Abstract
We obtained 3,372 tentative unique transcripts (TUTs) from a cDNA library of Fusarium oxysporum. A cDNA array with 3,158 TUTs was produced to analyze gene expression profiles in conidial germination. It seems that ras and other signaling genes, e.g., ccg, cooperatively initiate conidial germination in Fusarium by increasing protein synthesis.
Fusarium oxysporum, one of the most important phytopathogens, is widely distributed in every type of soil worldwide. Some strains tend to penetrate the host roots directly without producing fully differentiated infection structures, such as the appressoria of Magnaporthe grisea (11). Unlike Fusarium graminearum (Gibberella zeae), it has no known sexual stage (7, 8). The molecular mechanisms of pathogenicity and symptom induction caused by F. oxysporum are poorly understood thus far (15). Some fungal species, such as F. oxysporum, reproduce asexually through the production of spores called conidia, which is the most common means of dispersion for the filamentous fungi. Environmental factors are required to trigger their germination (14).
For the study presented herein, we performed the first large-scale expressed sequence tag (EST) sequencing of F. oxysporum and used cDNA arrays to analyze gene expression profiles during the conidial germination process in order to elucidate this asexual filamentous fungus at the molecular level during fungal development.
Fungal culture conditions.
Strain ATCC 16416 is F. oxysporum f. sp. cucumerinum, and strain AFu68 is F. oxysporum f. sp. radicis-cucumerinum (18). To obtain conidia, the fungal strains were grown on potato dextrose agar plates at 27°C for 8 days, harvested by washing the surfaces of the plates with distilled water and filtering the water through four layers of filter paper, and then collected in the filtrate. For both strains, >90% of the collected conidia were microconidia and the others were macroconidia, as determined by visible microscopy (Nikon, Japan). For the induction of germ tubes, the harvested conidia were resuspended in germination water (0.5 g MgSO4 · 7H2O, 1 g NH4NO3, 1 g KH2PO4, and 15 g glucose in 1 liter of water) at a concentration of about 106 conidia per milliliter and shaken slowly at 27°C for 6 h. More than 80% of the conidia from both strains germinated synchronously into germ tubes. To obtain mycelia that were growing vigorously before conidiation, the germ tubes were kept in the germination water with gentle shaking at 27°C for 30 h. The total RNAs were extracted from all samples of both strains.
cDNA library construction and EST sequencing.
We constructed a cDNA library using mixed RNA samples from the three contiguous conidial development stages of strain ATCC 16416 and sequenced reconstructed plasmids from the 5′ ends of insert genes. After trimming off the vector and poor-quality DNA segments, we retained 6,448 ESTs with sequence reading lengths of >100 nucleotides (nt). Among these ESTs, the average reading length was 362 nt, while >43% were longer than 400 nt. After assembling the sequences, 2,551 singletons (only one EST in a cluster) and 821 contigs (two or more ESTs in a cluster) were defined as tentative unique transcripts (TUTs) (Table 1). Compared with the 11,640 predicted genes in F. graminearum and the 11,109 predicted genes in M. grisea (Broad Institute), the 3,372 TUTs in our library represent roughly one-fourth of the F. oxysporum genes. After performing BLASTx searches (E values, ≤1e−3), we found that 1,769 of these TUTs share homology with proteins in the UniProt databases. Of these, 924 TUTs have known protein functions. Twenty TUTs were the most abundantly expressed genes in our library because each of their clusters included >20 ESTs. Four of these were homologous to the ribosomal protein genes (S25, S26E, L39, and P2).
TABLE 1.
Summary statistics of ESTs obtained from Fusarium oxysporum cDNA libraryb
| Parameter | Value | % of total TUTs |
|---|---|---|
| Total no. of EST clones | 6,448 | |
| Avg EST length (nt) | 362 | |
| G + C content (%) | 50.8 | |
| Total no. of TUTs | 3,372 | 100 |
| Singletons (one EST) | 2,755 | 81.7 |
| Contigs (two or more ESTs) | 617 | 18.3 |
| TUTs exhibiting homology to encoded protein sequences (UniProt proteins of known function and hypothetical proteins)a | 1,769 | 52.46 |
| TUTs exhibiting homology to encoded protein sequences (UniProt proteins of known function)a | 924 | 27.40 |
The criterion for BLASTx was an E value of ≤1E−3.
All information about the F. oxysporum cDNA library, ESTs, and TUTs can be downloaded from www.estarray.org.
cDNA array detection of gene expression profiles during conidial germination.
A total of 3,158 PCR products from TUT clones were printed on Immobilon-Ny+ transfer membranes (Millipore, Bedford, MA). The RNA samples of two strains, ATCC 16416 and AFu68, in the conidium, germ tube, and mycelium stages were labeled with [33P]dCTP during first-strand reverse transcription (RT) reactions and then hybridized with the F. oxysporum cDNA arrays. The t test approach (2, 9) in the CyberT program was used to determine gene expression changes between samples. After the genes with significant differences (P < 0.01; change of twofold or more) were selected for each of the strains, only those with the same expression pattern trends in both of the strains were considered to be specially expressed genes of a certain developmental stage. Some of them which have been annotated are listed in Table 2, where they are categorized by class.
TABLE 2.
Differently expressed genes during conidial development of Fusarium oxysporum strains ATCC 16416 and AFu68a
| Clone no. and class | Mean fold change in global normalized array data
|
Annotationb | Uniprot no. | E value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Conidia
|
Germ tubes
|
Mycelia
|
|||||||
| 416 | 68 | 416 | 68 | 416 | 68 | ||||
| Class I: high expression in conidia | |||||||||
| FU022b10 | 4.94 | 4.93 | 2.43 | 1.90 | 1.23 | 1.95 | Phosphate permease PHO89 (Saccharomyces cerevisiae) | P38361 | 3E−17 |
| FU030f08 | 3.65 | 5.46 | 0.95 | 0.31 | 0.76 | 0.21 | Hypothetical 30.7-kDa protein in RVS161-ADP1 intergenic region (S. cerevisiae) | P25613 | 4E−35 |
| FU032e11 | 1.65 | 1.99 | 0.16 | 0.11 | 0.15 | 0.17 | Phosphate-repressible phosphate permease (Neurospora crassa) | P15710 | 2E−51 |
| FU042d01 | 2.14 | 1.17 | 0.44 | 0.31 | 0.28 | 0.26 | Similar to hypothetical 17.2-kDa protein in PCT1-ADE3 intergenic region (S. cerevisiae) | P42937 | 4E−07 |
| FU054d09 | 4.59 | 12.6 | 2.28 | 1.77 | 2.19 | 0.48 | Nitrite reductase (N. crassa) | P38681 | 1E−33 |
| FU098e01 | 1.84 | 0.75 | 0.68 | 0.17 | 0.62 | 0.29 | Similar to maleylacetate reductase (Burkholderia cepacia) | Q45072 | 0.001 |
| FU101d09 | 8.17 | 8.35 | 2.79 | 3.39 | 0.79 | 2.22 | P68-like protein (Schizosaccharomyces pombe) | P24782 | 8E−55 |
| FU101g06 | 5.39 | 4.67 | 1.90 | 0.72 | 0.65 | 0.56 | Glyxoxylate pathway regulator (Yarrowia lipolytica) | P41943 | 2E−17 |
| FU112e10 | 4.29 | 5.62 | 1.66 | 2.00 | 0.93 | 1.49 | Similar to hypothetical 25.3-kDa protein in TIM23-ARE2 intergenic region (S. cerevisiae) | P53721 | 7E−06 |
| Class II: low expression in conidia | |||||||||
| FU058f02 | 0.13 | 0.28 | 0.66 | 1.94 | 0.64 | 2.4 | ATP synthase beta chain, mitochondrial precursor (N. crassa) | P23704 | 1E−20 |
| FU067h12 | 0.71 | 0.17 | 1.58 | 1.42 | 1.85 | 1.63 | O-Acetylhomoserine (Emericella nidulans) | P50125 | 3E−14 |
| FU077a03 | 1.10 | 0.79 | 3.30 | 3.22 | 2.58 | 2.84 | Similar to clock-controlled protein 6 (N. crassa) | Q01302 | 3E−5 |
| Class III: high expression in germ tubes | |||||||||
| FU017d03 | 0.33 | 0.68 | 0.96 | 2.14 | 0.44 | 0.34 | 60S ribosomal protein L37 (E. nidulans) | Q9C0T1 | 2E−31 |
| FU037g10 | 1.07 | 0.76 | 2.65 | 3.04 | 0.82 | 1.22 | 40S ribosomal protein S29 (N. crassa) | Q9C2P2 | 4E−24 |
| FU044h11 | 1.26 | 1.35 | 2.9 | 6.89 | 0.64 | 2.05 | Probable 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase (S. pombe) | Q9UT19 | 1E−40 |
| FU048e10 | 0.34 | 0.45 | 1.1 | 1.14 | 0.25 | 0.41 | Similar to 60S ribosomal protein L36 (Trichoderma hamatum) | Q9HFR7 | 2E−08 |
| FU066a05 | 1.41 | 1.44 | 3.87 | 6.96 | 1.2 | 3.06 | Similar to hypothetical protein (N. crassa) | Q7S2C2 | 6E−06 |
| FU079a07 | 2.44 | 2.12 | 6.07 | 4.27 | 0.48 | 0.95 | 60S acidic ribosomal protein P2 (Fusarium culmorum) | Q8TFM9 | 3E−18 |
| FU082e01 | 0.7 | 0.49 | 1.94 | 1.53 | 0.41 | 0.46 | 60S ribosomal protein L44 (Pichia jadinii) | P52809 | 1E−52 |
| FU084a11 | 3.29 | 5.57 | 7.96 | 12.7 | 1.06 | 3.75 | 60S acidic ribosomal protein P2 (Alternaria alternata) | P42037 | 9E−23 |
| FU110d07 | 0.65 | 0.58 | 1.85 | 1.19 | 0.22 | 0.21 | 60S ribosomal protein L38 (N. crassa) | Q9C2B9 | 2E−14 |
| FU117f01 | 1.05 | 1.28 | 2.94 | 7.99 | 1.22 | 2.41 | Anucleate primary sterigmata protein A (E. nidulans) | Q00083 | 3E−21 |
| Class IV: low expression in germ tubes | |||||||||
| FU026c01 | 1.74 | 2.15 | 0.65 | 0.82 | 1.83 | 1.89 | Leptomycin B resistance protein Pmd1 (S. pombe) | P36619 | 2E−26 |
| FU090g08 | 1.26 | 3.02 | 0.2 | 0.12 | 0.59 | 0.26 | Similar to hypothetical 30.7-kDa protein in RVS161-ADP1 intergenic region (S. cerevisiae) | P25613 | 3E−09 |
| Class V: high expression in mycelia | |||||||||
| FU055h10 | 4.70 | 6.09 | 2.72 | 5.32 | 16.1 | 12.7 | Ras-related protein Rab-11B (Rattus norvegicus) | O35509 | 9E−16 |
| FU063c02 | 0.59 | 0.36 | 0.48 | 0.51 | 12.6 | 1.43 | Similar to protein FDD123 (Coriolus versicolor) | O74631 | 7E−04 |
| Class VI: low expression in mycelia | |||||||||
| FU002a03 | 4.81 | 2.89 | 4.26 | 0.82 | 0.87 | 0.37 | Similar to nitrate reductase (NADPH) (Fusarium oxysporum) | P39863 | 1E−08 |
| FU007c04 | 0.85 | 0.92 | 2.04 | 1.49 | 0.25 | 0.25 | 60S ribosomal protein L38 (N. crassa) | Q9C2B9 | 4E−27 |
| FU007e11 | 1.00 | 1.21 | 1.95 | 3.06 | 0.39 | 0.6 | 40S ribosomal protein S18 (S. cerevisiae) | P35271 | 8E−64 |
| FU009c09 | 1.45 | 0.79 | 1.38 | 0.78 | 0.50 | 0.33 | Similar to probable eukaryotic translation initiation factor 3 subunit 11 (Drosophila melanogaster) | Q9W2D9 | 2E−10 |
| FU011c11 | 3.52 | 15.8 | 4.66 | 7.69 | 0.61 | 1.55 | Flavohemoprotein (Bordotella pertussis) | Q7TTP0 | 5E−34 |
| FU013c02 | 2.70 | 2.13 | 1.69 | 2.22 | 0.53 | 1.04 | l-Lactate dehydrogenase A (Rhizopus oryzae) | Q9P4NB6 | 5E−30 |
| FU040f06 | 0.99 | 0.91 | 1.06 | 0.80 | 0.36 | 0.33 | Hypothetical UPF0327 protein NCU06495.1 (N. crassa) | Q7RYI0 | 2E−29 |
| FU040g03 | 3.26 | 2.25 | 1.98 | 1.40 | 0.8 | 0.7 | Similar to arsenical resistance protein ACR3 (S. cerevisiae) | Q06598 | 1E−06 |
| FU045g01 | 3.29 | 3.01 | 2.37 | 1.77 | 0.62 | 0.73 | 1-Phosphatidylinositol-4,5-bisphosphate phosphodiesterase 1 (Candida albicans) | O13433 | 2E−16 |
| FU055a07 | 2.78 | 6.38 | 2.25 | 2.11 | 0.77 | 0.16 | Flavohemoprotein (Pseudomonas derugtnosa) | Q9I0H4 | 2E−14 |
| FU055e09 | 5.48 | 28.8 | 6.80 | 5.90 | 2.55 | 2.93 | Nitrite reductase (Leptosphaeria maculans) | P43504 | 2E−31 |
| FU071b09 | 0.82 | 0.89 | 0.79 | 2.93 | 0.21 | 0.43 | 60S ribosomal protein L18-B (S. pombe) | Q8TFH1 | 4E−50 |
| FU073c12 | 0.38 | 0.43 | 0.83 | 0.53 | 0.17 | 0.07 | 40S ribosomal protein S24 (Rhizomucor racemosus) | P14249 | 2E−16 |
| FU079a05 | 2.92 | 3.19 | 5.34 | 2.12 | 0.68 | 0.72 | Ubiquitin (S. cerevisiae) | P61864 | 1E−36 |
| FU079a07 | 2.44 | 2.12 | 6.07 | 4.27 | 0.48 | 0.95 | 60S acidic ribosomal protein P2 (F. culmorum) | Q8TFM9 | 3E−18 |
| FU083h08 | 1.54 | 2.07 | 1.36 | 0.88 | 0.44 | 0.43 | Clathrin coat assembly protein AP17 (R. norvegicus) | P62744 | 3E−28 |
| FU085f01 | 1.72 | 1.21 | 3.02 | 1.07 | 0.22 | 0.13 | 60S ribosomal protein L33-A (S. pombe) | Q9USX4 | 2E−38 |
| FU088a11 | 2.30 | 1.16 | 4.59 | 1.56 | 0.55 | 0.35 | 60S acidic ribosomal protein P1 (A. alternata) | P49148 | 8E−28 |
| FU089a10 | 0.94 | 0.92 | 0.97 | 0.75 | 0.18 | 0.25 | 60S ribosomal protein L5 (N. crassa) | O59953 | 1E−110 |
| FU097c03 | 0.77 | 0.82 | 1.04 | 1.07 | 0.36 | 0.18 | 60S ribosomal protein L14-A (S. cerevisiae) | P36105 | 5E−13 |
| FU100e11 | 0.85 | 0.58 | 2.01 | 1.00 | 0.24 | 0.18 | 60S ribosomal protein L29 (S. cerevisiae) | P05747 | 7E−14 |
| FU107b04 | 1.15 | 1.37 | 1.49 | 0.65 | 0.33 | 0.22 | NHP2/L7aE family protein YEL026W homolog (Caenorhabditis elegans) | Q21568 | 2E−30 |
| FU107d04 | 1.33 | 1.11 | 2.37 | 0.93 | 0.58 | 0.36 | 40S ribosomal protein S28 (N. crassa) | Q7S6W5 | 1E−22 |
The differentially expressed genes were calculated by the program CyberT (http://visitor/ics.uci.edu/genex/cybert/), and the thresholds were P values (lnp) of <0.01 and changes of more than twofold.
The annotation “similar to” means that the E value was from 10−3 to 10−10, and annotations without these words means that the E value was <10−10.
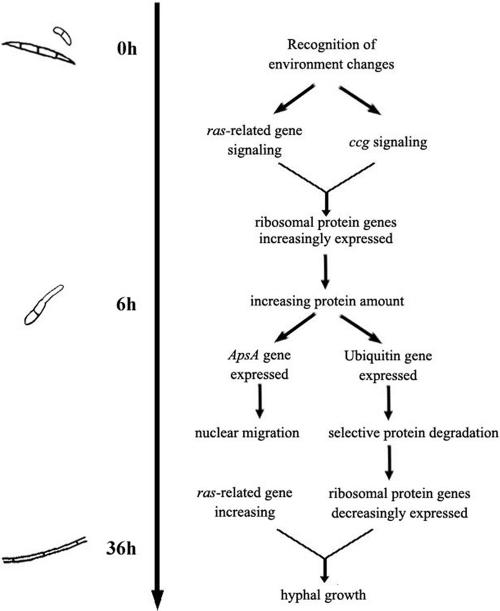
Through the EST and comparative cDNA array analyses, we found that ribosomal protein genes were highly redundant in our library and that some of them fluctuated during development. These genes included the 60S acidic ribosomal protein P2 gene (Table 2, class III) (FU084a11), which was expressed at greater levels in germ tubes than during the other two stages. Many studies have shown that protein synthesis becomes highly active during early Aspergillus nidulans and Neurospora crassa conidial germination (5, 13). In our study, the ribosomal protein genes were highly expressed in the germ tubes, and many genes were inactive in the mycelia. This finding indicated that germinating conidia must synthesize a large number of proteins to maintain their rapid growth but require fewer proteins for mycelial growth after germination. This conclusion also corresponds with the expression trend of the ubiquitin gene (FU079a07), which plays an essential role in intracellular protein turnover (12).
The clock-controlled gene (ccg) reportedly plays a role in fungal conidial development (4). Inactivation of this gene results in altered conidiophore morphology and abolishes the normal circadian rhythm of asexual macroconidial development of N. crassa (16). In this study, a gene similar to ccg6 (FU077a03) was expressed at higher levels in the germ tube and mycelium than in the conidium. This suggested that ccg could play dual regulatory roles in the circadian clock and during fungal spore development.
The normalized signal values for the ras-related gene encoding the protein Rab-11B (FU055h10) in our cDNA arrays were 4.70, 2.72, and 16.1, respectively, for the conidium, germ tube, and mycelium stages for strain ATCC 16416 and 6.09, 5.32, and 12.7, respectively, for strain AFu68. These data were far above the average expression level of all genes in the cDNA array (because of global normalization, the mean value of each cDNA array was 1). This shows that the ras-related gene is abundantly expressed during the entire conidial development process, but interestingly, it was most active in the mycelium. In eukaryotes, from yeast to humans, the ras gene has been implicated in transducing growth and differentiation signals (1). However, whether ras is necessary for spore germination is still arguable. An activated mutant form of ras induced conidial germination of A. nidulans in the absence of a carbon source (13), whereas deletion of the ras homologue smco7 from N. crassa did not inhibit germination, although the hyphal growth rate of the mutants was about 1/10 that of wild-type cells (10). Through the results of our arrays, it can be deduced that the main function of the ras-related gene is to regulate hyphal growth, while this and other signal genes, such as ccg, can cooperatively initiate conidial germination by increasing protein synthesis.
Anucleate primary sterigmata protein A (ApsA) regulates nuclear migration during hyphal growth (17). The ApsA mutant form of A. nidulans almost completely blocks entry of the nuclei into primary buds during conidiophore development, which results in developmental arrest (6). Herein, we found that the ApsA gene (FU026c01) was highly expressed in the germ tubes of F. oxysporum, indicating that ApsA is expressed abundantly before rapid vegetative growth of the germ tube. The relationships of the genes discussed above to conidial development are illustrated in Fig. 1.
FIG. 1.
Sketch map of conidial development of F. oxysporum.
The other genes listed in Table 2, including those for which there are no annotations, were initially regarded as development-related genes whose relationships to the conidial development of F. oxysporum require additional studies.
Beckman and Roberts first described the time course of the major events during F. oxysporum pathogenesis (3). The overall development process is outlined clearly, but details of the major events, especially interactions with the host plant in vivo, remain unfocused. Since F. oxysporum does not produce fully differentiated infection structures upon infection, interpreting in vitro gene expression data must be conducted with caution, given that there is no known link with the in vivo situation. Nevertheless, the differences in gene expression patterns during fungal development do provide a framework for developing hypothesis-driven experiments to investigate specific aspects of host-pathogen interactions.
Confirmation of cDNA array results by quantitative real-time RT-PCR.
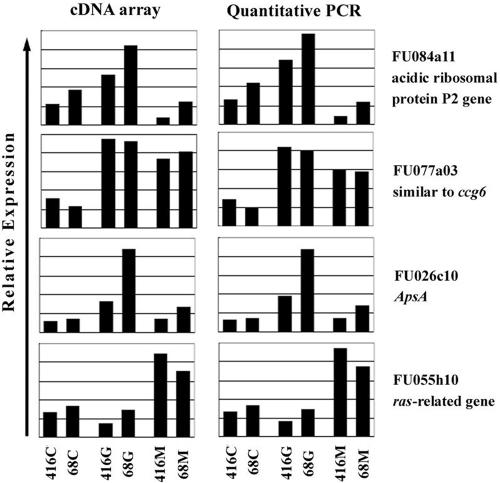
Four TUTs (FU084a11, FU077a03, FU026c10, and FU055h10) which were specifically expressed during certain developmental stages, as revealed by cDNA arrays, were utilized to perform quantitative real-time RT-PCR experiments in order to validate the array results. In general, the relative mRNA levels determined by quantitative real-time RT-PCR were in accordance with those obtained by cDNA arrays (Fig. 2).
FIG. 2.
Validation of array results by quantitative real-time RT-PCR. 416C, conidium of ATCC 16416; 68C, conidium of AFu68; 416G, germ tube of ATCC 16416; 68G, germ tube of AFu68; 416M, mycelium of ATCC 16416; 68M, mycelium of AFu68.
All the details of methods, EST sequences, and original data for cDNA arrays can be found at www.estarray.org.
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (grant no. 2002BA711A15) and the Sino-Greek Science and Technology Cooperative Project [grant no. 2003 (63)].
We thank Kangle Zheng (China National Rice Research Institute) and Baoshan Chen (Guangxi University of China) for critical readings of the manuscript and for many helpful suggestions.
REFERENCES
- 1.Adams, T. H., J. K. Wieser, and J. H. Yu. 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldi, P., and G. W. Hatfield. 2002. DNA microarrays and gene expression—from experiments to data analysis and modeling. Cambridge University Press, Cambridge, United Kingdom.
- 3.Beckman, C. H., and E. M. Roberts. 1995. On the nature and genetic basis for resistance and tolerance to fungal wilt diseases of plants. Adv. Botan. Res. 21:36-77. [Google Scholar]
- 4.Bell-Pedersen, D., J. C. Dunlap, and J. J. Loros. 1992. The Neurospora circadian clock-controlled gene, ccg-2, is allelic to eas and encodes a fungal hydrophobin required for formation of the conidial rodlet layer. Genes Dev. 6:2382-2394. [DOI] [PubMed] [Google Scholar]
- 5.D'Enfert, C. 1997. Fungal spore germination: insights from the molecular genetics of A. nidulans and N. crassa. Fungal Genet. Biol. 21:163-172. [Google Scholar]
- 6.Fischer, R., and W. E. Timberlake. 1995. Aspergillus nidulans apsA (anucleate primary sterigmata) encodes a coiled-coil protein required for nuclear positioning and completion of asexual development. J. Cell Biol. 128:485-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fravel, D., C. Olivain, and C. Alabouvette. 2003. Fusarium oxysporum and its biocontrol. New Phytologist 157:493. [DOI] [PubMed] [Google Scholar]
- 8.Garrett, S. D. 1970. Pathogenic root-infection fungi. Cambridge University Press, London, United Kingdom.
- 9.Hatfield, G. W., S. P. Hung, and P. Baldi. 2003. Differential analysis of DNA microarray gene expression data. Mol. Microbiol. 47:871-877. [DOI] [PubMed] [Google Scholar]
- 10.Kana-uchi, A., C. T. Yamashiro, S. Tanabe, and T. Murayama. 1997. A ras homologue of Neurospora crassa regulates morphology. Mol. Gen. Genet. 254:427-432. [DOI] [PubMed] [Google Scholar]
- 11.Mendgen, K., M. Hahn, and H. Deising. 1996. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 34:367-386. [DOI] [PubMed] [Google Scholar]
- 12.Noventa-Jordao, M. A., A. M. do Nascimento, M. H. Goldman, H. F. Terenzi, and G. H. Goldman. 2000. Molecular characterization of ubiquitin genes from Aspergillus nidulans: mRNA expression on different stress and growth conditions. Biochim. Biophys. Acta 1490:237-244. [DOI] [PubMed] [Google Scholar]
- 13.Osherov, N., and G. May. 2000. Conidial germination in Aspergillus nidulans requires RAS signaling and protein synthesis. Genetics 155:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osherov, N., and G. S. May. 2001. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 199:153-160. [DOI] [PubMed] [Google Scholar]
- 15.Recorbet, G., C. Steinberg, C. Olivain, V. Edel, S. S. G. Trouvelot, E. Dumas-Gaudot, S. Gianinazzi, and C. Alabouvette. 2003. Wanted: pathogenesis-related marker molecules for Fusarium oxysporum. New Phytologist 159:73. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara, M. L., A. Correa, D. Bell-Pedersen, J. C. Dunlap, and J. J. Loros. 2002. Neurospora clock-controlled gene 9 (ccg-9) encodes trehalose synthase: circadian regulation of stress responses and development. Eukaryot. Cell 1:33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suelmann, R., N. Sievers, and R. Fischer. 1997. Nuclear traffic in fungal hyphae: in vivo study of nuclear migration and positioning in Aspergillus nidulans. Mol. Microbiol. 25:757-769. [DOI] [PubMed] [Google Scholar]
- 18.Vakalounakis, D. J., and G. A. Fragkiadakis. 1999. Genetic diversity of Fusarium oxysporum isolates from cucumber: differentiation by pathogenicity, vegetative compatibility, and PAPD fingerprinting. Phytopathology 89:161-168. [DOI] [PubMed] [Google Scholar]