Pseudomonas agar were confirmed as P. aeruginosa according to color, ammonia production, and oxidase activity. TVCs were used as the definitive measure of total microbial contamination of the water passing through the DUWS. This was compared with the U.S. guideline for DUWS of ≤200 CFU · ml−1 as recommended by the American Dental Association (1). All of the participants used the same microbiological methods (46). The limit of detection was 10 CFU · ml−1.
Analysis of biofilm accumulation.
Biofouling was assessed by measuring the TVCs of defined areas of the inner surface of the tubing as previously described (49). The DUWS tubing was sectioned horizontally and rinsed twice in nonflowing sterile PBS to remove nonadhered cells, and the surface biofilm was removed by scraping it into 1 ml of sterile PBS with a sterile dental probe. The TVC was then determined as described above, and the limit of detection was 10 CFU · cm−2.
Evaluation of disinfectant agents in GDP DUWS.
Disinfectants (Table 1) were selected from a previous laboratory model evaluation study according to their ability to kill microbial cells and remove biofilm from the inner surfaces of DUWS tubing (47, 48). Products were supplied and used either intermittently or continuously (see below) according to the manufacturers' instructions. Treatment was administered each evening prior to the closing of the surgery, and on weekends, for up to 8 weeks. Where necessary, the disinfectant was removed from the system by flushing the following morning before patient treatment began. The removal of disinfectant was confirmed either by observing the loss of product color from the outflowing water or from a negative potassium iodide detection test for hydrogen peroxide-based products.
TABLE 1.
Summary of active agents, concentrations, type of application, and sources of the products evaluated
| Product name | Active agent(s) | Effective concn (%) | Applicationa | Manufacturer |
|---|---|---|---|---|
| Alpron BRS Solutionb | Sodium hypochlorite, citric acid | 1-2, 70 | Once | Alpro Dental Products GmbH, St. Georgen, Germany |
| Alpron Mintb | Sodium-p-toluol-sulfonechloramide, EDTA | <0.2, 1-5 | Continuous | |
| BioBlue | Ethanol, chlorhexidine | 12, 0.12 | Intermittent | Micrylium, Toronto, Canada |
| Dentosept P | Hydrogen peroxide, silver ions | 0.014 | Continuous | Sirona, Bensheim, Germany |
| Oxygenal 6 | Hydrogen peroxide, silver ions | 0.02 | Continuous | KaVo Dental GmbH, Biberach, Germany |
| Sanosil Super 25 | Hydrogen peroxide, silver ions | 5 | Intermittent | Sanosil Ltd., Hombrechtikon, Switzerland |
| Sterilex Ultra | Alkaline peroxide | 5 | Intermittent | Sterilex Corporation, Maryland |
| Ster4Spray | Sodium perborate, EDTA | 2 | Intermittent | Castellini S.p.a., Bologna, Italy |
Method of application used in this study.
Alpron treatment consists of initial use of Alpron BRS Solution followed by Alpron Mint.
Intermittent application of disinfectants.
At the end of the working day, 200 to 250 ml of disinfectant was placed in the reservoir bottle that supplied the dental unit with water, and the solution was run through the system for 2 minutes. The unit was then turned off, and the disinfectant was left overnight in situ. At the beginning of the next working day, the bottle was disconnected and the residual solution poured from the bottle without rinsing. The bottle was filled with water and connected to the unit, after which the system was flushed until all of the remaining solution had been discharged from the outflow of the DUWS (residual product was detected as described above). The dental unit was then ready for daily use.
For intermittent disinfection of mains water-supplied systems, the unit was disconnected from the mains water supply and connected to the bottle with disinfectant by an externally mounted purge system (see “Modification of DUWS” above). The product was administered as described above, and at the beginning of the next working day the DUWS was reconnected to the mains supply for use in daily practice. The disinfectants applied intermittently were BioBlue, Sanosil, Sterilex Ultra, and Ster4Spray (Table 1).
Continuous application of disinfectants.
The DUWS water reservoir bottle was filled with disinfectant and connected to the unit, and the disinfectant was run through the system for 2 minutes. The dental unit was then ready for daily use, and the disinfecting agent was present in the outflowing water throughout the day. The disinfectants used for continuous use were Alpron, Oxygenal, and Dentosept (Table 1). The products were left in situ overnight.
Statistical analysis.
The results for pre- and postdisinfection for each product were compared using a Mann-Whitney test for two independent groups of samples. To test for statistical differences between all products, a Kruskall-Wallis test was used. Mann-Whitney tests were subsequently used to compare products with all other products separately (SPSS 12.0.1 for Windows; SPSS, Chicago, IL).
RESULTS
Dental unit water systems and general dental practices.
Seven different disinfection products were tested in seven European countries in a total of 134 dental units (Table 2). The average period of disinfection was 6.9 weeks; due to methodological issues, some GDPs had to withdraw units from the disinfection period earlier than the intended 8 weeks. Alpron was tested in 37 units (28% of total units), Sanosil in 30 units (22%), BioBlue in 26 units (19%), Oxygenal in 15 units (11%), and Dentosept, Sterilex, and Ster4Spray in 11 (8%), 10 (7%), and 5 (4%) units, respectively (Table 2). Source water TVCs were measured previously and ranged from 0.77 to 2.31 log CFU · ml−1 (46). In the general dental practices visited, the TVCs of the water flowing from the three-in-one syringe ranged from 0 (i.e., no detectable colonies) to 5.41 log CFU · ml−1 during baseline pretreatment measurements. Before treatment, 64% of all baseline TVCs measured in samples from all countries (n = 585) were above the ADA recommendation of ≤200 CFU · ml−1, while during treatment, 83% of all measurements (n = 926) were below the ADA guideline. Baseline values from units in Germany were excluded from this analysis because these DUWS had an integrated disinfection system which provided an automatic continuous disinfection of the unit, making it impossible to determine nontreated baseline values.
TABLE 2.
Criteria used for evaluation of efficacies of the disinfection products in all countries
| Product | Total no. (%) of DUWS tested | Reduction (log CFU · ml−1) in median water TVC before and after treatment with disinfectants (% reduction of medians of absolute TVC values)a | % of water samples during treatment with disinfectant having a TVC of <200 CFU · ml−1 | No. of units with one or more high TVC values during longitudinal analysis of disinfection/totalb |
|---|---|---|---|---|
| Alpron | 37 (28) | 2.10† (99) | 87 | 3/29 |
| BioBlue | 26 (19) | 1.79† (99) | 74 | 4/21 |
| Dentosept | 11 (8) | 3.11† (>99) | 91 | 5/11 |
| Oxygenal | 15 (11) | 1.24† (97) | 91 | 3/14 |
| Sanosil | 30 (22) | 1.57† (98) | 83 | 5/24 |
| Sterilex | 10 (8) | 1.50† (98) | 78 | 3/7 |
| Ster4Spray | 5 (4) | 0.69 (80) | 60 | 3/5 |
| Total | 134 (100) | 26/111 |
Only units that were tested for 6 weeks or more were included.
†, statistically significant difference (P ≤ 0.05).
Effect of disinfection.
The following products were tested for their ability to produce TVCs compliant with the ADA recommendation of ≤200 CFU · ml−1 (≤2.3 log CFU · ml−1) in outflow water of the DUWS (Table 1).
(i) Alpron.
Alpron was tested in a continuous mode in 37 DUWS (Table 2). The use of Alpron resulted in a significant decrease in microbial counts to below 200 CFU · ml−1 (P < 0.01) in comparison to the baseline values prior to treatment (Fig. 1). Eighty-seven percent of treated water samples achieved the ADA guideline, and the change in median TVC between the baseline and treated water samples was 2.10 log CFU · ml−1 (Table 2).
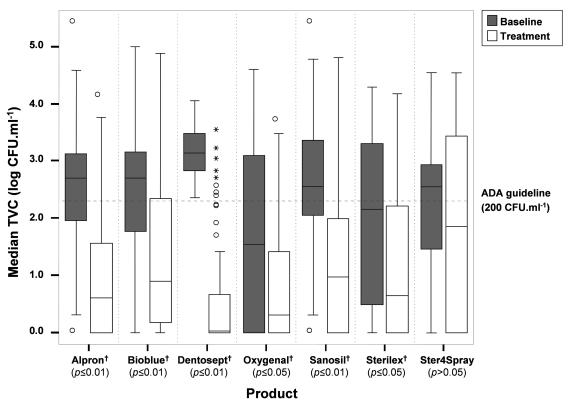
FIG. 1.
Median water TVCs before (▪) and after (□) the start of disinfection of DUWS for all products. Median values are shown as black lines in the boxes. The boxes represent the interquartile ranges, and the whiskers indicate the minimum and maximum values up to 1.5 times the IQR (made with SPSS 12.0.1 for Windows). ○, outlier value (between 1.5 and 3 times the IQR); *, extreme value (more than 3 times the IQR); †, statistically significant change.
(ii) BioBlue.
BioBlue was tested in intermittent mode in 26 DUWS (Table 2), and a significant decrease in microbial counts (P < 0.01) compared to the baseline results was found (Fig. 1). The mean percentage of treated water samples delivering water containing less than 200 CFU · ml−1 was 74%, and the change in median TVC between the baseline and treated water samples was 1.79 log CFU · ml−1 (Table 2). The error bars in Fig. 1 representing the range of values demonstrate that a number of samples (mainly in Ireland) showed high values during treatment, indicating an inconsistent effect of the disinfectant.
(iii) Dentosept.
Dentosept was tested in 11 dental units (Table 2) in a continuous mode. There was a significant difference (P < 0.01) between the baseline and treatment values (Fig. 1), with 91% of the treated water samples having a TVC of ≤200 CFU · ml−1 and a change of 3.11 log CFU · ml−1 between the baseline and treated water samples (Table 2). Extreme and outlier values that were measured (Fig. 1) indicate the occurrence of occasional high values during treatment. An outlier value was defined as being between 1.5 and 3 times the interquartile ranges (IQR), while extreme values were more than 3 times the IQR.
(iv) Oxygenal.
Oxygenal was tested in 15 DUWS (Table 2) in a continuous mode. A significant difference (P < 0.05) between pre- and posttreatment TVCs was found, and 91% of the treated water samples had a TVC of ≤200 CFU · ml−1, with a change between the median baseline and treated water samples of 1.24 log CFU · ml−1 (Table 2). One extreme value that was measured in The Netherlands (Fig. 1) was due to a problem with the application of the product to the DUWS, and the dentist stopped participating in the study after this high value was measured.
(v) Sanosil.
Sanosil was tested in intermittent mode in 30 dental units (Table 2). A significant decrease (P < 0.01) between results for baseline and treated water samples was found. The percentage of treated water samples that achieved the ADA guideline was 83%, and the change in median TVC between the baseline and test water samples was 1.57 log CFU · ml−1 (Table 2). The broad range of the error bars (Fig. 1), indicating a variable effect of the product, was caused mainly by two units in Spain in which high values were found during treatment; these values were defined statistically as outliers in the longitudinal study (Fig. 2).
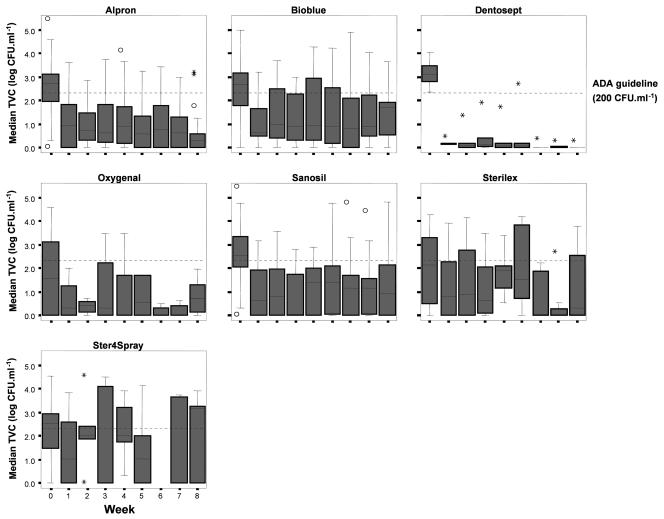
FIG. 2.
Longitudinal analysis of water TVCs during 8 weeks of disinfection of DUWS. Disinfection starts at week 1; week 0 represents the median baseline value. Median values are shown as black lines in the boxes. The boxes represent the interquartile ranges, and the whiskers indicate the minimum and maximum values up to 1.5 times the IQR (made with SPSS 12.0.1 for Windows). ○, outlier value (between 1.5 and 3 times the IQR); *, extreme value (more than 3 times the IQR).
(vi) Sterilex Ultra.
Sterilex Ultra was tested in 10 DUWS in intermittent mode (Table 2). The overall reduction in median TVC values of the baseline and the treated water samples was statistically significant (P < 0.05) (Fig. 1). Seventy-eight percent of the treated water samples were found to have less than 200 CFU · ml−1, with a change of 1.50 log CFU · ml−1 between the median values of the baseline and treated water samples (Table 2). There was a considerable difference in effect between countries, and the broad range of the data indicated by the error bars was caused mainly by TVCs from units in Ireland (one value was defined as an extreme outlier [Fig. 2]). However, the median TVC during treatment in Ireland was below 200 CFU · ml−1, with 68% of the treated water samples being below this limit.
(vii) Ster4Spray.
Ster4Spray was tested in intermittent mode in five units (Table 2). The median TVC was lower than the ADA guideline after treatment, but this difference was not statistically significant (Fig. 1). The distance between the error bars, representing the range of the values, indicates an inconsistent effect of the product (Fig. 1). Sixty percent of treated water samples were found to have TVCs of less than 200 CFU · ml−1, with a change of 0.69 log CFU · ml−1 between the median values of baseline and treated water samples (Table 2).
Longitudinal analysis of total viable counts during treatment.
A week-by-week comparison of products was performed to see if products differed in their patterns of disinfection, as it was hypothesized that some products might show a more rapid or sustained effect on TVC reduction than others (Fig. 2). Dentosept gave a marked and consistent reduction in median TVC values during the treatment period. The use of Alpron and, to a lesser extent, Oxygenal also caused a steady decline in median TVCs over time, whereas the other products did not have this effect (Fig. 2).
Dental units in which a product was tested for 6 weeks or more were included in a longitudinal analysis of the weekly TVCs to assess the presence of solitary high values in an otherwise low-value series. Samples treated with products in a series that yielded a median TVC of greater than 200 CFU · ml−1 were excluded from this analysis. A solitary high TVC value flanked by low values in the weeks before and after such an occurrence was found for 26 of 111 samples (23%) (Table 2). Of these DUWS, seven units (6%) showed a second high value during the same testing period. The analysis showed that this phenomenon occurred with all products tested. Alpron was the product least prone to incidental high values, with 26 out of 29 units showing no evidence of high solitary TVC values. BioBlue, Sanosil, and Oxygenal all suffered from these high values in about 20% of dental units. Single high TVCs were present in almost half of the units that used Sterilex and Dentosept and in three of the five units that used Ster4Spray (Table 2).
Biofilm.
Biofilm samples from 28 DUWS in two countries (Greece and the United Kingdom) were evaluated before and after disinfection with the products Alpron, BioBlue, and Sanosil. Comparison of baseline and posttreatment values showed a significant decrease in median TVCs (P < 0.01) for all products tested. Baseline median TVCs were 1.45 log CFU · cm−2 for Alpron and 1.04 log CFU · cm−2 for both Bioblue and Sanosil. All products reduced the median TVCs after the treatment period to levels below the detection limit, corresponding to a median percent reduction of >99% for all three products (data not shown).
Detection of pseudomonads.
Pseudomonads were detected in Germany only. Three units were positive before the product evaluation period, and two of those units tested positive during treatment as well. Counts ranged from 5 to 125 CFU · ml−1 (data not shown). All pseudomonads found were confirmed to be Pseudomonas aeruginosa.
Ease of use and adverse events.
A number of practical issues associated with the use of particular products were identified. These issues included dissolution of tubing and a lightweight nonpolypropylene plastic bottle on one unit (Sterilex Ultra), blocking of the tubing in the dental unit (Sterilex Ultra, three units; Ster4Spray, two units), and foaming and brownish discoloration of the dental unit water and staining of dental equipment surfaces in four units (Alpron). All Micrylium purge dispenser systems fitted to mains-fed DUWS that participated in this study had to be repaired at least once due to mechanical failures.
Assessment of overall efficacy of disinfectants.
A statistically significant difference in median TVC reduction (P < 0.01) was found between products used either continuously or intermittently (Table 1). The baseline median values for these two groups showed no significant difference, with 2.54 log CFU · ml−1 for continuously used products and 2.49 log CFU · ml−1 for intermittently used products. Treatment median values were 0.83 log CFU · ml−1 for continuously used products and 1.25 log CFU · ml−1 for intermittently used products.
There were significant differences between agents in terms of water TVCs (P value from Kruskal-Wallis-H). Further statistical tests showed that Dentosept was significantly more effective than all other agents (P = 0.0001). Oxygenal was significantly more effective than all agents except Dentosept (P = 0.001).
DISCUSSION
The biofilm on the inner surface of the tubing of dental units provides a continuous reservoir for microorganisms (6, 33, 41, 50-52). Not only patients but also dentists and dental personnel are at risk of being infected with opportunistic pathogens such as Pseudomonas or Legionella species by means of cross-infection or following aerosol formation from water emanating from DUWS (8, 27, 55).
Various products have now been developed to reduce the number of bacteria delivered to the patient by decontamination of the water used in DUWS (12, 13, 17, 18, 20, 31, 36, 38, 40, 49), and although these have been tested in a variety of settings, no multiproduct comparison in GDP surgeries has been done until now. In this trial, seven antimicrobial products approved for use in dental units were tested in seven EU countries for their ability to reduce TVCs to meet the ADA guideline of 200 CFU · ml−1 in the outflowing water (1) (Tables 1 and 2). Other criteria examined were the presence of occasional high TVC values in a series of low values, material incompatibility issues, the extent of the reduction of water TVCs during treatment compared to baseline TVCs in the same unit, and, where possible, the effect of disinfectants on biofilm TVCs.
Alpron.
Alpron has been tested in a number of studies. Smith et al. (39) found that Alpron reduced the TVC to a level similar to that in drinking water when used continuously over a period of 6 to 13 weeks. In the present study, during continuous use of Alpron, the bacterial counts of the outflowing water were reduced to below the ADA threshold of 200 CFU · ml−1 in 87% of samples. Biofilm samples also showed a significant reduction in TVCs when measured before disinfection and after 8 weeks of disinfection with Alpron. This agrees with a previous finding that Alpron completely removes the biofilm in dental tubing (48). Others have shown that Alpron is also effective in reducing TVCs to acceptable levels (i.e., <200 CFU · ml−1) when used intermittently (25).
BioBlue.
Kettering et al. (15) tested BioBlue (then named Bio2000) using a discontinuous regimen and concluded that it was not able to reduce the TVC to below 200 CFU · ml−1 when used in combination with tap water. Although BioBlue achieved levels of less than 200 CFU · ml−1 in 74% of samples in the present study, in some DUWS the measurements fluctuated, indicating an inability of the product to consistently maintain the TVC at below 200 CFU · ml−1 between disinfection periods. A factor that might explain these findings could be the presence of weakened biofilm remnants after overnight treatment that slough off during the day. In this study, BioBlue reduced biofilm TVCs by >99%, although in a previous study in a laboratory model, BioBlue achieved only a 53% reduction of the tubing's inner surface biofilm coverage (48).
Dentosept.
Overall, Dentosept was very effective in reducing TVCs and maintaining the microbial load to levels well below 200 CFU · ml−1 during this study, although occasional incidental high values were found. This product delivered the greatest reduction in TVCs, which resulted in the combined lowest number of samples which failed to meet the ADA guidelines during the treatment period. This is consistent with previous data which showed that daily application of Dentosept reduced TVCs in dental units to meet the ADA guideline (14).
Oxygenal.
Oxygenal has been evaluated previously in a laboratory DUWS biofilm model (48) and demonstrated a 99.2% reduction of biofilm coverage of dental tubing, while no TVC could be detected in the outflowing water after a single treatment. In the present study, Oxygenal achieved a statistically significant reduction in TVC, resulting in only a small percentage of samples that exceeded the ADA guidelines during the longitudinal study; the extent of the decrease was not very large, probably as a result of the low baseline values (Fig. 1).
Sanosil and Sterilex Ultra.
Tuttlebee et al. (43) compared Sanosil (5 to 8 weeks) and Sterilex Ultra (7 to 20 weeks) in GDPs using once-weekly disinfection of the DUWS, and both products reduced TVCs significantly to below the ADA guideline of ≤200 CFU · ml−1. Using microscopy imaging techniques, a significant reduction in biofilm coverage has also been observed (43, 48). Similar results were reported for both products in laboratory models of DUWS (22, 48). In the present study, Sanosil and Sterilex Ultra significantly reduced or maintained TVCs below ADA guidelines; Sanosil also had a significant effect on biofilm TVC, as described previously (48). Unfortunately, the application of Sterilex Ultra was stopped in three DUWS due to material incompatibility issues. The problem of blocking of DUWS tubing when using Sterilex Ultra has been described before (43) and may be caused by sedimentation of excess Sterilex Ultra that has not dissolved settling onto the tubing's narrow inner surface. In a previous study, Sterilex Ultra initially reduced bacterial counts to levels below the ADA guideline but failed to maintain low TVCs during an 8-week follow-up period (16). This finding concurs with the relatively high prevalence of incidental high values that was found in the present study (Table 2), which may have been caused by regrowth and detachment of the biofilm.
Ster4Spray.
Ster4Spray was tested in only five units and showed variable TVCs. The product seemed prone to incidental high values, but further research with more DUWS should be carried out in order to obtain more conclusive data. A previous study which tested six units reported that Ster4Spray reduced the TVC to below 200 CFU · ml−1 in 90% of samples collected over 10 days (23). However, the number of units tested was also small, and the testing period was too short to be able to conclusively assess the long-term efficacy of the product.
Low median baseline values and percent reduction.
The antimicrobial efficacy of a disinfectant should be assessed by both the reduction in TVC (log CFU · milliliter−1) and the mean percent reduction in absolute TVC (Table 2). This is because even a relatively small reduction in log CFU · ml−1 can correspond to a high percent reduction in absolute TVC when the initial (baseline) median TVCs are low. This effect was clearly visible in the Oxygenal data in Table 2, where a mean reduction in log CFU · milliliter−1 of 1.24 corresponds with an absolute TVC reduction of 97%. Median baseline TVC values in both countries where Oxygenal was tested (Ireland and The Netherlands) were lower than 200 CFU · ml−1, and in this case the percent reduction was a more accurate indicator of the efficacy of the product than the absolute reduction of medians in log CFU · milliliter−1.
Longitudinal analysis of TVCs.
The incidental high TVC values found in the longitudinal analysis of the data during disinfection may have been caused either by contamination of the water sample by biofilm detaching from the inner surface of the dental tubing or by a regrowth of bacteria due to a lack of compliance by dental staff in applying the disinfectant according to the manufacturer's instructions. Compliance has been shown to be an issue when nonautomated procedures are in place (53). Several chair manufacturers, e.g., KaVo and Siemens, now integrate an automated water disinfection system with electronic monitoring in their DUWS. This reduces the occurrence of problems that may be the result of accidental noncompliance. The occurrence of the aforementioned occasional high TVC values has not been described before and can go unnoticed if a unit is tested only periodically. Such levels of bacteria might pose a threat to dentists and patients. They also indicate that the water quality of units should be tested at several time points so that an accurate assessment of the numbers of microorganisms in the outflowing water can be made.
The nature of this multicountry, multiproduct study using dental units of various design did not make it possible to have equal numbers of identical units using each disinfectant. However, the longitudinal analysis gave insights into the extent and consistency of the antimicrobial effects of particular agents. Continuous use of Dentosept (and to some extent Alpron and Oxygenal) produced a marked and sustained reduction in water TVC (Fig. 2).
Ease of use and adverse events.
A number of practical issues associated with the use of particular products were reported by the dentists. Alpron required a 3-hour time commitment for the three-stage initial application phase, and in some practices, its use resulted in foaming, staining, and a brown discoloration of the water. Ster4Spray and Sterilex Ultra caused blocking of the tubing in some DUWS. However, material compatibility issues are often recognized by manufacturers, who may subsequently recommend specific products to be used with their equipment; for example, the manufacturers of Sterilex Ultra advise against the use of nonpolypropylene bottles. Therefore, dental practitioners must consult with the manufacturer of their DUWS prior to introducing any chemical agent, as this may otherwise invalidate their warranty. Other studies have raised additional concerns. Brass coupling connectors used in domestic water systems can be corroded by too harsh a chemical treatment, and this may lead to leaks and failure of the DUWS (44). Disinfectants could come into contact with the oral cavity, and this might affect the adhesion of resins to both enamel and dentin, so that restorations could fail prematurely (36, 42). The risk of chemical exposure of the patient and health care workers also has to be considered when using disinfectant products in DUWS (17).
In GDPs supplied by the mains water supply, independent water dispensers (Micrylium, Toronto, Canada) were retrofitted to the mains supply line in order for disinfectants to be added to the DUWS. All dispensers had to be repaired or replaced at some time during the study. Bottle-fed units needed no adaptation, and few problems were reported.
Evaluation of product efficacy.
In conclusion, this study, in which a number of disinfectants have been compared under realistic conditions of use in GDPs in several countries, has identified products that reduced the TVCs in DUWS to levels recommended by the ADA. When further statistical analyses were performed, in which all agents were compared with each other, the most effective products were Dentosept and Oxygenal. However, Dentosept gave the most consistent and sustained antimicrobial effect over time. Overall, the continuously applied products performed better than the intermittently applied products.
Acknowledgments
This investigation was supported by (i) the Primary Dental Care R&D Programme and National Research Register research grant PDC97-213 from the NHS Executive, North West, Warrington, United Kingdom, and (ii) the European Commission, specific RTD program “Quality of Life and Management of Living Resources 4.1 Environment and Health.”
We acknowledge the assistance of the disinfectant manufacturers for the supply of disinfectants and of the dental teams for their time and for allowing access to the DUWS.
No conflicts of interest are reported by any of the authors.
REFERENCES
- 1.Anonymous. 1996. ADA statement on dental unit waterlines. J. Am. Dent. Assoc. 127:185-186. [Google Scholar]
- 2.Anonymous. 1998. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Offic. J. Eur. Comm. L330:32-54. [Google Scholar]
- 3.Anonymous. 1994. The microbiology of water 1994. 1. Drinking water. Her Majesty's Stationery Office, London, United Kingdom.
- 4.Atlas, R. M., J. F. Williams, and M. K. Huntington. 1995. Legionella contamination of dental-unit waters. Appl. Environ. Microbiol. 61:1208-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagg, J., C. Sweeney, K. M. Roy, T. Sharp, and A. Smith. 2001. Cross infection control measures and the treatment of patients at risk of Creutzfeldt Jakob disease in UK general dental practice. Br. Dent. J. 191:87-90. [DOI] [PubMed] [Google Scholar]
- 6.Barbeau, J. 2000. Waterborne biofilms and dentistry: the changing face of infection control. J. Can. Dent. Assoc. 66:539-541. [PubMed] [Google Scholar]
- 7.Barbeau, J., C. Gauthier, and P. Payment. 1998. Biofilms, infectious agents, and dental unit waterlines: a review. Can. J. Microbiol. 44:1019-1028. [DOI] [PubMed] [Google Scholar]
- 8.Bennett, A. M., M. R. Fulford, J. T. Walker, D. J. Bradshaw, M. V. Martin, and P. D. Marsh. 2000. Microbial aerosols in general dental practice. Br. Dent. J. 189:664-667. [DOI] [PubMed] [Google Scholar]
- 9.Blake, G. C. 1963. Incidence and control of bacterial infection in dental spray reservoirs. Br. Dent. J. 115:413-416. [DOI] [PubMed] [Google Scholar]
- 10.Challacombe, S. J., and L. L. Fernandes. 1995. Detecting Legionella pneumophila in water systems: a comparison of various dental units. J. Am. Dent. Assoc. 126:603-608. [DOI] [PubMed] [Google Scholar]
- 11.Fotos, P., H. Westfall, I. Snyder, R. Miler, and B. Mutchler. 1985. Prevalence of legionella-specific IgG and IgM antibody in a dental clinic population. J. Dent. Res. 64:1382-1385. [DOI] [PubMed] [Google Scholar]
- 12.Karpay, R. I., T. J. Plamondon, and S. E. Mills. 1999. Comparison of methods to enumerate bacteria in dental unit water lines. Curr. Microbiol. 38:132-134. [DOI] [PubMed] [Google Scholar]
- 13.Karpay, R. I., T. J. Plamondon, S. E. Mills, and S. B. Dove. 1999. Combining periodic and continuous sodium hypochlorite treatment to control biofilms in dental unit water systems. J. Am. Dent. Assoc. 130:957-965. [DOI] [PubMed] [Google Scholar]
- 14.Kettering, J. D., C. A. Munoz-Viveros, J. A. Stephens, W. P. Naylor, and W. Zhang. 2002. Reducing bacterial counts in dental unit waterlines: distilled water vs. antimicrobial agents. J. Calif. Dent. Assoc. 30:735-741. [PubMed] [Google Scholar]
- 15.Kettering, J. D., J. A. Stephens, C. A. Munoz-Viveros, and W. P. Naylor. 2002. Reducing bacterial counts in dental unit waterlines: tap water versus distilled water. J. Contemp. Dent. Pract. 3:1-9. [PubMed] [Google Scholar]
- 16.Larsen, T., and N. E. Fiehn. 2003. The effect of Sterilex Ultra for disinfection of dental unit waterlines. Int. Dent. J. 53:249-254. [DOI] [PubMed] [Google Scholar]
- 17.Lee, T. K., E. J. Waked, L. E. Wolinsky, R. S. Mito, and R. E. Danielson. 2001. Controlling biofilm and microbial contamination in dental unit waterlines. J. Calif. Dent. Assoc. 29:679-684. [PubMed] [Google Scholar]
- 18.Linger, J. B., J. A. Molinari, W. C. Forbes, C. F. Farthing, and W. J. Winget. 2001. Evaluation of a hydrogen peroxide disinfectant for dental unit waterlines. J. Am. Dent. Assoc. 132:1287-1291. [DOI] [PubMed] [Google Scholar]
- 19.Martin, M. V. 1987. The significance of the bacterial contamination of dental unit water systems. Br. Dent. J. 163:152-154. [DOI] [PubMed] [Google Scholar]
- 20.Meiller, T. F., L. G. Depaola, J. I. Kelley, A. A. Baqui, B. F. Turng, and W. A. Falkler. 1999. Dental unit waterlines: biofilms, disinfection and recurrence. J. Am. Dent. Assoc. 130:65-72. [DOI] [PubMed] [Google Scholar]
- 21.Meiller, T. F., J. I. Kelley, A. A. Baqui, and L. G. DePaola. 2000. Disinfection of dental unit waterlines with an oral antiseptic. J. Clin. Dent. 11:11-15. [PubMed] [Google Scholar]
- 22.Meiller, T. F., J. I. Kelley, A. A. Baqui, and L. G. DePaola. 2001. Laboratory evaluation of anti-biofilm agents for use in dental unit waterlines. J. Clin. Dent. 12:97-103. [PubMed] [Google Scholar]
- 23.Montebugnoli, L., and G. Dolci. 2002. A new chemical formulation for control of dental unit water line contamination: an in vitro and clinical study. Br. Med. Coun. Oral Health. 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oppenheim, B. A., A. M. Sefton, O. N. Gill, J. E. Tyler, M. C. O'Mahony, J. M. Richards, P. J. Dennis, and T. G. Harrison. 1987. Widespread Legionella pneumophila contamination of dental stations in a dental school without apparent human infection. Epidemiol. Infect. 99:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozcan, M., Y. Kulak, and E. Kazazoglu. 2003. The effect of disinfectant agents in eliminating the contamination of dental unit water. J. Oral Rehabil. 30:290-294. [DOI] [PubMed] [Google Scholar]
- 26.Palleroni, N. J. 1981. Introduction to the family Pseudomonadaceae, p. 655-665. In M. Starr, H. Stolp, H. Truper, A. Balows, and H. Schlegel (ed.), The prokaryotes: a handbook on habitats, isolation and identification of bacteria. Springer-Verlag, New York, N.Y.
- 27.Pankhurst, C. L. 2003. Risk assessment of dental unit waterline contamination. Prim Dent. Care 10:5-10. [DOI] [PubMed] [Google Scholar]
- 28.Pankhurst, C. L., W. Coulter, J. J. Philpott-Howard, T. Harrison, F. Warburton, S. Platt, S. Surman, and S. Challacombe. 2003. Prevalence of legionella waterline contamination and Legionella pneumophila antibodies in general dental practitioners in London and rural Northern Ireland. Br. Dent. J. 195:591-594. [DOI] [PubMed] [Google Scholar]
- 29.Pankhurst, C. L., W. Coulter, J. N. Philpott-Howard, S. Surman-Lee, F. Warburton, and S. Challacombe. 2005. Evaluation of the potential risk of occupational asthma in dentists exposed to contaminated dental unit waterlines. Prim. Dent. Care 12:53-59. [DOI] [PubMed] [Google Scholar]
- 30.Pankhurst, C. L., N. W. Johnson, and R. G. Woods. 1998. Microbial contamination of dental unit waterlines: the scientific argument. Intern. Dent. J. 48:359-368. [DOI] [PubMed] [Google Scholar]
- 31.Pederson, E. D., M. E. Stone, J. C. Ragain, Jr., and J. W. Simecek. 2002. Waterline biofilm and the dental treatment facility: a review. Gen. Dent. 50:190-195. [PubMed] [Google Scholar]
- 32.Porter, S. R. 2002. Prions and dentistry. J. R. Soc. Med. 95:178-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putnins, E. E., D. Di Giovanni, and A. S. Bhullar. 2001. Dental unit waterline contamination and its possible implications during periodontal surgery. J. Periodontol. 72:393-400. [DOI] [PubMed] [Google Scholar]
- 34.Reinthaler, F., and F. Mascher. 1986. Demonstration of Legionella pneumophila in dental units. Zentralbl. Bakteriol. Mikrobiol. Hyg. B 183:86-88. [PubMed] [Google Scholar]
- 35.Reinthaler, F. F., F. Mascher, and D. Stunzner. 1988. Serological examinations for antibodies against Legionella species in dental personnel. J. Dent. Res. 67:942-943. [DOI] [PubMed] [Google Scholar]
- 36.Roberts, H. W., R. I. Karpay, and S. E. Mills. 2000. Dental unit waterline antimicrobial agents effect on dentin bond strength. J. Am. Dent. Assoc. 131:179-183. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd, P. A., M. A. Shojaei, P. D. Eleazer, A. Van Stewart, and R. H. Staat. 2001. Clearance of biofilms from dental unit waterlines through the use of hydroperoxide ion-phase transfer catalysts. Quintessence Int. 32:755-761. [PubMed] [Google Scholar]
- 38.Smith, A. J., J. Hood, J. Bagg, and F. T. Burke. 1999. Water, water everywhere but not a drop to drink? Br. Dent. J. 186:12-14. [DOI] [PubMed] [Google Scholar]
- 39.Smith, A. J., S. McHugh, I. Aitken, and J. Hood. 2002. Evaluation of the efficacy of Alpron disinfectant for dental unit water lines. Br. Dent. J. 193:593-596. [DOI] [PubMed] [Google Scholar]
- 40.Smith, A. J., S. McHugh, L. McCormick, R. Stansfield, A. McMillan, and J. Hood. 2002. A cross sectional study of water quality from dental unit water lines in dental practices in the West of Scotland. Br. Dent. J. 193:645-648. [DOI] [PubMed] [Google Scholar]
- 41.Tall, B. D., H. N. Williams, K. S. George, R. T. Gray, and M. Walch. 1995. Bacterial succession within a biofilm in water supply lines of dental air-water syringes. Can. J. Microbiol. 41:647-654. [DOI] [PubMed] [Google Scholar]
- 42.Taylor-Hardy, T. L., R. H. Leonard, Jr., S. M. Mauriello, and E. J. Swift, Jr. 2001. Effect of dental unit waterline biocides on enamel bond strengths. Gen. Dent. 49:421-425. [PubMed] [Google Scholar]
- 43.Tuttlebee, C. M., M. J. O'Donnell, C. T. Keane, R. J. Russell, D. J. Sullivan, F. Falkiner, and D. C. Coleman. 2002. Effective control of dental chair unit waterline biofilm and marked reduction of bacterial contamination of output water using two peroxide-based disinfectants. J. Hosp. Infect. 52:192-205. [DOI] [PubMed] [Google Scholar]
- 44.Wagner, D., W. Fischer, and H. H. Paradies. 1992. Copper deterioration in a water distribution system of a county hospital in Germany caused by microbial influenced corrosion 2. Simulation of the corrosion process in two test rigs installed in this hospital. Werkst. Korr. 43:496-502. [Google Scholar]
- 45.Walker, J. T., D. J. Bradshaw, A. M. Bennett, M. R. Fulford, M. V. Martin, and P. D. Marsh. 2000. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl. Environ. Microbiol. 66:3363-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker, J. T., D. J. Bradshaw, M. Finney, M. R. Fulford, E. Frandsen, O. S. E., J. M. Ten Cate, W. R. Moorer, A. J. Schel, A. Mavridou, J. J. Kamma, G. Mandilara, L. Stosser, S. Kneist, R. Araujo, N. Contreras, P. Goroncy-Bermes, D. O'Mullane, F. Burke, A. Forde, M. O'Sullivan, and P. D. Marsh. 2004. Microbiological evaluation of dental unit water systems in general dental practice in Europe. Eur. J. Oral Sci. 112:412-418. [DOI] [PubMed] [Google Scholar]
- 47.Walker, J. T., D. J. Bradshaw, M. R. Fulford, and P. D. Marsh. 2003. Control of biofilms and microbial contamination in dental unit water systems in general dental practice, p. 227-235. In D. Allison, A. McBain, M. Brading, A. Rickard, J. Verran, and J. T. Walker. (ed.), Biofilm communities—order from chaos. BioLine, Cardiff, United Kingdom.
- 48.Walker, J. T., D. J. Bradshaw, M. R. Fulford, and P. D. Marsh. 2003. Microbiological evaluation of a range of disinfectant products to control mixed-species biofilm contamination in a laboratory model of a dental unit water system. Appl. Environ. Microbiol. 69:3327-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker, J. T., D. J. Bradshaw, M. R. Fulford, M. V. Martin, and P. D. Marsh. 2001. Controlling mixed species biofilm contamination in dental unit water systems (DUWS) using a laboratory simulation model—a choice of products, p. 333-340. In P. Gilbert, D. Allison, M. Brading, J. Verran, and J. Walker (ed.), Biofilm community interactions: chance or necessity. BioLine, Cardiff, United Kingdom.
- 50.Whitehouse, R. L., E. Peters, J. Lizotte, and C. Lilge. 1991. Influence of biofilms on microbial contamination in dental unit water. J. Dent. 19:290-295. [DOI] [PubMed] [Google Scholar]
- 51.Williams, H. N., M. L. Baer, and J. I. Kelley. 1995. Contribution of biofilm bacteria to the contamination of the dental unit water supply. J. Am. Dent. Assoc. 126:1255-1260. [DOI] [PubMed] [Google Scholar]
- 52.Williams, H. N., A. Johnson, J. I. Kelley, M. L. Baer, T. S. King, B. Mitchell, and J. F. Hasler. 1995. Bacterial contamination of the water supply in newly installed dental units. Quintessence Int. 26:331-337. [PubMed] [Google Scholar]
- 53.Williams, H. N., J. Kelley, D. Folineo, G. C. Williams, C. L. Hawley, and J. Sibiski. 1994. Assessing microbial contamination in clean water dental units and compliance with disinfection protocol. J. Am. Dent. Assoc. 125:1205-1211. [DOI] [PubMed] [Google Scholar]
- 54.Williams, J. F., N. Andrews, and J. I. Santiago. 1996. Microbial contamination of dental unit waterlines: current preventive measures and emerging options. Compend. Cont. Ed. Dent. 17:691-694. [PubMed] [Google Scholar]
- 55.Williams, J. F., J. A. Molinari, and N. Andrews. 1996. Microbial contamination of dental unit waterlines: origins and characteristics. Compend. Cont. Ed. Dent. 17:538-540. [PubMed] [Google Scholar]
- 56.Zanetti, F., S. Stampi, G. De Luca, P. Fateh-Moghadam, M. Antonietta, B. Sabattini, and L. Checchi. 2000. Water characteristics associated with the occurrence of Legionella pneumophila in dental units. Eur. J. Oral Sci. 108:22-28. [DOI] [PubMed] [Google Scholar]