Abstract
Magnaporthe grisea, a destructive ascomycetous pathogen of rice, secretes cell wall-degrading enzymes into a culture medium containing purified rice cell walls as the sole carbon source. From M. grisea grown under the culture conditions described here, we have identified an expressed sequenced tag, XYL-6, a gene that is also expressed in M. grisea-infected rice leaves 24 h postinoculation with conidia. This gene encodes a protein about 65% similar to endo-β-1,4-d-glycanases within glycoside hydrolase family GH10. A M. grisea knockout mutant for XYL-6 was created, and it was shown to be as virulent as the parent strain in infecting the rice host. The proteins secreted by the parent strain and by the xyl-6Δ mutant were each fractionated by liquid chromatography, and the collected fractions were assayed for endo-β-1,4-d-glucanase or endo-β-1,4-d-xylanase activities. Two protein-containing peaks with endo-β-1,4-d-xylanase activity secreted by the parent strain are not detectable in the column eluant of the proteins secreted by the mutant. The two endoxylanases (XYL-6α and XYL-6β) from the parent were each purified to homogeneity. N-terminal amino acid sequencing indicated that XYL-6α is a fragment of XYL-6β and that XYL-6β is identical to the deduced protein sequence encoded by the XYL-6 gene. Finally, XYL-6 was introduced into Pichia pastoris for heterologous expression, which resulted in the purification of a fusion protein, XYL-6H, from the Pichia pastoris culture filtrate. XYL-6H is active in cleaving arabinoxylan. These experiments unequivocally established that the XYL-6 gene encodes a secreted endo-β-1,4-d-xylanase.
Magnaporthe grisea (Hebert) Barr (anamorph, Pyricularia oryzae Cav. or Pyricularia grisea) is the causal agent of the devastating rice blast disease (31). M. grisea primarily infects rice but also infects other Poaceae such as wheat and barley. There have been extensive studies of the cellular and molecular basis underlying M. grisea-plant interactions, particularly of the signal transduction pathways leading to early events of fungal infection (for a review, see reference 31). The recent publication of the M. grisea genome sequence is a cornerstone upon which the molecular interactions between a major crop killer and its plant hosts may be elucidated (9). Also, the ongoing accumulation and increased accessibility of large numbers of expressed sequence tags (ESTs) is greatly expediting the identification of individual proteins and the elucidation of gene expression profiles acquired under various environmental and culture conditions (14, 22, 23). We now describe the identification of an endoglycanase gene from a pool of ESTs of M. grisea grown on rice cell walls (RCWs) as its carbon source.
The primary walls of plant cells (6) are pivotal battlegrounds between microbial pathogens and their plant hosts (8, 13, 19, 32-34, 38). Microbial pathogens secrete an array of cell wall-degrading enzymes (CWDEs) capable of depolymerizing the noncellulosic polysaccharides of primary cell walls (2, 10, 11, 18, 19, 37). For example, the recently published M. grisea genome sequence unveiled the possible presence of as many as 20 xylanase genes, which encode six glycoside hydrolase family 10 (GH10), five GH11, and nine GH43 members (reference 9 and unpublished genome-mining data of this laboratory). This high level of redundancy is an indication that xylanase activity is essential for the vitality of M. grisea, either saprophytically or pathogenetically or both.
We previously described the purification, cloning, and gene knockout analyses of two endo-β-1,4-d-xylanases (EC 3.2.1.8) secreted by M. grisea (35, 37). We also provided evidence of the presence of at least three other xylanases encoded by M. grisea (37). PCR using degenerated oligonucleotide primers also allowed amplification and cloning of three putative xylanase genes, XYL-3, XYL-4, and XYL-5 (GenBank accession numbers AY144348 to 144350) (manuscript in preparation). We now show that one of the M. grisea ESTs encodes a hitherto-undiscovered family GH10 endo-β-1,4-d-xylanase that also possesses a class III (fungal) carbohydrate-binding domain (fCBD) (13, 20, 27).
MATERIALS AND METHODS
Fungal strain and host plant.
The protocols for growth, maintenance, and infection assays of both the rice BLAST fungus, M. grisea (Herbert) Barr strain CP987, and its host plant, Oryza sativa variety Sariceltik, were the same as previously described (37).
Construction of a cDNA library.
M. grisea (strain CP987) was grown in a basic medium containing purified rice cell walls as the sole carbon source, as described by Wu et al. (35). Fungal mycelia were harvested after 5 days of culture and used to extract a total RNA sample as previously described (35, 37). Polyadenylated mRNA species were purified using the Oligotex mRNA kit from QIAGEN, Inc. (Valencia, CA) according to the manufacturer's instructions. A cDNA library was constructed from 2 μg of the fungal mRNA using the λ bacteriophage vector Uni-Zap XR according to the manufacturer's manual (Stratagene Corp., La Jolla, CA). The packaged λ phage was amplified once on agar medium plates and stored at −80°C in 7% dimethyl sulfoxide as described in the manual.
Phagemids containing the cloned M. grisea cDNA were excised from randomly selected λ phages in the cDNA library as described in the manufacturer's manual (http://www.stratagene.com/manuals/211204.pdf). The M. grisea cDNA species cloned in the phagemids were subjected to nucleotide sequencing using the T3 and/or T7 primers (see the Uni-Zap XR manual) by the University of Georgia Integrated Biotechnology Laboratories. DNA sequence data were assembled using the software programs in the Wisconsin Package (Genetics Computer Group, Madison, WI) and deposited in GenBank.
Generation of a XYL-6 knockout mutant.
The strategy for creating a XYL-6 knockout mutant was the same as previously described (37). The XYL-6 gene was isolated by screening a M. grisea genomic library using EST RCW105 as a probe (see Table 2). A 5-kb Hind III/XhoI DNA fragment containing XYL-6 was subcloned from the genomic clone to the phagemid pBluescript II SK(+) (Stratagene). The resulting plasmid (pX6) was digested with restriction enzyme SphI to remove a 2-kb DNA fragment that encodes the entire transcript of XYL-6. This SphI-cleaved plasmid was then ligated in the presence of an oligonucleotide gap-filler, pTCGACATG (Oligo103 in Table 1), to a 3-kb SalI fragment encoding a mutated M. grisea acetolactate synthase gene (30). This mutated acetolactate synthase gene was named Sur for its ability to confer sulfonylurea resistance (plasmid pCB1637 that carries Sur was a generous gift from Jim Sweigard of The DuPont Company, Wilmington, DE). The resulting knockout vector, pX6Sur, was transformed into protoplasts of the M. grisea strain CP987 as described by Wu et al. (37). Sulfonylurea-resistant M. grisea transformants were screened by PCR for knockout mutants using oligonucleotide primers Oligo116 and Oligo117 (Table 1). These two primers are located immediately upstream and downstream, respectively, of the 2.0-kb XYL-6 gene being deleted. Therefore, PCR on DNA samples of the M. grisea transformants using Oligo116 and Oligo117 will amplify both the 2.0-kb XYL-6 gene and the 3.0-kb Sur gene if construct pX6Sur were integrated into the M. grisea chromosome via nonhomologous recombination (resulting in “ectopic” transformants). If the integration of pX6Sur into the chromosome occurs via homologous recombination that results in a “knockout” mutant, Oligo116 and Oligo117 will only amplify the 3.0-kb Sur gene. One xyl-6ΔSur mutant, designated strain X7601, was isolated out of a total of 73 transformants. Southern blot analyses were then performed using the 2-kb XYL-6 and the 3-kb Sur gene as probes to confirm the gene replacement as described previously (37).
TABLE 2.
Putative functions of M. grisea ESTs
| Type and sequence | Accession no. | Putative functiona | Matchb |
|---|---|---|---|
| Cell wall-degrading enzymes and sugar transportation | |||
| RCW5 | AA415060 | α-l-Arabinofuranosidase (Afs1) | AL021411 |
| RCW20 [2]c | AA415086 | β-Glucosidase (EC 3.2.1.21) | S52771 |
| RCW22 | L37530 | endo-β-1,4-Xylanase (EC 3.2.1.8) (XYL-2) | L37530 |
| RCW105d | AA415141 | Group 10 glycohydrolase (XYL-6) | P46239 |
| RCW109 | L81126 | endo-β-1,4-Xylanase (EC 3.2.1.8) (XYL-3) | L81126 |
| RCW18 | AA415084 | Sugar transporter protein (STP1) | P18631 |
| RCW69 | AA415111 | Sugar transporter protein (STP2) | P108708 |
| RCW79 | AA415117 | Sugar transporter protein (STP3) | U64903 |
| Filamentous fungus-specific extracellular proteins | |||
| RCW8 [4] | AA415063 | Unknown | BI190440 |
| RCW58 | AA415101 | Phytotoxin | AL513410 |
| RCW59 | AA415102 | Unknown | BI189031 |
| Development | |||
| RCW100 | AA415137 | Sporulation-specific protein SPS2 (Ces1) | S54039 |
| RCW108 | AA415143 | Yeast meiosis-specific SPO14 (phospholipase D) | P36126 |
| Metabolism | |||
| RCW6 | AA415061 | NADP-dependent mannitol dehydrogenase | AF387300 |
| RCW7 | AA415062 | tRNA-guanine transglycosylase (EC 2.4.2.29) | P44594 |
| RCW11 | AA415064 | Eukaryotic initiation factor 4A | Z74132 |
| RCW19 [8] | AA415085 | Ergosterol synthase (C-4 sterol methyl oxidase) | U31885 |
| RCW26 | AA415091 | Triacylglycerol lipase (EC 3.1.1.3) | S49236 |
| RCW35 | AA415071 | Ubiquinol-cytochrome c reductase | P07056 |
| RCW37 | AA415072 | Stearoyl-CoA desaturase (EC 1.14.99.5) | S52745 |
| RCW50 | AA415081 | 26S protease regulator subunit 8 (factor SUGI) | S24016 |
| RCW55 | AA415099 | Nuclear control of ATPase mRNA expression (NCA3) | D63817 |
| RCW65 [6] | AA415108 | NAD-dependent formate dehydrogenase (EC 1.2.1.2) | Q07103 |
| RCW88 | AA415125 | Aspartate aminotransferase (EC 2.6.1.1) | P23542 |
| RCW91 | AA415128 | Multicatalytic endopeptidase complex, subunit PRE2 | S43739 |
| RCW92 | AA415129 | d-2-Hydroxy acid dehydrogenase (EC 1.1.99.6) | P30799 |
| RCW112 | AA415145 | Mitochondial +H transporting ATP synthase (EC 3.6.1.34) | S28794 |
| Housekeeping | |||
| RCW16 | AA415082 | 30-kDa heat shock protein 1 | P19752 |
| RCW25 | AA415090 | Clathrin assembly protein | U44890 |
| RCW110 | AA415144 | Flavohemoprotein | P39662 |
| RCW94 | AA415131 | 60S ribosomal protein LI 6 | Q10157 |
| RCW96 | AA415133 | Frequency clock protein | S44457 |
| RCW101 | AA415138 | Histone H3 | P07041 |
| RCW60 | AA415103 | Cross-pathway control protein 1 | A30208 |
| RCW68 | AA415110 | 60S ribosomal protein LI 7 | P04451 |
| RCW82 | AA415120 | 30-kDa heat shock protein 2 | P40920 |
| RCW83 [2] | AA415121 | Ubiquitin precursor | X13140 |
| Hypothetical | |||
| RCW12 [2] | AA415065 | Schizosaccharomyces pombe 35.9-kDa hypothetical protein | Q10256 |
| RCW23 | AA415088 | Probable membrane protein (Neurospora crassa) | BF072630 |
| RCW49 | AA415073 | Erysiphe graminis gEghL-6 clone homolog | L40637 |
| RCW54 | AA415098 | Emericella nidulans hypothetical protein homolog | M59935 |
| RCW63 | AA415106 | Schizosaccharomyces pombe hypothetical 8.2-kDa protein | Z69240 |
| RCW102 | AA415139 | Yeast hypothetical protein (AMDI 5′ region) | S49741 |
BLAST similarity search against GenBank databases (1). EC numbers and encoding genes are listed in parentheses.
Accession number of a matched entry with the highest probability or smallest E value (1).
Numbers in brackets indicate the number of redundancies in the EST pool.
Subject of this paper; its corresponding genomic sequence, XYL-6, has been deposited in GenBank (AY124591).
TABLE 1.
Oligonucleotide primersa
| Primer | Nucleotide sequence | Expt |
|---|---|---|
| Oligo62 | CACACTCTGATCTGGCACAGTC | XYL-2 RT-PCR |
| Oligo65 | GTAGTTGCTGTCGAACAACAGA | XYL-2 RT-PCR |
| Oligo100 | CTCATGCGCGGTAGCTTCAAGTCG | Ces1 RT-PCR |
| Oligo167 | CACAAATTTACACCGTCGCGGAC | Ces1 RT-PCR |
| Oligo102 | GCATGAGCTTGCCGTTGGCCGTG | XYL-6 RT-PCR |
| Oligo168 | GGTGCCAAGACTTGCGTCTCTGG | XYL-6 RT-PCR |
| Oligo169 | CTGAACGTTGATGTTGCTGTTTCC | XYL-1 RT-PCR |
| Oligo170 | CACGTACTCGGGCACCTTTAACC | XYL-1 RT-PCR |
| Oligo103 | pTCGACATGb | pX6Sur construction |
| Oligo116 | CACCCAACCTATCACACACC | Mutant screening |
| Oligo117 | CGATTGACGAGTTGTATTGCC | Mutant screening |
| Oligo350 | pCTAGGGAACAAAAACTCATCTCAGAAGAGGATCTGAATAGCGCCG | pPicH construction |
| Oligo351 | pGGCCGCTACTTACTCAATGATGATGATGATGATGGTCGACGGCGC | pPicH construction |
| Oligo352 | pTCGACCATCATCATCATCATCATTGAGTAAGTAGC | pPicH construction |
| Oligo227 | TTTACGTAACCACCATGCGTACTCCCGC | pHX6 cloning |
| Oligo359 | TTTCCTAGGCAAAGCGTTCATGATGGCGGTG | pHX6 cloning |
All primers were synthesized by the Integrated Biotechnology Laboratories of the University of Georgia.
The italic lowercase p preceding the nucleotide sequence represents a 5′ phosphorylation.
Enzyme purification and activity assay.
The extracellular proteins present in the culture media of the parent M. grisea strain (CP987) and the mutant strain (X7601) were fractionated as described by Wu et al. (37), except that the Mono-S column (Pharmacia Biotech, Piscataway, NJ) was replaced by a 1-ml HiTrap SP column (Pharmacia Biotech). The final purification step utilized hydrophobic interaction chromatography to purify the target endo-β-1,4-xylanases to apparent homogeneity. The enzyme-containing fractions that were collected following the HiTrap SP chromatography were diluted with 1 volume of a 2× ammonium sulfate buffer (3.4 M ammonium sulfate in 50 mM sodium acetate, pH 5.0). The sample (2 ml) was injected onto a Phenyl-Superose HR 10/10 column (Pharmacia Biotech) that was irrigated with buffer A (1.7 M ammonium sulfate in 50 mM sodium acetate, pH 5.0) until UV absorption was reduced to that of the baseline. Column-bound proteins were eluted with a linear gradient from 0 to 50% of buffer B (50 mM sodium acetate, pH 5.0) in 100 min with a flow rate of 0.5 ml/min.
An aliquot (5 μl) of each fraction (1 ml from the HiTrap SP column and 0.5 ml from the Phenyl-Superose column) was assayed for endo-β-1,4-xylanase as described by Wu et al. (37), except that the assay was conducted with 100 mM 2-(N-morpholino)-ethane sulfonic acid buffer at pH 6.0, the optimum pH for XYL-6 (data not shown). endo-β-1,4-Glucanase activity was determined by the PAHPAH method (35), which measures the reducing sugar formed from the carboxymethyl cellulose substrate (Sigma). The assay buffer for the endo-β-1,4-glucanase was 50 mM sodium acetate at pH 5.0.
Construction of a Pichia expression vector.
Plasmid pPicH was constructed by ligation of oligonucleotide primers Oligo350, Oligo351, and Oligo352 (Table 1) with Avr II- and NotI-cleaved pPIC3.5k vector (Multi-Copy Pichia Expression Vector, catalogue no. K1750-01; Invitrogen Corp., Carlsbad, CA). After transformation of the ligated product into Escherichia coli, plasmids harboring the correctly inserted DNA sequences were screened by SalI digestion (Oligo351 includes a SalI restriction site that is not present in vector pPIC3.5k) and confirmed by DNA sequencing. The cloning resulted in a 74-bp insertion encoding a c-myc epitope and a His6 tag. Therefore, a protein expressed in Pichia pastoris (7) using pPicH is a fusion protein with the c-myc epitope and the His6 tag at its C terminus (16, 25).
Heterologous expression of XYL-6.
Expression of XYL-6 in Pichia pastoris was performed according to the Invitrogen manual Multi-Copy Pichia Expression (7). A 1.2-kb cDNA fragment containing the entire coding region of the XYL-6 gene was amplified by RT-PCR (3, 36) from an mRNA sample using oligonucleotide primers Oligo227 and Oligo359 (Table 1). The mRNA sample was the same as the one used for the construction of the cDNA library. The PCR fragment was then digested with SnaBI and AvrII and inserted into SnaBI/AvrII-cleaved pPicH. The resulting plasmid, pHXyl6, was multiplied in E. coli strain Top10 (Invitrogen), and determined by DNA sequencing to be error free. The plasmid was then linearized by SacI digestion and electrotransformed into Pichia cells. Transformants resistant to a minimum of 1.0 mg of antibiotic G418 sulfate (Geneticin)/ml were selected for protein expression in 10 ml buffered complex methanol medium according to the Invitrogen manual. Aliquots (each, 1 ml) of the culture were taken every 24 h following induction and centrifuged in a microcentrifuge for 5 min at full speed to remove the Pichia cells. Samples (each, 10 μl) of the cell-free supernatant were assayed for endo-β-1,4-xylanase activity as described above with blue-dyed RBB-xylan used as a substrate (37). For large-scale induction, Pichia cells at a density of 2 U of optical density at 600 nm per ml were grown in 1 liter of buffered complex methanol medium at 30°C for 3 days. After induction, the culture medium that contained secreted proteins was concentrated to about 100 ml and subjected to nickel-chelate affinity column chromatography (25) using HisLink resin (catalogue no. V8821; Promega Corp., Madison WI), according to the manufacturer's manual. Western blot analysis using the alkaline phosphatase-conjugated anti-myc antibody (catalogue no. R950-25; Invitrogen Corp.) was performed on the purified protein according to the accompanying instructional manual.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the nucleotide sequences described in this paper are AA415057 to AA415145 and AY124591.
RESULTS
Analyses of ESTs from M. grisea.
The fungus was grown in a basal medium containing RCWs as the sole carbon source. An mRNA sample isolated from the culture was used to prepare a cDNA library. Partial or complete nucleotide sequences of 112 random cDNA clones, or ESTs, were determined and subjected to similarity comparison with the BLAST software to all entries in publicly available sequence databases (1). It was assumed that the similarity between two sequences was significant if the E value resulting from the comparison was <1e − 10, i.e., 10−10 (1). Based on this criterion, this 112-EST pool contained 86 singletons. Only half of these singletons encode proteins with significant matches in the databases (Table 2). The other 43 singletons did not have significant similarity to any entries in public protein databases. In agreement with the growth conditions in which polysaccharides (the major component of RCWs) are used as the carbon source, eight ESTs (∼10% of all singletons) encoded polypeptides involved in polysaccharide catabolism and sugar transport. This statistic was similar to that derived from a larger EST pool (14).
XYL-6 encodes a member of family GH10 endoglycanases.
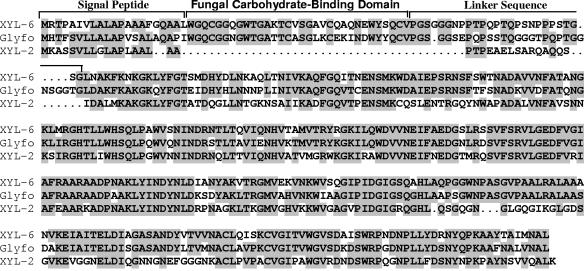
Among the ESTs, RCW105 is a partial transcript that encodes a protein that is 55 to 80% similar to family GH10 glycohydrolases (13, 21, 27-29). The gene, XYL-6, was isolated from a genomic library of M. grisea (35) using RCW105 as a probe. The nucleotide sequence of XYL-6 (GenBank accession number AY124591) includes four introns and five exons that encode a polypeptide of 380 amino acids (Fig. 1). Excluding the putative signal peptide, XYL-6p had a molecular mass of 38.8 kDa and a theoretical pI of 8.5. The mature peptide starts with a typical class III fCBD (21, 27), which is connected through a proline-glycine-rich linker sequence to the catalytic domain (20). XYL-6p does not contain any putative N-glycosylation sites. In comparison to other family GH10 endoglycanases, the amino acid sequence of XYL-6p is 79.2% similar to a putative endo-β-1,4-glycanase from Fusarium oxysporum (28, 29) but is only 60.3% similar to XYL-2p from M. grisea (35).
FIG. 1.
Comparison of family GH10 endoglycanases. The amino acid sequence of XYL-6p was deduced from the five exons encoded by the XYL-6 gene (GenBank accession number AY124591) and compared, using software programs included in the Wisconsin Package (Genetics Computer Group, Madison, WI), to M. grisea XYL-2 (formerly XYN33) (35) and a putative endo-β-1,4-glycanase from Fusarium oxysporum (Glyfo) (28, 29). Identical amino acid residues among the compared sequences are highlighted.
XYL-6 is expressed in culture and in infected rice leaves.
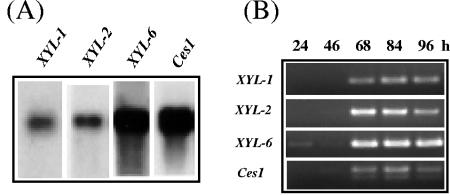
Gene expression of XYL-6 was analyzed by Northern blot analysis and reverse transcriptase-mediated PCR (RT-PCR) (Fig. 2) (35-37). The detected XYL-6 mRNA signal was strong in M. grisea cells growing in basal medium with RCW as the only carbon source; it was about 10 times stronger than that for XYL-1 mRNA and about 8 times stronger than that for XYL-2 mRNA (Fig. 2A). In infected rice seedlings, XYL-6 transcripts were detectable as early as 24 h postinoculation with M. grisea conidia and accumulated to a PCR-saturated level 68 h postinoculation. In comparison, XYL-1 and XYL-2 transcripts were barely detectable at 46 h, accumulated to maximum at 84 h, and declined in amount by 96 h postinoculation.
FIG. 2.
Northern blot and RT-PCR analysis of XYL-6 transcripts. (A) The total RNA sample for construction of the cDNA library was subjected to Northern blot analysis using digoxigenin-labeled cDNA fragments as probes according to the manufacturer's instructions (Roche Applied Sciences catalogue no. 1636090, 1363514, and 1603558). Probes XYL-1 (transcript = 1.1 kb) and XYL-2 (transcript = 1.3 kb) have been described previously (35, 37); probe XYL-6 is EST RCW105 (transcript = 1.5 kb); and probe Ces1 is EST RCW100 (transcript = 1.7 kb). The Ces1 gene encodes a homolog of a yeast sporulation-related gene, SPS2 (26). Ces1 is constitutively transcribed in M. grisea culture (unpublished observations). (B) RT-PCR measurement of gene transcripts in infected rice leaves. Seedlings of rice cultivar Sariceltik were grown for 14 days in a growth chamber and inoculated with an aqueous conidial suspension (107/ml) of M. grisea strain CP987 as previously described (37). Total RNA samples were isolated from the infected seedlings 24, 46, 68, 84, or 96 h postinoculation. The RNA samples were treated with DNase I prior to RT-PCR as previously described (36). RT-PCR was performed using gene-specific primers (Table 1) designed for fragments of XYL-1 (397 bp), XYL-2 (621 bp), XYL-6 (325 bp), and Ces1 (349 bp), respectively.
Generation of a xyl-6Δ knockout mutant.
In an attempt to identify any biological roles XYL-6 may play in M. grisea, a knockout mutant, X7601, was generated from the M. grisea parent strain, CP987, by the same strategy as previously described (see Materials and Methods for screening details) (37). In X7601, the entire coding sequence of XYL-6 was replaced by a selection marker gene that encodes a sulfonylurea-resistant acetolactate synthase (Sur). In comparison with the wild-type strain, the xyl-6ΔSur mutant did not appear to exhibit any morphological abnormality. For example, it grew normally in medium containing either RCW or xylan as the sole carbon source and infected rice hosts nearly as efficiently as the parent strain (Table 3). Thus, under the defined experimental conditions (37), XYL-6 is not required for either saprophytic or pathogenic growth of M. grisea.
TABLE 3.
Saprophytic growth and pathogenicity of M. grisea xylanase mutants
| Growth and pathogenicity | Strain and genotype
|
||
|---|---|---|---|
| CP987, wild-type | X7231,axyl-1ΔHygrxyl-2ΔBenr | X7601, xyl-6Δsur | |
| Wild-type | |||
| Fresh wt (mg)b on: | |||
| CM | 107 ± 8 | 94 ± 9 | 111 ± 12 |
| Vogel/RCW | 86 ± 9 | 48 ± 4 | 83 ± 7 |
| Vogel/OSX | 71 ± 7 | 29 ± 6 | 69 ± 6 |
| Pathogenicityc | |||
| No. of lesions | 166 | 158 | 149 |
| Lesion length (mm) | 3.1 ± 1.0 | 2.7 ± 0.8 | 3.2 ± 1.2 |
X7231 is a double-knockout strain for the XYL-1 and XYL-2 genes (37).
Mycelia (fresh weight) were taken from 5-day-old cultures as described by Wu et al. (37). Growth media are complete media (CM) and Vogel's basic salt medium with rice cell walls (RCW) or oat spelt xylan (OSX; Sigma) as the sole carbon source. Medium recipes and sampling methodology were the same as described previously (37).
An infection assay was performed on 17-day-old rice seedlings (compatible cultivar Sariceltik) as described previously (37). The number of lesions was the total from 10 fourth-folial leaves, and lesion length was the average of 30 randomly selected lesions from the 10 fourth-folial leaves 5 days postinoculation of fungal conidial suspension (2 × 104/ml).
Two Xylanases are absent from the xyl-6ΔSur mutant.
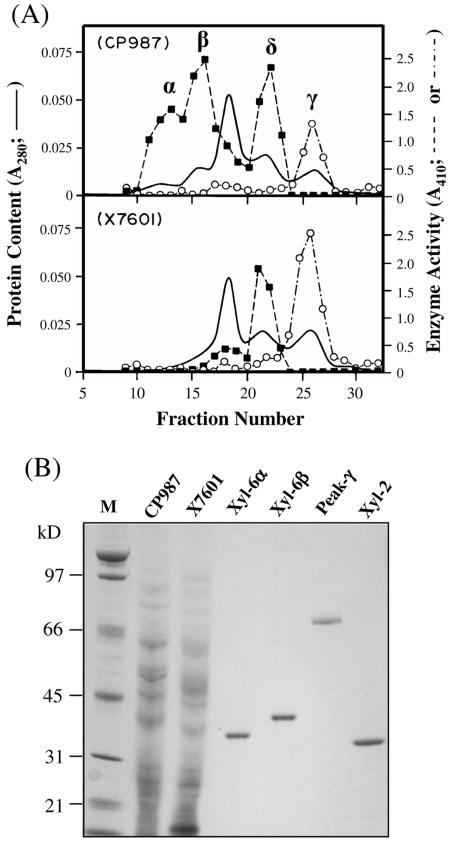
It is known that family GH10 glycanases may be endo-β-1,4-xylanase, endo-β-1,4-glucanase, or both (20, 21). To determine the enzymatic activity of XYL-6p, secreted proteins in the culture filtrates of the RCW-grown (35, 37) parent strain (CP987) and the xyl-6ΔSur mutant (strain X7601) were separately subjected to cation-exchange chromatography. The collected fractions were assayed for both endo-β-1,4-glucanase and endo-β-1,4-xylanase activities. The results, summarized in Fig. 3, showed glucanase activity in fractions eluting in a single peak (Fig. 3A, peak γ) present in the proteins secreted by both CP987 and X7601. Fractions containing xylanase activities were separated into three peaks (α, β, and δ) from the culture filtrate of CP987. Peak δ, which contained both XYL-1 and XYL-2, has been previously described by Wu et al. (35, 37). Peaks α and β, however, were both missing from strain X7601. Therefore, the deletion of the XYL-6 gene from M. grisea eliminates the secretion of two xylanases, but not the glucanase activity.
FIG. 3.
Purification of XYL-6p. (A) Cation-exchange chromatograms of secreted proteins. Secreted protein samples from 200 ml of fungal cultures were processed as previously described (35). The protein samples (∼2 mg each) were subjected to cation-exchange chromatography using a HiTrap SP column (Pharmacia Biotech) at pH 5.0. The column-bound proteins were eluted with a linear salt gradient from 0 to 1.0 m sodium chloride (fractions 1 to 120). Peak α was eluted by ∼0.045 M NaCl; peak β was eluted by ∼0.065 M NaCl; peak δ was eluted by ∼0.09 M NaCl, and peak λ was eluted by ∼0.11 M NaCl. A fifth peak eluted by ∼0.6 M NaCl and present in both CP987 and X7601 is not shown in this figure. This fifth peak contained an endo-β-1,4-xylanase, XYL-3, that has been described previously (37). Each fraction was assayed for endo-β-1,4-xylanase (▪) and endo-β-1,4-glucanase (○) activities using oat spelt xylan (Sigma catalog no. X-0627) dissolved in 100 mM 2-(N-morpholino)-ethane sulfonic acid buffer (pH 6.0) and carboxymethylcellulose (Sigma catalog no. C-0806) dissolved in 50 mM sodium acetate buffer (pH 5.0), respectively, as the substrates (see Materials and Methods) (35). (B) Gel analysis of the purified enzymes. Samples CP987 (2 μg) and X7601 (2 μg) are total extracellular proteins from the culture filtrate of M. grisea strains CP987 and X7601, respectively. XYL-6α (100 ng) and XYL-6β (100 ng) were purified, respectively, from peaks α and β shown in panel A by hydrophobic interaction chromatography (see Materials and Methods). Peak γ (40 ng) is the endo-β-1,4-glucanase-containing peak shown in panel A. XYL-2 (100 ng) is a 33-kDa xylanase purified previously (35). The protein samples were separated by SDS-PAGE in a 4 to 12% NuPAGE Bis-Tris precast gel (Invitrogen) as previously described (35, 37) and stained with a Colloidal Blue Staining kit (Invitrogen). Lane M, molecular mass ladder.
The xylanases eluting in the fractions of peaks α and β were purified to apparent homogeneity by two rounds of hydrophobic interaction chromatography as previously described (37). The yield was low, with about 4 μg of XYL-6α and 7 μg of XYL-6β obtained from 200 ml of culture (Fig. 3). Denaturing gel analysis showed that the purified XYL-6α had a molecular mass of about 34 kDa and that XYL-6β had a molecular mass of about 40 kDa (Fig. 3B). The latter agreed with the calculated molecular weight of mature XYL-6p based on its amino acid sequence (Fig. 1). The purified XYL-6α and XYL-6β were subjected to amino acid sequencing. The N-terminal amino acid sequences of XYL-6α and XYL-6β were determined to be WGQCGGXGWT and WXQCGGQXXTGA, respectively, where X represents a single, unidentified amino acid residue. Except for the unidentified X residues, these two N-terminal sequences were identical to the deduced N terminus of XYL-6p (Fig. 1). Thus, XYL-6α and XYL-6β are most likely two isoforms encoded by the same XYL-6 gene, with XYL-6α being a fragment of XYL-6β.
Heterologous expression of XYL-6 generated endo-β-1,4-xylanase activity.
Heterologous expression is another approach employed to unequivocally confirm the enzyme activity of XYL-6p. A full-length XYL-6 cDNA was amplified by PCR from an mRNA preparation of the RCW-grown CP987 mycelia (Table 1). The amplified XYL-6 cDNA was ligated into the SnaBI and AvrII restriction sites of a Pichia pastoris expression vector, pPicH, which was created by inserting a 74-bp DNA fragment into the AvrII and NotI restriction sites of the commercial vector pPIC3.5K (see Materials and Methods). Since the 74-bp DNA sequence encodes a c-myc epitope and a His6 tag, a protein expressed in Pichia pastoris using pPicH will have the tandem c-myc-His6 tag fused at its C terminus (16, 25).
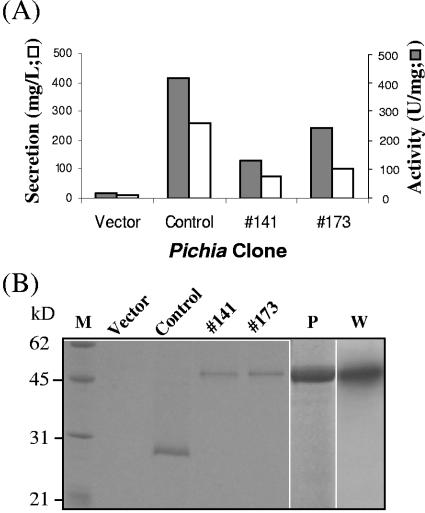
The resulting construct, pHXyl-6, was transformed into Pichia pastoris cells, followed by selection of two independent transformants for protein induction. The culture media of both Pichia clones contained endo-β-1,4-xylanase activity and a unique protein band of approximately 47 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). As shown in Fig. 4, the enzyme activity and the intensity of the 47-kDa protein band of clone 141 were both slightly weaker than those from clone 173. These results were consistent with the measured level of secretion for clone 141 (75 mg/liter), clone 173 (100 mg/liter), and the vector-transformed clone (12 mg/liter). Thus, XYL-6H was the predominant component of the secreted proteins (Fig. 4). The expressed fusion protein (XYL-6H) was purified from the 1 liter of induction medium of clone 173 by nickel-chelate affinity chromatography (25) with a recovery of approximately 2 mg and a specific endo-β-1,4-d-xylanase activity of 301 U/mg on RBB-xylan substrate (for a definition of the unit, see reference 37). The purified XYL-6H bound specifically to the c-myc antibody by a Western blot analysis (Fig. 4). It is noteworthy that XYL-6H is, by SDS-PAGE, approximately 6 kDa larger than the predicted molecular mass of 41.3. Therefore, it is possible that XYL-6H is posttranslationally modified. Consistent with the data shown in Fig. 3, XYL-6H had no detectable endo-β-1,4-d-glucanase (cellulase) activity (data not shown).
FIG. 4.
Pichia expression of XYL-6H. (A) XYL-6-transformed Pichia clones (clones 141 and 173), a positive control-expressing M. grisea xylanase XYL-4 (GenBank no. AY144349; manuscript in preparation), and a clone carrying the empty vector pPic3.5K were induced for protein expression in 1 liter of induction medium for 80 h. Specific endo-β-1,4-xylanase activity in the culture filtrate was assayed with 100 mM phosphate, pH 6.0, using RBB-xylan (Sigma) as the substrate according to Wu et al. (37). Total protein content was measured using Protein Assay Reagent purchased from Bio-Rad Laboratories (Hercules, CA). (B) SDS-PAGE and Western blot analysis of secreted proteins in the culture filtrate (30 μl). Lane M, molecular mass markers; lane P, 0.5 μg of HisLink-purified XYL-6H from the culture filtrate of Pichia clone 173; lane W, Western blot analysis of lane P using an alkaline phosphatase-conjugated anti-myc antibody.
DISCUSSION
Recent progress in genomics has resulted in exponential expansion of the number of identified genes (9). The pilot analysis of 112 ESTs that are unique to M. grisea growing on RCWs as the sole carbon source revealed expression profiles similar to those of a larger study (14). Among the sequences analyzed, five encode plant CWDEs and another three are responsible for sugar transport across the plasma membrane (Table 2). It is most likely that the CWDEs and sugar transporters are expressed in response to the culture environment of the RCW carbon source and are needed for depolymerizing the RCW into monosaccharides that can be utilized by the fungus as nutrients. Among the identified ESTs that encode CWDEs is XYL-6, whose function is the focus of this study.
XYL-6 is expressed more strongly than XYL-1 and XYL-2 (35, 37) both in culture with RCW as a carbon source and in infected rice leaves. Nonetheless, we noticed that the accumulation pattern of XYL-1, XYL-2, and XYL-6 transcripts in planta was similar to that in culture. In other words, under both conditions, XYL-6 mRNA had the highest level of accumulation, XYL-2 mRNA had a lower level, and XYL-1 mRNA had the lowest level. In addition, transcripts of these three genes were not detectable by RT-PCR in culture using sucrose as the carbon source (data not shown). Thus, although each xylanase gene is uniquely expressed, they may be regulated by a coordinated mechanism (10, 11, 18, 28). In line with this thought, the M. grisea genome (9) encodes two homologs (MG01414 and MG02880) of XlnR, a transcription factor that regulates most xylan-degrading enzymes in Aspergillus niger (for a review, see reference 11).
The protein fractionation profiles in Fig. 3 indicate that the biochemical properties of XYL-6 are different from those of XYL-1, XYL-2, and XYL-3. For example, XYL-6 was eluted by low-salt buffer (XYL-6α by 0.045 M and XYL-6β by 0.065 M NaCl), whereas XYL-1 and XYL-2 (Fig. 3, peak δ) were eluted by 0.09 M NaCl, and XYL-3 (not shown in Fig. 3) was eluted by 0.6 M NaCl. Also, XYL-6 appears to have an optimal pH of 6 instead of 7.2 for XYL-1, XYL-2, and XYL-3 (37). Structurally, XYL-6 includes an N-terminal fCBD, while the other characterized xylanases do not (35). The successful purification and/or heterologous expression makes it possible to investigate and compare the enzymatic mode of action and substrate specificity (5, 12, 24, 27) of these various isoforms of M. grisea xylanases.
The conclusion that the 34-kDa XYL-6α and the 40-kDa XYL-6β are the same gene product of XYL-6 arises from the following two facts. (i) Deletion of the XYL-6 gene eliminates both α and β activities. (ii) Both XYL-6α and XYL-6β have exactly the same N terminus as the one predicted from the nucleotide sequence of XYL-6. Analysis of protein purification results also indicated that a good portion of XYL-6 was fragmented into the smaller 34-kDa polypeptide that is still active at hydrolyzing xylan substrate. Therefore, the proteolytic degradation must be rather specific, and the cleaved C terminus (mass of 6 kDa or ∼55 amino acid residues) must not be required for xylanase activity. It has been predicted (and in some cases shown) that the catalytic site of group 10 xylanases from other microbial species involves conserved glutamic acid (Glu) residues (5, 20, 25). There are no Glu residues within the ∼55 C-terminal amino acids, as shown in Fig. 1. However, it remains to be determined whether the 34-kDa and the 40-kDa XYL-6 differ in their mode of enzymatic action and/or substrate specificity.
Heterologous expression of XYL-6 unequivocally confirmed that XYL-6 is an endo-β-1,4-xylanase (Fig. 4). This experiment also indicated for the first time that the native XYL-6 signal peptide (Fig. 1) directs protein secretion from Pichia cells into the culture medium (Fig. 4). The secretion level of 75 to 100 mg/liter is similar to that of an Aspergillus niger xylanase directed by the built-in Saccharomyces cerevisiae α-factor (4). Using the same strategy, we also successfully expressed a number of genes in Pichia encoding putative secreted proteins from M. grisea (unpublished data). Therefore, it is most likely that M. grisea signal peptides in general are recognized by the Pichia secretory machinery.
The inclusion of an His6 tag for heterologous protein expression greatly facilitates purification of the expressed protein by a one-column affinity chromatography. However, the yield of 2 mg of pure XYL-6H by nickel-chelate chromatography out of an estimated 100 mg secreted into the culture media is extremely low. It is possible that the C-terminal fusion of the His6 tag leads to an XYL-6H conformation that limits exposure of the tandem histidine residues to the nickel ligand, resulting in low binding of XYL-6H to the HisLink resins. It is also possible that the purification process will have to be optimized for maximum yield, which could include tests of buffer conditions and various affinity media.
endo-β-1,4-Xylanases from various microbial sources are being intensively investigated. Most of these studies focus on xylanase's potential in industrial applications, such as in paper pulping and bleaching (27). A few studies attempted to elucidate the role of xylanases in microbial pathogenesis (2, 10, 17, 18, 33, 37), as arabinoxylan is the quantitatively predominant hemicellulosic component of the cell walls of the Poaceae (6, 15). These studies have established that plant pathogenic fungi secrete multiple xylanases when infecting plant tissues as well as when growing in pure culture with arabinoxylan as the carbon source. The strong transcription of XYL-6 in both the culture and rice leaves during the early infection stage further supports these claims, although irrefutable evidence that xylanases are pathogenicity factors has yet to be obtained. The xyL-6Δ mutant, like previously investigated xyl-1Δ and xyl-2Δ mutants, is not required for pathogenicity under the defined growth chamber conditions. However, our investigation of the M. grisea genome sequence indicates the presence of as many as 20 xylanase genes, including at least genes encoding six family GH10 members, five family GH11 members, and nine family GH43 members (9, 29, 37; unpublished data). It is possible that any of the xylanases, other than XYL-1, XYL-2 and XYL-6, is required for pathogenicity, or a member of the xylanases lost is complemented by the others. Alternatively, pathogenicity may partially depend on the fungus's ability to depolymerize cell wall xylan, which could require two or more xylanases working in concert during infection growth in host tissues. Xylanases, working alone or with other inhibiting proteins, may also be indirectly involved in fungus-plant interactions by generating structure-specific xylan oligosaccharide fragments that are recognized by the plant host as elicitor signal molecules (8, 13, 19). Our growing collection of purified or Pichia-expressed M. grisea xylanases, as well as their knockout mutants, allows us to continue investigation of these possibilities.
Acknowledgments
This project was supported by U.S. Department of Energy (DOE) grant DE-FG02-96ER20221 and the DOE-funded (DE-FG05-93ER20097) Center for Plant and Microbial Complex Carbohydrates.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apel-Birkhold, P. C., and J. D. Walton. 1996. Cloning, disruption, and expression of two endo-β-1,4-xylanase genes, XYL2 and XYL3, from Cochliobolus carbonum. Appl. Environ. Microbiol. 62:4129-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 2002. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 4.Berrin, J. G., G. Williamson, A. Puigserver, J. C. Chaix, W. R. McLauchlan, and N. Juge. 2000. High-level production of recombinant fungal endo-β-1,4-xylanase in the methylotrophic yeast Pichia pastoris. Protein Expr. Purif. 19:179-187. [DOI] [PubMed] [Google Scholar]
- 5.Biely, P., M. Vrsanska, M. Tenkanen, and D. Kluepfel. 1997. Endo-β-1,4-xylanase families: differences in catalytic properties. J. Biotechnol. 57:151-166. [DOI] [PubMed] [Google Scholar]
- 6.Carpita, N. C., and M. McCann. 2000. The cell wall, p. 25-109. In B. B. Buchanan, W. Gruissem, and R. J. Jones (ed.), Biochemistry and molecular biology of plants. American Society of Plant Biologists, Rockville, Md.
- 7.Cregg, J. M., J. L. Cereghino, J. Shi, and D. R. Higgins. 2000. Recombinant protein expression in Pichia pastoris. Mol. Biotechnol. 16:23-39. [DOI] [PubMed] [Google Scholar]
- 8.Darvill, A., C. Augur, C. Bergmann, R. W. Carlson, J.-J. Cheong, S. Eberhard, M. G. Hahn, V.-M. Ló, V. Marfà, B. Meyer, D. Mohnen, M. A. O'Neill, M. D. Spiro, H. Van Halbeek, W. S. York, and P. Albersheim. 1992. Oligosaccharins—oligosaccharides that regulate growth, development and defence responses in plants. Glycobiology 2:181-198. [DOI] [PubMed] [Google Scholar]
- 9.Dean R., et al. 2005. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980-986. [DOI] [PubMed] [Google Scholar]
- 10.Degefu, Y., K. Lohtander, and L. Paulin. 2004. Expression patterns and phylogenetic analysis of two xylanase genes (htxyl1 and htxyl2) from Helminthosporium turcicum, the cause of northern leaf blight of maize. Biochimie 86:83-90. [DOI] [PubMed] [Google Scholar]
- 11.de Vries, R. P. 2003. Regulation of Aspergillus genes encoding plant cell wall polysaccharide-degrading enzymes; relevance for industrial production. Appl. Microbiol. Biotechnol. 61:10-20. [DOI] [PubMed] [Google Scholar]
- 12.Ducros, V., S. J. Charnock, U. Derewenda, Z. S. Derewenda, Z. Dauter, C. Dupont, F. Shareck, R. Morosoli, D. Kluepfel, and G. J. Davies. 2000. Substrate specificity in glycoside hydrolase family 10. Structural and kinetic analysis of the Streptomyces lividans xylanase 10A. J. Biol. Chem. 275:23020-23026. [DOI] [PubMed] [Google Scholar]
- 13.Durand, A., R. Hughes, A. Roussel, R. Flatman, B. Henrissat, and N. Juge. 2005. Emergence of a subfamily of xylanase inhibitors within glycoside hydrolase family 18. FEBS J. 272:1745-1755. [DOI] [PubMed] [Google Scholar]
- 14.Ebbole, D. J., Y. Jin, M. Thon, H. Pan, E. Bhattarai, T. Thomas, and R. Dean. 2004. Gene discovery and gene expression in the rice blast fungus, Magnaporthe grisea: analysis of expressed sequence tags. Mol. Plant-Microbe Interact. 17:1337-1347. [DOI] [PubMed] [Google Scholar]
- 15.Edashige, Y., and T. Ishii. 1998. Hemicellulosic polysaccharides from bamboo shoot cell-walls. Phytochemistry 49:1675-1682. [DOI] [PubMed] [Google Scholar]
- 16.Evan, G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giesbert, S., H.-B. Lepping, K. B. Tenberge, and P. Tudzynski. 1998. The xylanolytic system of Claviceps purpurea: cytological evidence for secretion of xylanases in infected rye tissue and molecular characterization of two xylanase genes. Phytopathology 88:1020-1030. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Gomez, E., M. C. Ruiz-Roldan, A. Di Pietro, M. I. Roncero, and C. Hera. 2002. Role in pathogenesis of two endo-beta-1,4-xylanase genes from the vascular wilt fungus Fusarium oxysporum. Fungal Genet. Biol. 35:213-222. [DOI] [PubMed] [Google Scholar]
- 19.Ham, K.-S., S.-C. Wu, A. G. Darvill, and P. Albersheim. 1997. Fungal pathogens secrete an inhibitor protein that distinguishes isoforms of plant pathogenesis-related endo-β-1,3-glucanases. Plant J. 11:169-179. [Google Scholar]
- 20.Henrissat, B. 1993. Hidden domains and active site residues in β-glycanase-encoding gene sequences. Gene 125:199-204. [DOI] [PubMed] [Google Scholar]
- 21.Henrissat, B., and A. Bairoch. 1993. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamakura, T., J. Xiao, W. Choi, T. Kochi, S. Yamaguchi, T. Teraoka, and I. Yamaguchi. 1999. cDNA subtractive cloning of genes expressed during early stage of appressorium formation by Magnaporthe grisea. Biosci. Biotechnol. Biochem. 63:1407-1413. [DOI] [PubMed] [Google Scholar]
- 23.Kim, S., I.-P. Ahn, and Y.-H. Lee. 2001. Analysis of genes expressed during rice-Magnaporthe grisea interactions. Mol. Plant-Microbe Interact. 14:1340-1346. [DOI] [PubMed] [Google Scholar]
- 24.Liab, K., P. Azadi, R. Collins, J. Tolan, J. S. Kim, and K. L. Eriksson. 2000. Relationships between activities of xylanases and xylan structures. Enzyme Microbe Technol. 27:89-94. [DOI] [PubMed] [Google Scholar]
- 25.Lonnerdahl, B., and C. L. Keen. 1982. Metal chelate affinity chromatography of proteins. J. Appl. Biochem. 4:203-208. [Google Scholar]
- 26.Percival-Smith, A., and J. Segall. 1987. Increased copy number of the 5′ end of the SPS2 gene inhibits sporulation of Saccharomyces cerevisiae. Mol. Cell. Biol. 7:2484-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polizeli, M. L., A. C. Rizzatti, R. Monti, H. F. Terenzi, J. A. Jorge, and D. S. Amorim. 2005. Xylanases from fungi: properties and industrial applications. Appl. Microbiol. Biotechnol. 67:577-591. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Roldán, M. C., A. Di Pietro, M. D. Huertas-González, and M. I. G. Roncero. 1999. Two xylanase genes of the vascular wilt pathogen Fusarium oxysporum are differentially expressed during infection of tomato plants. Mol. Gen. Genet. 261:530-536. [DOI] [PubMed] [Google Scholar]
- 29.Sheppard, P. O., F. J. Grant, P. J. Oort, C. A. Sprecher, D. C. Foster, F. S. Hagen, A. Upshall, G. L. McKnight, and P. J. O'Hara. 1994. The use of conserved cellulase family-specific sequences to clone cellulase homologue cDNAs from Fusarium oxysporum. Gene 150:163-167. [DOI] [PubMed] [Google Scholar]
- 30.Sweigard, J. A., F. G. Chumley, A. M. Carroll, L. Farrall, and B. Valent. 1995. A series of vectors for fungal transformation. Fungal Genet. Newsl. 44:52-53. [Google Scholar]
- 31.Talbot, N. J. 2003. On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 57:177-202. [DOI] [PubMed] [Google Scholar]
- 32.Ten Have, A., W. Mulder, J. Visser, and J. A. van Kan. 1998. The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol. Plant-Microbe Interact. 11:1009-1016. [DOI] [PubMed] [Google Scholar]
- 33.Tonukari, N. J., J. S. Scott-Craig, and J. D. Walton. 2000. The Cochliobolus carbonum SNF1 gene is required for cell wall-degrading enzyme expression and virulence on maize. Plant Cell 12:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walton, J. D. 1994. Deconstructing the cell wall. Plant Physiol. 104:1113-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, S.-C., S. Kauffmann, A. G. Darvill, and P. Albersheim. 1995. Purification, cloning and characterization of two endo-β-1,4-xylanases from Magnaporthe grisea, the rice blast fungus. Mol. Plant-Microbe Interact. 8:506-514. [DOI] [PubMed] [Google Scholar]
- 36.Wu, S.-C., J. B. Blumer, A. G. Darvill, and P. Albersheim. 1996. Characterization of an endo-β-1,4-glucanase gene induced by auxin in elongating pea epicotyls. Plant Physiol. 110:163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, S.-C., K.-S. Ham, A. G. Darvill, and P. Albersheim. 1997. Deletion of two endo-β-1,4-xylanase genes reveals additional isozymes secreted by the rice blast fungus. Mol. Plant-Microbe Interact. 10:700-708. [Google Scholar]
- 38.Xu, H., and K. Mendgen. 1997. Targeted cell wall degradation at the penetration site of cowpea rust basidiosporelings. Mol. Plant-Microbe Interact. 10:87-94. [Google Scholar]