Abstract
Fifty strains representing 38 species of the genus Legionella were examined for biofilm formation on glass, polystyrene, and polypropylene surfaces in static cultures at 25°C, 37°C, and 42°C. Strains of Legionella pneumophila, the most common causative agent of Legionnaires' disease, were found to have the highest ability to form biofilms among the test strains. The quantity, rate of formation, and adherence stability of L. pneumophila biofilms showed considerable dependence on both temperature and surface material. Glass and polystyrene surfaces gave between two- to sevenfold-higher yields of biofilms at 37°C or 42°C than at 25°C; conversely, polypropylene surface had between 2 to 16 times higher yields at 25°C than at 37°C or 42°C. On glass surfaces, the biofilms were formed faster but attached less stably at 37°C or 42°C than at 25°C. Both scanning electron microscopy and confocal laser scanning microscopy revealed that biofilms formed at 37°C or 42°C were mycelial mat like and were composed of filamentous cells, while at 25°C, cells were rod shaped. Planktonic cells outside of biofilms or in shaken liquid cultures were rod shaped. Notably, the filamentous cells were found to be multinucleate and lacking septa, but a recA null mutant of L. pneumophila was unaffected in its temperature-regulated filamentation within biofilms. Our data also showed that filamentous cells were able to rapidly give rise to a large number of short rods in a fresh liquid culture at 37°C. The possibility of this biofilm to represent a novel strategy by L. pneumophila to compete for proliferation among the environmental microbiota is discussed.
Legionnaires' disease is a form of pneumonia caused by bacteria of the genus Legionella, of which Legionella pneumophila is the type species. This infectious disease involves inhalation of aerosols of water containing Legionella into the lung. Within the lung, the bacteria are internalized by alveolar macrophages, replicate within the phagosomes, and eventually lyse the host macrophages (27). The genus Legionella currently contains as many as 48 species, and at least 20 species are known to be pathogenic to humans (22). Despite this, the general perception is that the species L. pneumophila is the main causative agent of Legionnaires' disease, due to the much higher incidence of the disease reported to be caused by L. pneumophila than by the other species of the genus (5). One or more of the following reasons could account for this higher incidence. First, lack of diagnostic reagents sufficiently discriminative to identify non-L. pneumophila species could lead to inaccurate clinical statistics (22). Second, L. pneumophila may be inherently more virulent than other species. However, only a few aspects of its pathogenicity e.g., cytopathogenicity and intracellular multiplication, have currently been shown to be enhanced, compared to non-L. pneumophila species (1, 23, 30, 42), providing no strong support for this hypothesis. Third, L. pneumophila may be easier to transmit than other Legionella species. However, transmission of L. pneumophila from humans to humans has not been reported, despite many cases of outbreaks in the recent few decades. Transmission to humans has reportedly occurred only via mechanical means, such as air-conditioning units, showerheads, and sprinklers (27, 61); therefore, enhanced human-to-human transmissibility is an unlikely reason. Fourth and finally, L. pneumophila may have a higher chance than other Legionella species of coming into contact with humans. One speculation is that L. pneumophila has an innate ability to better survive in the water environment, especially man-made ones. Factors influencing survival of L. pneumophila have been studied previously (45), but its survival ability compared to that of other non-L. pneumophila species has not been explored.
The habitats for the genus Legionella are moist soil and aquatic environments, and outbreaks of legionellosis are often associated with contamination of man-made aquatic environments (for a review, see references 3, 22, and 27). Although Legionella bacteria do exist as free-living planktonic forms in the environment, they are more commonly found as intracellular parasites of protozoans such as Acanthamoeba spp., Hartmannella spp., and Tetrahymena spp. (3, 7) and as inhabitants of mixed-community biofilms (51, 62). Biofilms are assemblages of bacteria encased in extracellular polymeric matrices, attached to surfaces or phase boundaries. Being able to form such an adherent, hydrated, and structured community confers several advantages on bacteria for survival in harsh world, e.g., protection from harmful compounds and prevention of desiccation (for a review, see references 15 and 18). Legionella has been studied in the context of mixed-community biofilm experimental systems (9, 40, 52) or with the purpose of understanding the efficacy of biocides or biocidal treatments (26, 64, 65), but its ability to form biofilms per se has not been investigated.
The initial motivation for this study was to address the question: has L. pneumophila better survival ability in terms of biofilm formation than other Legionella species? We were particularly interested in differences that may exist at temperatures such as 37°C and 42°C, often encountered in man-made environments, e.g., hot spring spas, in contrast to the more ambient temperature of 25°C. In the course of the investigation, we found that a novel form of biofilm composed of filamentous cells of L. pneumophila exists, which may represent another aspect of survival strategy by this organism. Here, we shall report on these findings.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Legionella spp. were obtained from Gifu Type Culture Collection (GTC) (Table 1). Unless otherwise stated, all strains were grown in buffered yeast extract (BYE) broth (49) at 37°C with shaking or on buffered yeast extract charcoal agar (BYECA; BYE with 0.2% activated charcoal and 1.5% Bacto agar) (37) at 37°C for 3 days. Both types of media were supplemented with 0.4-g/liter of l-cysteine and 0.25-g/liter ferric pyrophosphate, and the pH was adjusted to 6.9 with 1 M potassium hydroxide. Kanamycin at 25 μg/ml was added where necessary.
TABLE 1.
Legionella strains used in this study
| Species (serogroup) | Strain (GTCa designation) | Numerical designation in Fig. 1 |
|---|---|---|
| L. pneumophila | ||
| L. pneumophila (1) | Philadelphia-1 (GTC 9134b) | 1 |
| L. pneumophila (1) | Knoxville-1 (GTC 9135) | 2 |
| L. pneumophila (2) | Togus-1 (GTC 9136) | 3 |
| L. pneumophila (3) | Bloomington-2 (GTC 9137) | 4 |
| L. pneumophila (4) | Los Angeles-1 (GTC 9246b) | 5 |
| L. pneumophila (5) | Dallas-1E (GTC 9139) | 6 |
| L. pneumophila (6) | Chicago-2 (GTC 9138) | 7 |
| L. pneumophila (7) | Chicago-8 (GTC 10064) | 8 |
| L. pneumophila (8) | Concord-3 (GTC 10065) | 9 |
| L. pneumophila (9) | IN-23-G1-C2 (GTC 10738) | 10 |
| L. pneumophila (10) | Leiden-1 (GTC 11369) | 11 |
| L. pneumophila (11) | 797-PA-H (GTC 11365) | 12 |
| L. pneumophila (5) | U8W (GTC 13567b) | 13 |
| Non-L. pneumophila species | ||
| L. bozemanae (1) | ATCCc33217 (GTC 9140b) | 14 |
| L. micdadei (NRd) | ATCC 33218 (GTC 9141b) | 15e |
| L. gormanii (NR) | ATCC 33297 (GTC 9142b) | 16f |
| L. dumoffii (NR) | ATCC 33343 (GTC 9244) | 17f |
| L. longbeachae (1) | ATCC 33462 (GTC 9245b) | 18f |
| L. dumoffii (NR) | ATCC 33279 (GTC 9247b) | 19 |
| L. oakridgensis (NR) | ATCC 33761 (GTC 10061b) | 20 |
| L. wadsworthii (NR) | ATCC 33877 (GTC 10062b) | 21 |
| L. feeleii (1) | ATCC 35072 (GTC 10063b) | 22g |
| L. longbeachae (2) | ATCC 33484 (GTC 10066) | 23 |
| L. sainthelensi (1) | ATCC 35248 (GTC 10392b) | 24 |
| L. bozemanae (2) | ATCC 35545 (GTC 10739) | 25 |
| L. hackeliae (1) | ATCC 35250 (GTC 10740b) | 26 |
| L. jamestowniensis (NR) | ATCC 35298 (GTC 10741b) | 27e |
| L. rubrilucens (NR) | ATCC 35304 (GTC 10743b) | 28 |
| L. feeleii (2) | ATCC 35849 (GTC 10744) | 29 |
| L. maceachernii (NR) | ATCC 35300 (GTC 10745b) | 30 |
| L. spiritensis (NR) | ATCC 35249 (GTC 11199b) | 31g |
| L. israelensis (NR) | ATCC 43119 (GTC 11367b) | 32 |
| L. hackeliae (2) | ATCC 35999 (GTC 11368) | 33 |
| L. parisiensis (NR) | ATCC 35299 (GTC 11745b) | 34 |
| L. erythra (NR) | ATCC 35303 (GTC 11748b) | 35g |
| L. birminghamensis (NR) | ATCC 43702 (GTC 11749b) | 36 |
| L. anisa (NR) | ATCC 35292 (GTC 12075b) | 37g |
| L. cincinnatiensis (NR) | ATCC 43753 (GTC 12201b) | 38 |
| L. quinlivanii (NR) | ATCC 43830 (GTC 12648b) | 39 |
| L. moravica (NR) | ATCC 43877 (GTC 12649b) | 40g |
| L. brunensis (NR) | ATCC 43878 (GTC 12655b) | 41e |
| L. tusconensis (NR) | ATCC 49180 (GTC 12656b) | 42 |
| L. jordanis (NR) | ATCC 33623 (GTC 12657b) | 43 |
| L. adelaidensis (NR) | ATCC 49625 (GTC 13562b) | 44e |
| L. fairfieldensis (NR) | ATCC 49588 (GTC 13563b) | 45 |
| L. gratiana (NR) | ATCC 49413 (GTC 13564b) | 46g |
| L. lansingensis (NR) | ATCC 49751 (GTC 13565b) | 47 |
| L. geestiana (NR) | ATCC 49504 (GTC 13568b) | 48 |
| L. londiniensis (NR) | ATCC 49505 (GTC 13635b) | 49 |
| L. nautarum (NR) | ATCC 49506 (GTC 13636b) | 50g |
| L. worsleiensis (NR) | ATCC 49508 (GTC 13638b) | 51g |
GTC, Gifu Type Culture Collection, Department of Microbiology, Gifu University School of Medicine, 1-1 Yanagido, Gifu 501-1194, Japan.
Type strain.
ATCC, American Type Culture Collection, 10801 University Blvd., Manassas, VA 20110-2209.
NR, not reported.
At 25°C, less-robust growth was observed on BYECA.
At 37°C, less-robust growth was observed on BYECA.
At 42°C, less-robust growth was observed on BYECA.
Biofilm formation and quantification.
Legionella strains were shaken at 37°C for 30 h and then diluted 1:10 to give a final optical density at 600 nm of 0.2 to 0.3. For general observation and quantification, biofilms were allowed to form from 2.2 ml of this diluted culture in round-bottom test tubes (diameter, 12 mm; height, 75 mm) of glass (Pyrex, Iwaki Glass, Japan), polystyrene (PS) (Falcon; Becton Dickinson), and polypropylene (PP) (Sarstedt, Germany), statically incubated in water vapor-saturated incubators set at 25°C, 37°C, or 42°C. For confocal laser scanning microscope (CLSM) observation, biofilms were allowed to form on Micro glass cover slides of 0.8 mm in thickness (Matsunami Glass Ind. Ltd., Japan), vertically immersed in the diluted culture in a thin-layer chromatography tank and similarly incubated at the indicated temperatures.
Quantification of biofilms were performed based on a protocol modified from that of O'Toole et al. (46). Briefly, a one-fifth volume of 0.25% crystal violet solution was added to the biofilm culture, and the biofilm was stained for 15 min. The biofilm was rinsed three times with deionized water, and the crystal violet stain was solubilized in 2 ml of 95% ethanol with shaking and then transferred as 200-μl aliquots (in triplicate) to polystyrene 96-well microtiter plates (Costar; Corning, Inc.). Absorbance at 600 nm was measured with a Wallac 1420 ARVOsx multilabel plate reader (Perkin-Elmer Life Sciences, Japan).
Plasmid construction, genetic manipulation, and recA mutant generation.
The plasmid pPZ1 carrying the green fluorescent protein (GFP) gene gfp-mut3* under the control of the LacI-repressible PΛ1/04/03 promoter (2) was constructed by standard recombinant DNA techniques (57). A NotI fragment from pJBA27 carrying PΛ1/04/03-gfp-mut3* (2) was inserted into the NotI site of pKM330, an RSF1010-based broad-host-range vector derived from pVI397 (58), with the carbenicillin resistance marker replaced by a kanamycin resistance marker (a kind gift from V. Shingler). To visualize L. pneumophila directly under CLSM, pPZ1 was introduced via electroporation into the strain, using a Gene-Pulser (Bio-Rad Laboratories Pte. Ltd., Japan).
For the generation of the recA mutant, the region encoding the L. pneumophila recA gene was PCR amplified with primers 5′-CCAGATTGCTTACTCATCTC-3′ and 5′-AGTTGTTCAAGCTCTGCAGC-3′, ligated into pGEM-Easy Vector (Promega), and inserted at its internal BamHI site, a 1.7-kb kanamycin resistance cassette from pUT-miniTn5-Km (14). The interrupted recA gene was then transferred into the NotI site of pLAW344, which harbors the sacB gene that confers sucrose sensitivity and allows counterselection for homologous recombination by double crossover (33). The plasmid was introduced into L. pneumophila JR32 (56) and selected for kanamycin and sucrose resistance, and the recombinants were confirmed for allelic replacement via PCR and increased UV sensitivity, as would be expected of a recA mutant (17).
Observation using CSLM, scanning electron microscope (SEM), and transmission electron microscope (TEM).
Glass slides with biofilms formed on both sides by L. pneumophila carrying pPZ1 were cleaned on one side with an alcohol swab and mounted on an Eclipse TE2000-U inverted microscope (Nikon) attached to a Radiance 2100 Laser Scanning system (Bio-Rad) to observe the biofilm attached on the intact side. Images were taken under an argon laser source with a dichroic mirror (excitation, 488 nm; emission, 515 nm) and processed by LaserSharp 2000 (Bio-Rad) software.
For EM observation, biofilms formed on the glass test tubes were transferred using a toothpick into glass petri dishes and washed briefly with deionized water. They were fixed with 2% glutaraldehyde for 2 to 3 h and then additionally with 1% OsO4 in the case of TEM observation. Subsequent dehydration was performed step wise using 50%, 70%, 90%, 95%, 99.5%, and 100% ethanol. For SEM observation, the ethanol was replaced by isoamyl acetate and then liquid CO2, which was allowed to vaporize gradually. Following this, the samples were coated with 60% gold-40% palladium alloy powder. For TEM observation, dehydrated samples were embedded in Epon resin, ultrathin sectioned, and stained with uranyl acetate, followed by lead citrate. The samples were examined with or without using a scanning image-observing device attached to a JEOL JEM 2000EX electron microscope.
Nucleoid staining of filamentous cells.
Filamentous cells from L. pneumophila biofilms were teased on glass slides to separate the strands and stained according to a modified version of HCl-Giemsa staining (50). Briefly, the preparation was fixed in OsO4 vapor for 45 min, treated with 1 M hydrochloric acid at 60°C for 10 min, rinsed, and stained with Giemsa stain for 5 min, followed by phosphate buffer (pH 6.4) for 5 min. The air-dried preparation was observed under an oil immersion lens on a light microscope (Olympus).
Monitoring distribution of filamentous and rod-shaped cells.
Biofilms formed on glass test tubes or colonies formed on agar were picked by toothpicks and spread extensively on glass slides for ease of examination of individual cells. For observation of cells in the medium surrounding biofilms, aliquots of the medium were taken with care to avoid disturbing the biofilms, centrifuged briefly to collect the cells in pellets, and then spread on glass slides. Cells from shaken culture were similarly collected and spread. Slides with these preparations were flame fixed, stained with Pfeiffer stain, and observed under oil immersion by a light microscope. Images in three different fields were processed with the Image-Pro 3D Suite (Media Cybernetics, Inc.) to quantify the number of pixel units for images of the filamentous and rod-shaped forms. The percentage of biovolume of filamentous cells was calculated as 100 × (pixel units for filamentous cells/pixel units for all cells).
Growth of cells from biofilm and planktonic culture.
Biofilms cultured for 4 days in glass test tubes were collected with Pasteur pipettes and rinsed briefly in sterile deionized water. They were then transferred into a test tube containing 5 ml of AC buffer [4 mM MgSO4, 0.4 mM CaCl2, 3.4 mM sodium citrate, 2.5 mM Na2HPO4, 2.5 mM KH2PO4, 20-mg/liter Fe(NH4)2(SO4)2 · 6H2O, pH 6.5] and shaken vigorously for 1 h to physically separate the filaments. A microscopic check of this suspension revealed that 90% of the cells were untangled from each other. For the “planktonic control,” a 30-h shaken culture in BYE was diluted 1:100 in 5 ml AC buffer and shaken similarly for 1 h. Aliquots (each, 4 ml) of the biofilm cell-derived and planktonic cell-derived suspensions were then transferred to conical flasks, each containing 66 ml of fresh BYE broth. Growth was monitored at 4-h intervals by counting the bacterial CFU on buffered yeast extract charcoal agar.
RESULTS
Biofilm formation by Legionella spp.
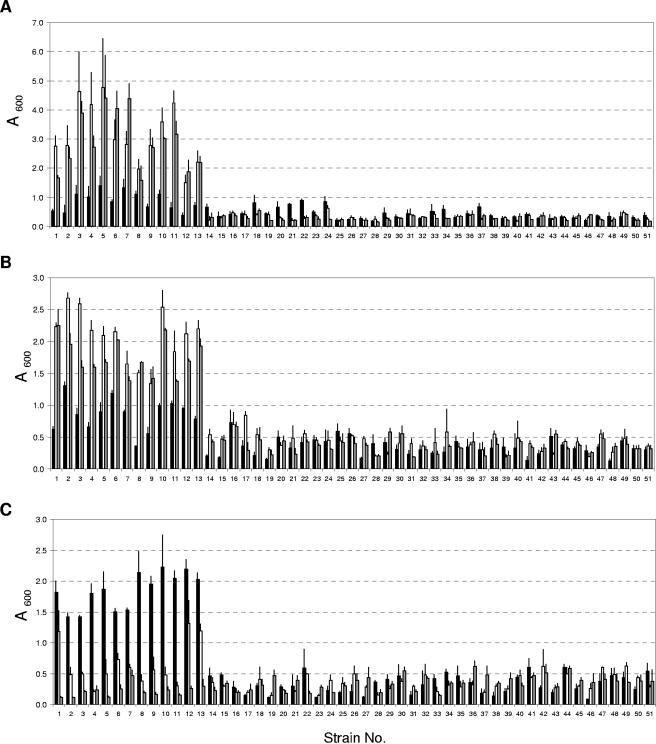
To assess the ability of Legionella spp. to form biofilms, particularly to compare L. pneumophila to non-L. pneumophila species, we utilized 13 strains of L. pneumophila and 38 strains of other Legionella spp. (Table 1). This gives a good representation of the genus Legionella. The experimental system is a static culture in the BYE broth medium, and three different materials (glass, PS, and PP) were chosen as surfaces for biofilm growth at three temperatures (25°C, 37°C, and 42°C). The growth of all test strains in BYE liquid and agar media at the experimental temperatures was checked to ensure that any inability to form a biofilm was not due to nonoptimal growth conditions. A preliminary experiment, in which biofilm formation was noted daily by eye over a period of 30 days, indicated that stable biofilms could be observed from day 11 at 25°C and from day 3 at 37°C or 42°C. The biofilms formed were therefore quantified by crystal violet staining on day 12 for the 25°C cultures and day 4 for the 37°C and 42°C cultures. The results are presented in Fig. 1. Statistical analysis of the data indicated that at 37°C and 42°C on glass and PS (Fig. 1A and B) and at 25°C on PP (Fig. 1C), L. pneumophila strains have significantly greater biofilm-forming capacity than all other Legionella spp.
FIG. 1.
Quantification of biofilms (as described in Materials and Methods) formed by Legionella spp. on surfaces of glass (A), polystyrene (B), and polypropylene (C) at incubation temperatures of 25°C (black bars), 37°C (white bars), and 42°C (gray bars). Strains or species are indicated as strain numbers and presented in Table 1. Values are presented as a means ± standard deviation (SD) of three to six independent experiments. Statistical analysis was performed using Student's t test to compare the differences between groups, and P values of <0.05 were considered statistically significant.
Quantity, speed of formation, and adherence stability of L. pneumophila biofilms.
In the above data, the quantity of the biofilms formed by L. pneumophila strains (Fig. 1, strains 1 to 13) was observed to be influenced by temperature. On glass and PS surfaces (Fig. 1A and B), biofilms were generally formed more extensively at the higher temperatures than at 25°C: biofilms at 37°C were two- to sevenfold higher (glass) and two- to fourfold higher (PS) in yield and at 42°C were three- to fivefold higher (glass) and two- to fivefold higher (PS) in yield. On PP surfaces, the situation was reversed: at 25°C, 2- to 7-fold and 3- to 16-fold more biofilms were formed than at 37°C and 42°C, respectively.
The temperature dependence could also be observed in the speed of formation and the adherence stability of the biofilms of L. pneumophila strains. In three to five independent experiments looking at biofilm formation by the 13 L. pneumophila strains in glass test tubes, we documented (i) the number of days it took for the appearance of stable biofilms, as an indicator of the speed of biofilm formation; and (ii) the day at which the biofilms were observed to naturally detach from the walls of the test tubes, as an indicator of the adherence stability. Statistical analysis revealed that all L. pneumophila strains showed similar trends: at 25°C, biofilms were formed more slowly (12 ± 1 days) but remained stably attached throughout the 30-day period of observation. At 37°C and 42°C, biofilms were formed faster (3 ± 1 days), but adherence stability was lower (7 ± 1 days). Similar trends in the speed of biofilm formation were observed when biofilms were grown on PS or PP, but the difference in adherence stability was not as apparent (data not shown).
Thickness and structure of L. pneumophila biofilms.
The temperature dependence may have biological significance related to possible survival advantages of L. pneumophila in man-made environments over non-L. pneumophila species. To look further into this, L. pneumophila strain Knoxville-1 was chosen as a representative of the 13 L. pneumophila strains for in-depth structural observation. We introduced pPZ1, which allows constitutive expression of the GFP into Knoxville-1 strain for visualization of its biofilms in the natural hydrated state by CLSM. The GFP-expressing Knoxville-1 strain showed biofilm formation properties at the three temperatures, similar to those of the wild-type Knoxville-1 strain (data not shown), indicating that the expression of GFP does not observably affect the gross phenomenon under study.
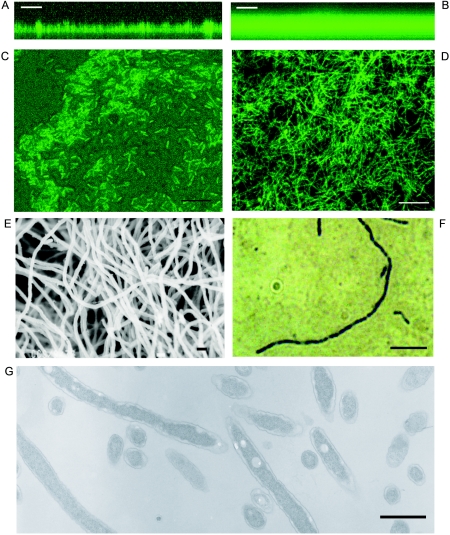
Biofilms were allowed to form on glass slides immersed in BYE broth and then observed directly under CLSM on the indicated days (chosen to arbitrarily represent an early and a mature stage of biofilm formation) to quantify the thickness (Table 2). Biofilms at 37°C and 42°C were found to be indeed thicker than that at 25°C by about twofold. In terms of structures, biofilms at 25°C (Fig. 2A) possessed features typical of biofilms reported to date, i.e., pillar- and mushroom-like structures and what seemed like water channels within (15). Biofilms at 37°C, however, showed an even and extensive mat of considerably greater cell density without the commonly observed water channel structures (Fig. 2B).
TABLE 2.
Thickness of GFP-expressing L. pneumophila Knoxville-1 biofilm
| Temperature (°C) | Day | Mean thicknessa (μm) |
|---|---|---|
| 25 | 9 | 20.4 ± 6.3 |
| 25 | 18 | 42.3 ± 10.9 |
| 37 | 3 | 37.5 ± 2.8 |
| 37 | 6 | 71.6 ± 4.4 |
| 42 | 3 | 40.3 ± 2.2 |
| 42 | 6 | 73.2 ± 3.5 |
Values were obtained as means ± SD of six to eight measurements of thickness. Statistical analysis was performed using Student's t test to compare the differences between groups, and P values of <0.05 were considered statistically significant.
FIG. 2.
Structure of L. pneumophila biofilms. The L. pneumophila Knoxville-1 strain expressing GFP was observed under CSLM (A to D). x-z plane projection of 25°C biofilm at day 18 (A) and 37°C biofilm at day 6 (B); x-y plane projection of 25°C biofilm at day 8 (C) and 37°C biofilm at day 4 (D). The L. pneumophila Knoxville-1 biofilm at 37°C on day 4 was observed under scanning electron microscope (E), and its filamentous cell stained with HCl-Giemsa was observed under the light microscope (F). (G) A section of the filamentous cells of L. pneumophila Philadelphia-1 strain was observed under the transmission electron microscope. Bars, 50 μm (A and B), 10 μm (C and D), 1 μm (E), 5 μm (F), and 1 μm (G). Images were processed and compiled with Adobe Photoshop 7.0 software.
Morphology of L. pneumophila cells in biofilms.
It was apparent that L. pneumophila biofilms at 37°C did not have the typical biofilm features (16) shown by most bacteria. At higher magnifications, cells within biofilm at 25°C showed the normal morphology of L. pneumophila, i.e., they were rod shaped (Fig. 2C). In contrast, the morphology of cells in biofilm at 37°C was filamentous (Fig. 2D). The meshwork of filaments resembled the mycelia of fungi; hence, we tentatively refer to it as a “mycelial mat-like biofilm.” Filamentation of bacterial cells is sometimes associated with physiological abnormalities such as mutations or overexpression of certain proteins (8, 13). To check if the filamentation was an artifact due to gfp overexpression, we collected the biofilm formed by the wild-type Knoxville-1 strain at 37°C and prepared it for observation under the SEM. The electron micrograph (Fig. 2E) showed a filamentous meshwork similar to that seen by CSLM, ruling out the possibility of artifacts. Hence, the morphology of L. pneumophila cells in the biofilms formed on glass (and likewise on PS and PP surfaces) (data not shown) appears to be regulated by temperature.
Distribution of rod-shaped and filamentous cells under different growth conditions.
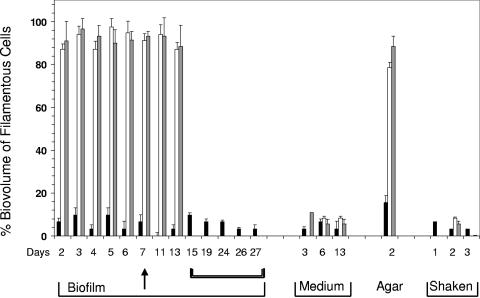
The distribution of the morphological forms under differing growth conditions was next examined. We systematically assessed the relative contribution of filamentous cells to the total biovolume (i) within biofilms grown on glass, (ii) in the surrounding media, (iii) in colonies grown on agar, and (iv) in shaken cultures. At 25°C (Fig. 3, black bars), the static culture showed a consistent dominance by rod-shaped cells, both within the biofilm and outside (i.e., in the medium), throughout the period of observation. At 37°C and 42°C (Fig. 3, white and gray bars) the biofilms showed that close to 90% of the total biovolume consisted of filamentous cells as early as day 2 and continued to be dominated by the filamentous form until day 7. Upon detachment from the vessel walls (day 11 and day 13), these detached biofilms retained their mycelial characteristics. However, within the surrounding media of biofilms at 37°C and 42°C, rod-shaped cells were in the majority. Shaken cultures were predominantly rod shaped; it should be noted that at 37°C and 42°C, predominance of rod-shaped cells was maintained even up to day 3. Agar plate culture, on the other hand, showed a profile for distribution of forms similar to that observed in biofilms at the three temperatures.
FIG. 3.
Percentage of biovolumes represented by filamentous cells within various L. pneumophila Knoxville-1 cultures. Black bars represent growth at 25°C, white bars represent growth at 37°C, and gray bars represent growth at 42°C. The arrow indicates the point at which detachment of biofilms at 37°C and 42°C occurred. Double brackets indicate days when only 25°C biofilms were assessed. Values are presented as means ± SD of triplicate data. Statistical analysis was performed using Student's t test to compare the differences between groups, and P values of <0.05 were considered statistically significant.
Filamentous cells are multinucleate and nonseptate.
The filamentous cells in L. pneumophila's biofilm were stained for their nucleoids and found to be clearly multinucleate (Fig. 2F). This indicates that the filamentous form within the biofilm is one whereby DNA replication has proceeded in the absence of cell division. Indeed, observation by TEM showed the total lack of septa within the filamentous cells (Fig. 2G).
Temperature-regulated filamentation in L. pneumophila is not RecA dependent.
The filaments formed as a result of nonseptation, observed with L. pneumophila biofilms when cultured at higher temperatures, is reminiscent of the phenotype of the thermosensitive recA mutants of Escherichia coli. RecA in E. coli is a regulator involved in the SOS response induced by DNA damage, and an abnormality in this regulator has been reported to result in filamentation at the elevated temperature of 40°C (10, 28). This is due to the control of RecA on factors involved in cell division, e.g., FtsZ (39) and SulA (20), both of which require well-regulated expression levels to ensure normal septation. A RecA homologue in L. pneumophila has been found to be able to functionally complement the E. coli counterpart and appears to be similarly regulated (17). The possible involvement of RecA in temperature-dependent filamentation by L. pneumophila cells was checked. An insertional null mutant of recA was generated in strain JR32, a L. pneumophila Philadelphia-1 derivative amenable to genetic manipulation (56) and observed to be able to form biofilms in a manner similar to that of the wild type, showing temperature-regulated morphological forms. Thus, the recA gene in L. pneumophila appears not to be responsible for the temperature-regulated filamentation phenomenon.
Growth of cells from mycelial biofilm.
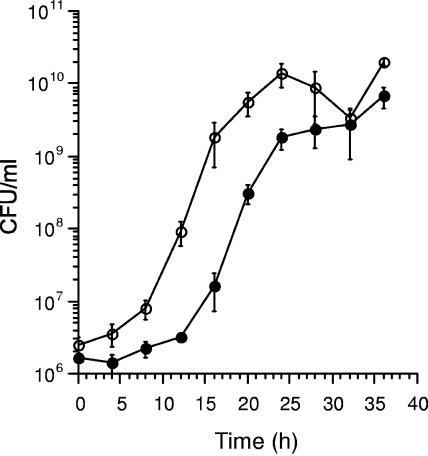
The fact that the filamentous form of L. pneumophila is multinucleate but nonseptate suggests that, given favorable conditions, the formation of septa may quickly give rise to more daughter cells than rod-shaped cells, since multiple DNA replication could be assumed to have already proceeded to completion during the filamentous state. To test this hypothesis, we mechanically “unraveled” a mature mycelial biofilm from a 37°C day 4 culture of Knoxville-1 strain by extensive shaking in a suitable buffer. We then followed the growth of its cells in fresh BYE medium shaken at 37°C over 36 h and compared it to that of the rod-shaped cells, seeded from a 30-h shaken culture (Fig. 4). The assumption is that one filamentous cell before septation will appear as one CFU. The rod-shaped cell culture continued in its lag phase until 12 h, but the culture from mycelial biofilm entered exponential phase by 8 h and mostly converted to rod-shaped cells. This shortened lag phase was remarkable, considering that cells from the biofilm were 4 days old and therefore expected to have an even longer lag phase than cells from a 30-h-old shaken culture. The growth rate (gradient at exponential phase) for the mycelial mat-like culture was not significantly higher than that for the rod-shaped cell culture during their respective exponential phases. Hence, the filamentous form of L. pneumophila seems to allow the organism to proliferate particularly rapidly in the initial stage, more than does the normal rod-shaped form.
FIG. 4.
Growth of L. pneumophila Knoxville-1 in BYE. The culture was shaken at 37°C and seeded from filamentous cells of a 37°C biofilm at day 4 (open circles) and rod-shaped planktonic cells of a 37°C culture shaken for 30 h (black circles). Data are means ± SD from three independent experiments.
DISCUSSION
The ability to form biofilms provide a bacterium with survival advantages in the environment, e.g., anchorage at a location where growth is favorable, protection from desiccation, and resistance to biocides and detergents (15, 16, 18). Under the conditions used in this study, the 13 strains of L. pneumophila tested showed enhanced biofilm formation compared to other non-L. pneumophila strains: at 37°C and 42°C on glass and PS surfaces (Fig. 1A and B) and at 25°C on PP surfaces (Fig. 1C). To some extent, this may be due to the less-robust growth of a few non-L. pneumophila strains at specific temperatures (Table 1, footnotes e to g) in a medium optimized for cultivation of L. pneumophila, but these are minor exceptions. Within the genus Legionella, L. pneumophila is the species most often associated with human clinical cases (22). One speculation is that it is better able to survive in man-made environments than non-L. pneumophila species and hence has a greater chance of crossing paths with the human population. Our finding that L. pneumophila is more proficient at biofilm formation than other Legionella species supports this speculation. Since the temperatures 37°C and 42°C are often encountered in man-made aquatic environments, e.g., air-conditioning cooling towers in the summer and hot spring spas, the enhanced ability by L. pneumophila to form biofilms on glass, PS, and possibly other surfaces would likely increase its chance of association with humans. On the other hand, the fact that biofilm formation is favored on PP at 25°C also suggests that the competitive edge conferred may be dependent on a combination of the temperature and the type of attachment surfaces.
It is interesting to note that the properties of L. pneumophila biofilms formed at 25°C differ considerably from those formed at 37°C and 42°C. At higher temperatures, the biofilms are formed faster, thicker, and spread wider. Most interestingly, at higher temperatures, they are mycelial mat like in structure. Bacterial biofilms reported to date commonly show structures of polymeric matrices interspersed with water channels (32). For rod-shaped bacteria, e.g., Pseudomonas spp. and Vibrio cholerae, cells within biofilms remain rod shaped (60), with only a rare appearance of filaments (38). In rivers and on riverbeds, mixed-community biofilms may have filamentous components (bacteria or fungi) among rods and cocci (35), but biofilms composed primarily of extensive meshwork of filaments, to our knowledge, have only been reported for Thiothrix spp. in cases of biofouling (6) and Methanosaeta spp. in anaerobic sludge granules (29). Furthermore, although filamentation in rod-shaped bacteria with physiological abnormalities has been reported (13, 28, 48, 66), temperature-regulated filamentation in a biofilm context is highly unusual.
One question that may arise with this novel form of biofilm is, can we truly consider it a “biofilm”? The definition given by Donlan and Costerton (16) states that a biofilm is “a microbially derived sessile community characterized by cells that are irreversibly attached to a substratum or interface or to each other, are embedded in a matrix of extracellular polymeric substances that they have produced, and exhibit an altered phenotype with respect to growth rate and gene transcription.” For our mycelial mat-like structure, the criteria of attachment is certainly satisfied; furthermore, a thin layer of ruthenium red-stainable substance (possibly exopolysaccharide) (24) coats the filaments (data not shown). The filamentous cells have a growth profile (Fig. 4) that indicates an altered phenotype from the planktonic, rod-shaped form and are expected to have considerable modification in gene expression compared to expression in a binary fission mode. Our current analysis of the transcriptional profiles of the biofilm filamentous versus planktonic L. pneumophila cells also substantiates this point (Z. Piao, unpublished data). Thus, all the criteria seem to have been met for the mycelial mat-like structure to be referred to as a biofilm.
L. pneumophila have long been known to be pleomorphic (31), varying its morphology with physiological conditions (36, 43) or infectious cycle phases (61). Species of Legionella have also been reported to form varied microcolonies within Vero cells, some appearing filamentous, although the septation status has not been addressed (44). L. pneumophila also “differentiates” into a few extracellular or intracellular forms with varying degrees of infectivity for HeLa cells (25), which can be distinguished by the ultrastructural properties of their cell envelopes (21). In our study, the electron micrograph of the filamentous L. pneumophila cell (Fig. 2G) shows cell wall structure that is consistent with that of the extracellular stationary phase rod forms reported by Faulkner et al. (21), confirming its “extracellular” status.
Extensive filamentation by L. pneumophila has previously been described in conditions of physiological stress such as exposure to antibiotics (19, 59) and nutrient limitation (63). In other rod-shaped bacteria, filamentation by mutants of global regulator (28) or cell division genes (13, 48) and when exposed to high salt concentrations (66) has also been reported. We considered, by analogy with the phenotype of an E. coli mutant (10), that our filamentation phenomenon could be due to the regulatory action of the recA gene. The thermosensitive mutation recA441 (formerly tif-1) of E. coli is known to convert cells to multinucleate, aseptate filaments at 40°C through the constitutive expression of the sulA gene that encodes an inhibitor of the cell division protein FtsZ (34). However, this possibility was ruled out for L. pneumophila because disruption of the recA homologue was found to be without effect on the phenotype. Another immediate possibility, suggested by analogy with E. coli ftsZ mutants (28, 47), is that L. pneumophila FtsZ protein might be thermosensitive. We have not yet been successful in substantiating this idea, but we intend to continue to dissect the mechanism of regulation in this novel form of biofilm. The recently completed L. pneumophila genome sequences of strains Philadelphia-1 (12) and Paris (11) (http://genome3.cpmc.columbia.edu/∼legion/seq_anno.html) indicate that the organism possesses not only recA and ftsZ but also homologues of other E. coli genes, e.g., ftsA and mreB, involved in cell division and maintenance of cell morphology (48). We are currently looking into how these genes are regulated and correlated with the biofilm phenotype of L. pneumophila.
Filamentation is often a consequence of some stress-induced disruption of normal cell division functions. However, in rare cases, it serves as a strategy to counter specific types of stress. Elongation of cells by nutrient-deprived Pseudomonas aeruginosa has been proposed to be a strategic response to enlarge their nutrient collection surface without substantially changing the surface area-to-volume ratio (60). Temperature-regulated filamentation in L. pneumophila biofilm is unusual in that it is clearly not a simple case of cell division inhibition by sublethal heat stress, e.g., as seen with Listeria monocytogenes (53). Induction of filamentation at 37°C or 42°C was not observed for L. pneumophila cells outside of the biofilms or in a shaken liquid medium. Instead, only surface-associated cells, i.e., within biofilm or on agar, exhibited filamentation (Fig. 3). Some other signal(s) besides temperature, e.g., attachment to surfaces, is possibly involved, and it is conceivable that filamentation is a strategic regulated response rather than an accidental by-product of stress. Our growth experiment showed that cell proliferation of the biofilm filamentous form is faster at the initial stage than the planktonic rod-shaped form (Fig. 4). During this initial stage, the multinucleate filamentous cells have converted into rod-shaped forms, after which no difference in growth rate could be observed. The filament-turned-rod-shaped cells therefore do not differ in terms of growth rate from the normal rod-shaped cells. Presumably, the time and energy required by a filamentous cell to increase in number involve only the formation of cell septa, whereas binary fission involves DNA replication and biomass generation, in addition to septa formation. Hence, filamentation may represent a novel strategy utilized by L. pneumophila to rapidly increase its population.
It is worth noting that the experimental system of our study made use of a rich medium. In low-nutrient medium, very little formation of biofilm could be observed (C. C. Sze, unpublished data). In the environment, the bacterium more often encounters low-nutrient status, so one may wonder at the relevance of this particular study. However, it should not be forgotten that the environment is dynamic and that bacteria generally experience cycles of “feast and famine.” The survival of a bacterium, therefore, not only involves living through a famine but also hinges on how well it exploits the rare but valuable encounter of a feast. L. pneumophila is a slow grower compared to other bacteria such as E. coli, P. aeruginosa, and V. cholerae and may seem to be in danger of losing out to these fast growers in nature. It has, in part, made up for its shortcomings by finding a niche as an intracellular parasite of free-living protozoans (4, 41, 54, 55). By forming mycelial mat-like biofilms, L. pneumophila may have hit upon another strategy to compete with fast growers in the environment, which allows it to (i) anchor itself at a location transiently flooded with nutrients, (ii) increase rapidly in biomass while able to access the nutrients by eliminating the process of septum formation, and then (iii) proliferate and disperse in great numbers when conditions are appropriate. We intend to explore this phenomenon of filamentous biofilms further, from both genetic and ecological points of view.
Acknowledgments
We thank H. Nakayama for valuable advice, H. Kajiwara and A. Takade for technical assistance, and V. Shingler and S. Molin for their generous gifts of plasmids.
C.C.S. was supported by grant 13-01132 from the Japan Society for Promotion of Science. This work was supported by grant 14370094 from the Ministry of Education, Science, Culture and Sports of Japan and grant H14-047 from the Ministry of Health, Labor and Welfare of Japan.
REFERENCES
- 1.Alli, O. A., S. Zink, N. K. von Lackum, and Y. Abu-Kwaik. 2003. Comparative assessment of virulence traits in Legionella spp. Microbiology 149:631-641. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, J. B., C. Sternberg, L. K. Poulsen, S. P. Bjorn, M. Givskov, and S. Molin. 1998. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl. Environ. Microbiol. 64:2240-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atlas, R. M. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283-293. [DOI] [PubMed] [Google Scholar]
- 4.Barker, J., and M. R. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. [DOI] [PubMed] [Google Scholar]
- 5.Benin, A. L., R. F. Benson, and R. E. Besser. 2002. Trends in Legionnaires disease, 1980-1998: declining mortality and new patterns of diagnosis. Clin. Infect. Dis. 35:1039-1046. [DOI] [PubMed] [Google Scholar]
- 6.Brigmon, R. L., H. W. Martin, and H. C. Aldrich. 1997. Biofouling of groundwater systems by Thiothrix spp. Curr. Microbiol. 35:169-174. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M. R., and J. Barker. 1999. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 7:46-50. [DOI] [PubMed] [Google Scholar]
- 8.Burnett, B. P., A. L. Horwich, and K. B. Low. 1994. A carboxy-terminal deletion impairs the assembly of GroEL and confers a pleiotropic phenotype in Escherichia coli K-12. J. Bacteriol. 176:6980-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cargill, K. L., B. H. Pyle, R. L. Sauer, and G. A. McFeters. 1992. Effects of culture conditions and biofilm formation on the iodine susceptibility of Legionella pneumophila. Can. J. Microbiol. 38:423-429. (Erratum, 38: 1089.) [DOI] [PubMed] [Google Scholar]
- 10.Castellazzi, M., J. George, and G. Buttin. 1972. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol. Gen. Genet. 119:139-152. [DOI] [PubMed] [Google Scholar]
- 11.Cazalet, C., C. Rusniok, H. Bruggemann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bouchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchrieser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36:1165-1173. [DOI] [PubMed] [Google Scholar]
- 12.Chien, M., I. Morozova, S. Shi, H. Sheng, J. Chen, S. M. Gomez, G. Asamani, K. Hill, J. Nuara, M. Feder, J. Rineer, J. J. Greenberg, V. Steshenko, S. H. Park, B. Zhao, E. Teplitskaya, J. R. Edwards, S. Pampou, A. Georghiou, I. C. Chou, W. Iannuccilli, M. E. Ulz, D. H. Kim, A. Geringer-Sameth, C. Goldsberry, P. Morozov, S. G. Fischer, G. Segal, X. Qu, A. Rzhetsky, P. Zhang, E. Cayanis, P. J. De Jong, J. Ju, S. Kalachikov, H. A. Shuman, and J. J. Russo. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966-1968. [DOI] [PubMed] [Google Scholar]
- 13.de Leeuw, E., B. Graham, G. J. Phillips, C. M. ten Hagen-Jongman, B. Oudega, and J. Luirink. 1999. Molecular characterization of Escherichia coli FtsE and FtsX. Mol. Microbiol. 31:983-993. [DOI] [PubMed] [Google Scholar]
- 14.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donlan, R. M. 2002. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyfus, L. A. 1989. Molecular cloning and expression in Escherichia coli of the recA gene of Legionella pneumophila. J. Gen. Microbiol. 135:3097-3107. [DOI] [PubMed] [Google Scholar]
- 18.Dunne, W. M., Jr. 2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott, T. S., and F. G. Rodgers. 1985. Morphological response and growth characteristics of Legionella pneumophila exposed to ampicillin and erythromycin. J. Med. Microbiol. 19:383-390. [DOI] [PubMed] [Google Scholar]
- 20.Ennis, D. G., J. W. Little, and D. W. Mount. 1993. Novel mechanism for UV sensitivity and apparent UV nonmutability of recA432 mutants: persistent LexA cleavage following SOS induction. J. Bacteriol. 175:7373-7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faulkner, G., and R. A. Garduno. 2002. Ultrastructural analysis of differentiation in Legionella pneumophila. J. Bacteriol. 184:7025-7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flieger, A., S. Gong, M. Faigle, H. Northoff, and B. Neumeister. 2001. In vitro secretion kinetics of proteins from Legionella pneumophila in comparison to proteins from non-pneumophila species. Microbiology 147:3127-3134. [DOI] [PubMed] [Google Scholar]
- 24.Fulcher, T. P., J. K. Dart, L. McLaughlin-Borlace, R. Howes, M. Matheson, and I. Cree. 2001. Demonstration of biofilm in infectious crystalline keratopathy using ruthenium red and electron microscopy. Ophthalmology 108:1088-1092. [DOI] [PubMed] [Google Scholar]
- 25.Garduno, R. A., E. Garduno, M. Hiltz, and P. S. Hoffman. 2002. Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infect. Immun. 70:6273-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green, P. N. 1993. Efficacy of biocides on laboratory-generated Legionella biofilms. Lett. Appl. Microbiol. 17:158-161. [DOI] [PubMed] [Google Scholar]
- 27.Harb, O. S., L.-Y. Gao, and Y. Abu-Kwaik. 2000. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ. Microbiol. 2:251-265. [DOI] [PubMed] [Google Scholar]
- 28.Hirota, Y., A. Ryter, and F. Jacob. 1968. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb. Symp. Quant. Biol. 33:677-693. [DOI] [PubMed] [Google Scholar]
- 29.Hulshoff Pol, L. W., S. I. de Castro Lopes, G. Lettinga, and P. N. Lens. 2004. Anaerobic sludge granulation. Water Res. 38:1376-1389. [DOI] [PubMed] [Google Scholar]
- 30.Joshi, A. D., and M. S. Swanson. 1999. Comparative analysis of Legionella pneumophila and Legionella micdadei virulence traits. Infect. Immun. 67:4134-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz, S. M., S. Hashemi, K. R. Brown, W. A. Habib, and J. M. Hammel. 1984. Pleomorphism of Legionella pneumophila. Ultrastruct. Pathol. 6:117-129. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence, J. R., D. R. Korber, B. D. Hoyle, J. W. Costerton, and D. E. Caldwell. 1991. Optical sectioning of microbial biofilms. J. Bacteriol. 173:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luneberg, E., B. Mayer, N. Daryab, O. Kooistra, U. Zahringer, M. Rohde, J. Swanson, and M. Frosch. 2001. Chromosomal insertion and excision of a 30 kb unstable genetic element is responsible for phase variation of lipopolysaccharide and other virulence determinants in Legionella pneumophila. Mol. Microbiol. 39:1259-1271. [DOI] [PubMed] [Google Scholar]
- 34.Lutkenhaus, J. F. 1983. Coupling of DNA replication and cell division: sulB is an allele of ftsZ. J. Bacteriol. 154:1339-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manz, W., K. Wendt-Potthoff, T. R. Neu, U. Szewzyk, and J. R. Lawrence. 1999. Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb. Ecol. 37:225-237. [DOI] [PubMed] [Google Scholar]
- 36.Mauchline, W. S., R. Araujo, R. Wait, A. B. Dowsett, P. J. Dennis, and C. W. Keevil. 1992. Physiology and morphology of Legionella pneumophila in continuous culture at low oxygen concentration. J. Gen. Microbiol. 138:2371-2380. [DOI] [PubMed] [Google Scholar]
- 37.Miyamoto, H., M. Ogawa, K. Maruta, Y. Nikaido, C. Yamamoto, H. Taniguchi, and S. Yoshida. 1995. Temperature effects on Legionella pneumophila killing by and multiplication in phagocytes of guinea pigs. Microbiol. Immunol. 39:647-654. [DOI] [PubMed] [Google Scholar]
- 38.Mizunoe, Y., S. N. Wai, A. Takade, and S. I. Yoshida. 1999. Isolation and characterization of rugose form of Vibrio cholerae O139 strain MO10. Infect. Immun. 67:958-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee, A., and J. Lutkenhaus. 1998. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 17:462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murga, R., T. S. Forster, E. Brown, J. M. Pruckler, B. S. Fields, and R. M. Donlan. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121-3126. [DOI] [PubMed] [Google Scholar]
- 41.Nagington, J., and D. J. Smith. 1980. Pontiac fever and amoebae. Lancet ii:1241. [DOI] [PubMed] [Google Scholar]
- 42.Neumeister, B., G. Reiff, M. Faigle, K. Dietz, H. Northoff, and F. Lang. 2000. Influence of Acanthamoeba castellanii on intracellular growth of different Legionella species in human monocytes. Appl. Environ. Microbiol. 66:914-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowicki, M., N. Bornstein, J. C. Paucod, P. Binder, and J. Fleurette. 1987. Effect of culture medium on morphology and virulence of Legionella pneumophila serogroup 1. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 264:167-177. [DOI] [PubMed] [Google Scholar]
- 44.Ogawa, M., A. Takade, H. Miyamoto, H. Taniguchi, and S. Yoshida. 2001. Morphological variety of intracellular microcolonies of Legionella species in Vero cells. Microbiol. Immunol. 45:557-562. [DOI] [PubMed] [Google Scholar]
- 45.Ohno, A., N. Kato, K. Yamada, and K. Yamaguchi. 2003. Factors influencing survival of Legionella pneumophila serotype 1 in hot spring water and tap water. Appl. Environ. Microbiol. 69:2540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 47.Phoenix, P., and G. R. Drapeau. 1988. Cell division control in Escherichia coli K-12: some properties of the ftsZ84 mutation and suppression of this mutation by the product of a newly identified gene. J. Bacteriol. 170:4338-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rico, A. I., M. Garcia-Ovalle, J. Mingorance, and M. Vicente. 2004. Role of two essential domains of Escherichia coli FtsA in localization and progression of the division ring. Mol. Microbiol. 53:1359-1371. [DOI] [PubMed] [Google Scholar]
- 49.Ristroph, J. D., K. W. Hedlund, and R. G. Allen. 1980. Liquid medium for growth of Legionella pneumophila. J. Clin. Microbiol. 11:19-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinow, C. F. 1960. Morphology of bacterial spores, their development and germination, p. 207-248. In I. C. Gunsalus and R. Y. Stanier (ed.), The Bacteria, vol. 1. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 51.Rogers, J., A. B. Dowsett, P. J. Dennis, J. V. Lee, and C. W. Keevil. 1994. Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl. Environ. Microbiol. 60:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers, J., and C. W. Keevil. 1992. Immunogold and fluorescein immunolabelling of Legionella pneumophila within an aquatic biofilm visualized by using episcopic differential interference contrast microscopy. Appl. Environ. Microbiol. 58:2326-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rowan, N. J., J. G. Anderson, and A. A. Candlish. 2000. Cellular morphology of rough forms of Listeria monocytogenes isolated from clinical and food samples. Lett. Appl. Microbiol. 31:319-322. [DOI] [PubMed] [Google Scholar]
- 54.Rowbotham, T. J. 1986. Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22:678-689. [PubMed] [Google Scholar]
- 55.Rowbotham, T. J. 1981. Pontiac fever, amoebae, and legionellae. Lancet i:40-41. [DOI] [PubMed] [Google Scholar]
- 56.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 58.Shingler, V., and T. Moore. 1994. Sensing of aromatic compounds by the DmpR transcriptional activator of phenol-catabolizing Pseudomonas sp. strain CF600. J. Bacteriol. 176:1555-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smalley, D. L., P. A. Jaquess, D. D. Ourth, and J. S. Layne. 1980. Antibiotic-induced filament formation of Legionella pneumophila. Am. J. Clin. Pathol. 74:852. [DOI] [PubMed] [Google Scholar]
- 60.Steinberger, R. E., A. R. Allen, H. G. Hansa, and P. A. Holden. 2002. Elongation correlates with nutrient deprivation in Pseudomonas aeruginosa-unsaturated biofilms. Microb. Ecol. 43:416-423. [DOI] [PubMed] [Google Scholar]
- 61.Steinert, M., U. Hentschel, and J. Hacker. 2002. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 26:149-162. [DOI] [PubMed] [Google Scholar]
- 62.Walker, J. T., D. J. Bradshaw, A. M. Bennett, M. R. Fulford, M. V. Martin, and P. D. Marsh. 2000. Microbial biofilm formation and contamination of dental-unit water systems in general dental practice. Appl. Environ. Microbiol. 66:3363-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warren, W. J., and R. D. Miller. 1979. Growth of Legionnaires' disease bacterium (Legionella pneumophila) in chemically defined medium. J. Clin. Microbiol. 10:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright, J. B., I. Ruseska, M. A. Athar, S. Corbett, and J. W. Costerton. 1989. Legionella pneumophila grows adherent to surfaces in vitro and in situ. Infect. Control Hosp. Epidemiol. 10:408-415. [DOI] [PubMed] [Google Scholar]
- 65.Wright, J. B., I. Ruseska, and J. W. Costerton. 1991. Decreased biocide susceptibility of adherent Legionella pneumophila. J. Appl. Bacteriol. 71:531-538. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida, S., T. Udou, Y. Mizuguchi, and T. Tanabe. 1986. Salt-induced filamentous growth of a Salmonella strain isolated from blood. J. Clin. Microbiol. 23:192-194. [DOI] [PMC free article] [PubMed] [Google Scholar]