Abstract
Polaromonas naphthalenivorans CJ2, found to be responsible for the degradation of naphthalene in situ at a coal tar waste-contaminated site (C.-O. Jeon et al., Proc. Natl. Acad. Sci. USA 100:13591-13596, 2003), is able to grow on mineral salts agar media with naphthalene as the sole carbon source. Beginning from a 484-bp nagAc-like region, we used a genome walking strategy to sequence genes encoding the entire naphthalene degradation pathway andadditional flanking regions. We found that the naphthalene catabolic genes in P. naphthalenivorans CJ2 were divided into one large and one small gene cluster, separated by an unknown distance. The large gene cluster (nagRAaGHAbAcAdBFCQEDJI′ORF1tnpA) is bounded by a LysR-type regulator (nagR). The small cluster (nagR2ORF2I"KL) is bounded by a MarR-type regulator (nagR2). The catabolic genes of P. naphthalenivorans CJ2 were homologous to many of those of Ralstonia U2, which uses the gentisate pathway to convert naphthalene to central metabolites. However, three open reading frames (nagY, nagM, and nagN), present in Ralstonia U2, were absent. Also, P. naphthalenivorans carries two copies of gentisate dioxygenase (nagI) with 77.4% DNA sequence identity to one another and 82% amino acid identity to their homologue in Ralstonia sp. strain U2. Investigation of the operons using reverse transcription PCR showed that each cluster was controlled independently by its respective promoter. Insertional inactivation and lacZ reporter assays showed that nagR2 is a negative regulator and that expression of the small cluster is not induced by naphthalene, salicylate, or gentisate. Association of two putative Azoarcus-related transposases with the large cluster and one Azoarcus-related putative salicylate 5-hydroxylase gene (ORF2) in the small cluster suggests that mobile genetic elements were likely involved in creating the novel arrangement of catabolic and regulatory genes in P. naphthalenivorans.
The degradation of naphthalene has been studied extensively in two Pseudomonas species that carry the archetypal catabolic plasmids, NAH7 (in P. putida G7) and pDTG1 (in P. putida NCIB9816-4) (6, 11, 41). In both strains, the nah dissimilatory genes are organized into two operons: one coding for the enzymes involved in the conversion of naphthalene to salicylate (naphthalene degradation upper pathway) and another coding for the conversion of salicylate to pyruvate and acetyl coenzyme A via meta-cleavage (naphthalene degradation lower pathway) (2, 42, 42, 54). In contrast to the nah naphthalene catabolic pathway in Pseudomonas species, the nag genes of Ralstonia sp. strain U2 encode the alternative gentisate pathway, which converts naphthalene to fumarate and pyruvate via salicylate and gentisate (14). The nag genes are organized in a single operon (56, 57). Despite the contrasts in structural genes and their arrangements, the nah and nag systems exhibit strikingly similar regulation. Transcription of nag and both nah operons is controlled by a single Lys-R-type regulatory protein (NagR and NahR, respectively). These proteins act as positive regulators for both pathways, and salicylate functions as an inducer (23, 34, 37, 39).
Detailed genetic and/or biochemical information on naphthalene metabolism have been obtained via studies of many bacteria: e.g., Burkholderia sp. strain DNT (45), Burkholderia sp. strain RP007 (27), Comamonas testosteroni GZ39 (16), Comamonas sp. strain JS765 (29), Cycloclasticus sp. strain A5 (25), and Sphingomonas sp. strain CHY-1 (8). As this information accrues, we have the opportunity to gain understanding about how these catabolic operons evolve. For instance, a recent study by Kulakov et al. (26) has shown that genetic rearrangements of nar genes have been important in the evolution of naphthalene metabolism in Rhodococcus. In the present study, we examine Polaromonas naphthalenivorans CJ2, a bacterium found to be responsible for the field biodegradation of naphthalene at a coal tar waste-contaminated site (21, 22). Here we report DNA sequences showing that the nag genes of P. naphthalenivorans CJ2 are similar to those of Ralstonia sp. strain U2, but they are divided into two clusters and exhibit one duplication and several deletions. Furthermore, regulatory control of the two clusters from strain CJ2 isnovel.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth condition.
All bacterial strains, vectors and plasmids used in the present study are listed in Table 1. P. naphthalenivorans CJ2 was grown at 20°C and maintained on mineral salts basal medium (MSB) (43) with either naphthalene vapor or a 0.5% (wt/vol) suspension of crystals (MSB-N) or 0.2% (wt/vol) pyruvate (MSB-P) as the sole carbon source. Other culture media were prepared according to the procedures described previously (20, 44). All Escherichia coli strains were grown at 37°C in Luria-Bertani (LB) medium in a shaking incubator (100 rpm). When required, the appropriate antibiotics and reagents were added to the media: X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside; 30 μg/ml), kanamycin (40 μg/ml), and salicylate (2.5 mM). Growth was monitored by measuring the optical density of the cultures at a wavelength of 600 nm after naphthalene crystals had settled. Spontaneous rifampin-resistant P. naphthalenivorans CJ2 was selected on R2A plates containing a rifampin gradient (0 to 200 μg/ml) (12, 44).
TABLE 1.
Bacterial strains, plasmids, and PCR primers used in this studya
| Name of strain, plasmid, or primer | Description or sequence | Length (bp) | Source or reference(s) |
|---|---|---|---|
| Strains | |||
| P. naphthalenivorans CJ2 | Naphthalene degrader | 21, 22 | |
| Strain CJN110 | P. naphthalenovorans CJ2 ΔnagR::kan LacZ+ | This study | |
| Strain CJM110 | P. naphthalenovorans CJ2 ΔR2::kan LacZ+ | This study | |
| Strain CJM112 | P. naphthalenovorans CJ2 R2-ORF2::LacZ+ fusion | This study | |
| E. coli SY327 λpir | λpir Δ(lac pro) argE(Am) recA56 nalA Rif(λpir); carries π protein for R6Kγ ori | 24 | |
| E. coli S17-1 λpir | trp SmrrecA thi pro hsdM+ RP4-2-Tc::Mu::Km Tn7 λpir; hsdR mutant | 24 | |
| INV-αF′ | Cloning host, F′ endA1 recA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA96 relA1 φ80lacZΔM15 Δ(lacZYA-argF)U169 λ− | Invitrogen | |
| Plasmids or vector | |||
| pVIK110 | lacZY for translational fusions, R6KoriV, suicide vector; Kmr | 24 | |
| pCJN110 | Internal nagR fragment (derived from pVIK112); Kmr | This study | |
| pCJM110 | Internal R2 fragment (derived from pVIK112); Kmr | This study | |
| pVIK112 | lacZY for transcriptional fusions, R6KoriV, suicide vector; Kmr | 24 | |
| pCJM112 | R2-ORF2::LacZ+ fusion (derived from pVIK112); Kmr | This study | |
| pCR2.1-TOPOa | Kmr Ampr; cloning vector | Invitrogen | |
| PCR primers | |||
| RT1-F | 5′-ACATCCTGGGCTTCTACGCC-3′ | 20 | |
| RT2-F | 5′-AATGGCCTCGTCCTACTACA-3′ | 20 | |
| RT2-R | 5′-CAGATCGATGCCTACTGTTG-3′ | 20 | |
| RT3-F | 5′-CTGGAGTCGCAGATTGTGAA-3′ | 20 | |
| RT3-R | 5′-ATTGCTCCCTGCTTTGTTCT-3′ | 20 | |
| RT4-F | 5′-CTGTTGGTGCAGGCTGTTAC-3′ | 20 | |
| RT4-R | 5′-GGCTTTGGCTTGGGCATTGA-3′ | 20 | |
| RT5-F | 5′-GCTTGTTCAGCAGTTCCATG-3′ | 20 | |
| RT5-R | 5′-ACATTGACCAGATGCCTGGA-3′ | 20 | |
| RT6-F | 5′-GCTGCTGTCGCTGACGAAC-3′ | 19 | |
| RT6-R | 5′-TCACATTGACCAGATGCCTG-3′ | 20 | |
| RT7-F | 5′-TCAGTTCCTGGACCCCTTCC-3′ | 20 | |
| RT7-R | 5′-GTCGAGGTCAGGAATTCGAC-3′ | 20 | |
| RT8-F | 5′-AACTGCCACCTGGTGCACTA-3′ | 20 | |
| RT8-R | 5′-CAGCACATCGAGCCAGATCA-3′ | 20 | |
| RT9-F | 5′-GATGGTGTTCCATGTGATCG-3′ | 20 | |
| RT9-R | 5′-TGGCAACCACCAGCTCCATT-3′ | 20 | |
| RT10-F | 5′-TGGGACTTGGGCAAGGACGT-3′ | 20 | |
| RT10-R | 5′-CATCTGCGGGTTGACGGCTT-3′ | 20 | |
| RT11-F | 5′-ACACCAGCGCTGTTGCCAGG-3′ | 20 | |
| RT11-R | 5′-TGATGGTCTCGAAAGCGAAG-3′ | 20 | |
| RT12-F | 5′-GTGTCCCAGCTCTTCACCTC-3′ | 20 | |
| RT12-R | 5′-GAGCAGTCGAAAACAGACGT-3′ | 20 | |
| RT13-F | 5′-CCCAGCGCATGCTGCACTTC-3′ | 20 | |
| RT13-R | 5′-GTGAGCTTGTTCAGATCGAA-3′ | 20 | |
| nrc-F | 5′-CCCTCTAGAGATCAAGCAAGCCATCCACT-3′ | 29 | |
| nrc-R | 5′-CCCGTCGACCATTCACCACACGCAACTCT-3′ | 29 | |
| mrc-F | 5′-CCCTCTAGACTGAGTTCGTTGTTCGTGCT-3′ | 29 | |
| mrc-R | 5′-CCCGTCGACAGTACGCCATCCTCCAGT-3′ | 27 | |
| sc-F | 5′-CCGGAATTCGCATGTGGTGCGGATTGTCG-3′ | 29 | |
| sc-R | 5′-CGCTCTAGACCAGCACCTTGACGGAAA-3′ | 27 | |
| onrc-F | 5′-AGTGGCGATGAGATCGGTAG-3′ | 20 | |
| omrc-F | 5′-GGAGCGGGAGTGAAGGCAG-3′ | 19 | |
| lacZ-R | 5′-CGCCAAGACTGTTACCCATC-3′ | 20 | |
| nahAc114-F | 5′-CTGGC(T/A)(T/A)TT(T/C)CTCAC(T/C)CAT-3′ | 19 | |
| nahAc595-R | 5′-TC(C/G)GC(G/A)GGTG(T/C)CTTCCAGTTG-3′ | 21 |
Nomenclature: o, outer; F, forward; R, reverse; pro, promoter. Restriction enzyme sites (for future study) are underlined. Kmr, kanamycin resistance; Ampr, ampicillin resistance.
Plasmid characterization and Southern hybridization.
Large plasmids were isolated from P. naphthalenivorans CJ2, Pseudomonas putida NCIB 9816-4, and Pseudomonas putida G7 according to a method described previously (44). Plasmid DNAs were separated on a 0.7% agarose gel in Tris-acetate-EDTA. The voltage was 30 V until the dye entered the gel, and then it was increased to 60 V. For Southern hybridizations of the isolated plasmid, nahAc probes were generated via PCR from strains CJ2, NCIB 9816-4, and G7 using nahAc-based degenerate primers (Table 1) as previously described (52). The probes were mixed 1:1:1. The primers were 5′ end labeled using [γ-32P]dATP and a T4 polynucleotide kinase (Invitrogen). Southern hybridizations were carried out according to the instructions of standard protocol (36). To seek evidence for a naphthalene-degrading plasmid, curing assays were performed by periodically subculturing the bacterium in the absence of naphthalene (on R2A medium [44]), by exposing growing cultures to ethidium bromide (7), and by cultivation on 1-chloronaphthalene (49). Genomic DNA isolation, digestion with restriction enzymes, electrophoresis, transfer onto nylon membranes, and Southern hybridizations were done according to standard protocols (36).
Nucleotide sequence determination and sequence analysis.
Nucleotide sequences were determined by the “genome walking” strategy using GenomeWalker Kit (Clontech) from a conserved, PCR-amplifiable (484 bp) region of the naphthalene dioxygenase (nahAc) gene as the starting point. Our first effort at genome walking allowed sequencing to proceed for approximately 25 kb, and the similarity to the operon of Ralstonia sp. strain U2 was noted. For unknown reasons, extending the sequence beyond nagI was unsuccessful. Then, using primers designed from the naphthalene operon of Ralstonia sp. strain U2, we found that P. naphthalenivorans CJ2 carried another copy of gentisate dioxygenase (nagI). Sequences of genes adjacent to both copies were obtained and were analyzed with the Lasergene software package (DNASTAR). BLASTX was used for the deduced amino acid identity search, and BLASTN was used for the nucleotide identity search (1). Transcription promoters and termination sequences of the nag gene clusters were analyzed by using web-based programs (http://www.softberry.com/; http://www.fruitfly.org/seq.tools/promoters.html.
RT-PCR.
Cells were grown on MSB-N agar plates. Total RNA was prepared from colonies by using RNeasy minicolumns (QIAGEN). The RNA was treated to remove any genomic DNA contamination by incubation with 1 U of RNase-free DNase I (Promega) and 1 U of RNasin (Promega) in 40 mM Tris-HCl (pH 7.9) containing 10 mM NaCl, 10 mM CaCl2, and 6 mM MgSO4 for 30 min at 37°C. The RNA preparation was cleaned by passage through an RNase minicolumn. Next, a reverse transcriptase-PCR (RT-PCR) reaction was carried out by using SuperScript II RT (Invitrogen/Life Technologies, Carlsbad, Calif.). The intergenic regions between the nag genes were amplified by using primer pairs of RT1-RT13 (Table 1). To confirm that cDNA synthesis occurred and that RNA preparation was free of genomic DNA, a negative control RT-PCR was performed with Taq polymerase (omitting the RT).
Insertional inactivation of nagR and nagR2 by homologous recombination.
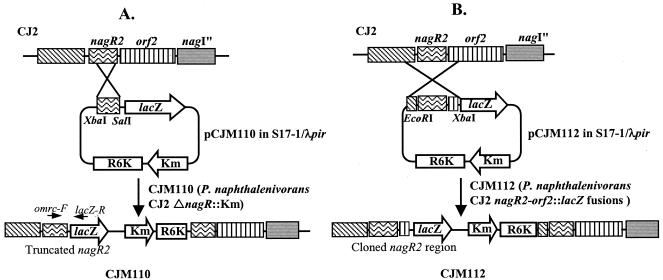
Strains with mutations in regulatory genes (nagR and nagR2) were prepared by using the suicide translational fusion vector, pVIK110 (containing the R6K oriV region so that it cannot replicate in the absence of the λpir replication system of E. coli SY327 λpir; Table 1). Campbell-type homologous recombination with nagR and nagR2 was achieved by PCR amplification of 396 and 322 bp, respectively, internal to nagR and nagR2. The primer pairs NRC-F/NRC-R and MRC-F/MRC-R) (Table 1) generated fragments with XbaI and SalI cohesive ends that were subcloned into the XbaI-SalI cloning site of pVIK110, creating plasmids pCJN110 and pCJM110, respectively. The pCJN110 and pCJM110 plasmids were introduced by electroporation into E. coli S17-1 λpir that has the tra region of RP4. Then, pCJN110 and pCJM110 were conjugated into rifampin-resistant P. naphthalenivorans CJ2 by mating on R2A agar media at 25°C for 16 h, as previously described (35). The transconjugants (strain CJN110 [P. naphthalenivorans CJ2 ΔnagR::kan] and strain CJM110 [P. naphthalenivorans CJ2 ΔR2::kan]) were selected on R2A plates containing kanamycin (40 μg/ml) and rifampin (200 μg/ml) at 20°C. Confirmation of the transconjugants was conducted by using PCR. Figure 1A shows the schematic diagram for creating strain CJM110 via homologous recombination.
FIG. 1.
Construction of mutants CJM110 and CJM112 via Campbell-type homologous recombination in the small cluster of P. naphthalenivorans strain CJ2 naphthalene degradation genes. (A) The mutant CJM110 was designed to create a polar knock out of regulator nagR2. (B) The mutant CJM112 reported transcriptional activity in the small cluster of the naphthalene degradation pathway.
Growth tests of regulatory mutant strains CJN110 and CJM110 and Northern blot analysis.
Cells of strains CJN110, CJM110, and CJ2, grown in MSB-P medium for 24 h at 20°C were inoculated (8% [vol/vol]) into MSB-N media with 0.5% naphthalene crystals. Growth was monitored by measuring the optical density at 600 nm of the cultures.
To measure mRNA expression of ORF2 in mutant CJM110 and wild-type CJ2, total RNA was isolated from exponentially growing cells in MSB-N media by using an RNeasy kit (QIAGEN) according to the manufacturer's instructions. The RNA concentration was measured by determining the absorbance at 260 nm. A total of 100 μg of total RNA per sample was transferred to nylon membranes (Schleicher & Schuell, Keene, NH) by using a Slot blotter (Schleicher & Schuell). Northern hybridization was carried out by using standard protocol (36). Random-primed DNA labeling with digoxigenin-dUTP was applied to PCR products (478 bp) amplified by using the primers ORF2-226-F and ORF2-703R (Table 2). Detection of the labeled DNA by enzyme immunoassay on nylon membranes was performed with a DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Applied Sciences) according to the manufacturer's instructions.
TABLE 2.
P. naphthalenivorans strain CJ2 genes and gene products
| Cluster and gene | Putative function | Length (aa)a | Most similar gene product(s) (species, accession no.) or reference | % Identity (aa) |
|---|---|---|---|---|
| Large gene cluster | ||||
| nagR | Transcription regulator (LysR type) | 301 (−) | LysR-like regulatory protein (Ralstonia sp. strain U2, AAG13636) | 80 |
| LysR-type regulatory protein (Burkholderia sp. strain DNT, AAP70493) | 79 | |||
| nagAa | Ferredoxin reductase | 328 (+) | Ferredoxin reductase (Ralstonia sp. strain U2, AAD12606) | 75 |
| DntAa (Burkholderia cepacia R34, AAL50024) | 75 | |||
| nagG | Salicylate-5-hydroxylase, large oxygenase component | 420 (+) | Salicylate-5-hydroxylase large oxygenase component (Ralstonia sp. strain U2, AAD12607) | 88 |
| ORF2 (Burkholderia sp. strain DNT, AAB09764) | 84 | |||
| nagH | Salicylate-5-hydroxylase, small oxygenase component | 161 (+) | Salicylate-5-hydroxylase small oxygenase component (Ralstonia sp. strain U2, AAD12608) | 86 |
| ORFX (Burkholderia sp. DNT, AAD15560) | 86 | |||
| nagAb | Ferredoxin | 112 (+) | Ferredoxin (Ralstonia sp. strain U2, AAD12609) | 78 |
| DntAb (Burkholderia cepacia R34, AAL50022) | 78 | |||
| nagAc | Naphthalene dioxygenase, large | 447 (+) | Large dioxygenase subunit (Comamonas testosterone H, AAF72976) | 95 |
| dioxygenase component | Large dioxygenase component (Ralstonia sp. strain U2, AAD12610) | 95 | ||
| nagAd | Naphthalene dioxygenase, small | 194 (+) | 2,4-DNT dioxygenase; DntAd (Burkholderia sp. DNT, AAS09912) | 88 |
| oxygenase component | Dioxygenase small subunit (Comamonas testosterone H, AAF72977) | 88 | ||
| nagB | cis-Naphthalene dihydrodiol dehydrogenase | 259 (+) | cis-Naphthalene dihydrodiol dehydrogenase (Comamonas testosterone H, AAF72978) | 86 |
| cis-Naphthalene dihydrodiol dehydrogenase (Ralstonia sp. strain U2, AAD12612) | 86 | |||
| nagF | Salicylaldehyde dehydrogenase | 483 (+) | Salicylaldehyde dehydrogenase (Ralstonia sp. strain U2, AAD12613) | 88 |
| Dehydrogenase (Pseudomonas aeruginosa Pak1, BAA12243) | 84 | |||
| nagC | 1,2-Dihydroxynaphthalene dehydrogenase | 302 (+) | 1,2-Dihydroxynaphthalene dioxygenase (Ralstonia sp. strain U2, AAD12614) | 94 |
| 1,2-Dihydroxynaphthalene dioxygenase (Pseudomonas putidaNCIB 9816-4) | 88 | |||
| nagQ | Putative aldolase | 212 (+) | Putative aldolase (Ralstonia sp. strain U2, AAD12615) | 91 |
| Dibenzothiophene oxidation protein (Pseudomonas sp. strain ND6, NP_943091) | 71 | |||
| nagE | trans-o-Hydroxybenzylidenepyruvate hydratase-aldolase | 345 (+) | trans-o-Hydroxybenzylidenepyruvate hydratase-aldolase (Ralstonia sp. strain U2, AAD12616) | 96 |
| trans-o-Hydroxybenzylidenepyruvate hydratase-aldolase (Pseudomonas putida NCIB 9816-4, NP_863078) | 87 | |||
| nagD | 2-Hydroxychromene-2-carboxylate isomerase | 197 (+) | 2-Hydroxychromene carboxylate isomerase (Ralstonia sp. strain U2, AAD12617) | 88 |
| 2-Hydroxychromene-2-carboxylate dehydrogenase (Pseudomonas stutzeri AN10, AAD02142) | 68 | |||
| nagJ | GST homolog (glutathione | 201 (+) | Glutathione S-transferase-like protein (Ralstonia sp. strain U2, AAD12618) | 93 |
| S-transferase-like protein) | Putative glutathione S-transferase (Pseudomonas putida NCIB 9816-4, NP_863083) | 76 | ||
| nagI′ | Gentisate 1,2-dioxygenase | 334 (+) | Gentisate 1,2-dioxygenase (Ralstonia sp. strain U2, AAD12619) | 82 |
| Putative 1,2-dioxygenase (Salmonella enterica serovar Typhimurium LT2, NP_461123) | 32 | |||
| ORF1 | Unknown | 289 (+) | 2-Keto-4-pentenoate hydratase/2-oxohepta-3-ene-1,7-dioic acid hydratase (catechol pathway) (Mesorhizobium sp. strain BNC1, ZP_00195913) | 44 |
| 2-Hydroxyhepta-2,4-diene-1,7-dioate isomerase (Bacillus halodurans C-125, NP_242871) | 41 | |||
| tnpA | Putative transposase | 344 (+) | Transposase and inactivated derivatives (Azotobacter vinelandii, ZP_00089601) | 49 |
| Probable transposase (Pseudomonas aeruginosa PAO1, NP_249136) | 49 | |||
| None | Putative terminator | Rho-independent terminator | ||
| Small gene cluster | ||||
| R2 | Putative transcriptional regulator (MarR type) | 161 (−) | Transcriptional regulatory protein (Bradyrhizobium japonicumUSDA110, NP_766748) | 42 |
| Transcriptional regulators (Burkholderia cepacia R1808, ZP_00213511) | 44 | |||
| ORF2 | Unknown | 400 (+) | Putative salicylate 5-hydroxylase (Azoarcus sp. strain EbN1) | 89 |
| 2-Polyprenyl 6-methoxyphenol hydroxylase and related FAD-dependent oxidoreductases (Ralstonia eutrophaJMP134) | 60 | |||
| nagI" | Gentisate 1,2-dioxygenase | 351 (+) | Gentisate 1,2-dioxygenase (Ralstonia sp. strain U2, AAD12619) | 82 |
| Putative 1,2-dioxygenase (Salmonella enterica serovar Typhimurium LT2, NP_461123) | 38 | |||
| nagK | Fumarylpyruvate hydrolase | 192 (+) | Fumarylpyruvate hydrolase (Ralstonia sp. strain U2, AAD12620) | 80 |
| Unnamed protein product (Sphingomonas sp. strain RW1, CAA73583) | 59 | |||
| nagL | Maleylpyruvate isomerase | 212 (+) | Maleylpyruvate isomerase (Ralstonia sp. strain U2, AAD12621) | 69 |
| Glutathione S-transferase (Ralstonia eutropha JMP134, ZP_00171533) | 55 |
aa, amino acids. The orientation of coding strands are indicated in parentheses.
Construction of nagR2-ORF2::lacZ fusions and β-galactosidase assays.
Figure 1B shows how the reporter plasmid was constructed. Suicide transcriptional fusion vector pVIK112 (containing a stop codon immediately downstream of multicloning sites and the R6KoriV region so that it cannot replicate without the λpir replication system) was maintained in E. coli SY327 λpir (Table 2). Using the procedures described above, pCJM112 was prepared (Fig. 1B). The R2-ORF2::lacZ fusion was achieved by introducing pCJM112 into E. coli S17-1 λpir and subsequent conjugation into strain CJ2 as described above.
For β-galactosidase assays, cultures were grown overnight in 5 ml of minimal medium containing 0.2% (wt/vol) sodium pyruvate with or without naphthalene, salicylate, and gentisate. Cells were lysed with chloroform and sodium dodecyl sulfate, and β-galactosidase activities were determined by using standard methods (30).
Nucleotide sequence accession numbers.
The nucleotide sequences of P. naphthalenivorans CJ2 have been deposited in GenBank under accession no. DQ167474 for the large gene cluster and accession no. DQ167475 for the small gene cluster.
RESULTS
Localization of the nag gene clusters on the chromosome DNA.
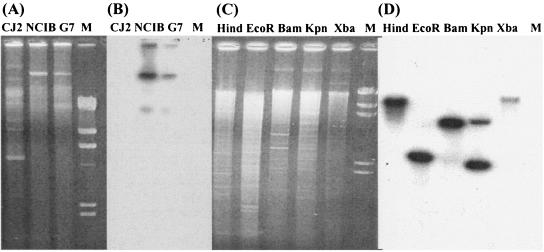
To examine whether the naphthalene catabolic genes reside on the chromosome or on a plasmid, the plasmids of strain CJ2 were subjected to electrophoresis and then transferred to a membrane which was probed with a genetically identical 488-bp 32P-labeled nahAc PCR product. The result showed that at least five plasmids in strain CJ2, but none of the plasmids, hybridized with the probe tested (Fig. 2), although positive-control plasmids from other naphthalene-degrading pseudomonads hybridized as expected. Instability of the Nah+ phenotype, often associated with curable catabolic plasmids, was examined as an additional way to detect a naphthalene catabolic plasmid. Strain CJ2 was not cured of a plasmid by traditional ethidium bromide or chloro-naphthalene treatment or by periodic subculturing in the absence of naphthalene. Southern blots of total genomic DNA digested with five different restriction enzymes probed positively for nahAc (Fig. 2C and D). Thus, despite the common occurrence of naphthalene catabolic genes on plasmids, our results indicated that the naphthalene degradation pathway in strain CJ2 is chromosomally encoded. These findings resemble those from P. stutzeri AN 10 (4).
FIG. 2.
Localization of naphthalene degradation genes in P. naphthalenivorans strain CJ2. (A) Plasmids retrieved from wild-type CJ2 and two positive-control pseudomonads carrying 80-kb naphthalene catabolic plasmids, P. putida NCIB 9816-4 and P. putida G7; (B) Southern hybridization of plasmids with nahAc probes; (C) the genomic DNA from strain CJ2 digested with HindIII, EcoRI, KpnI, and XbaI; (D) Southern hybridization of the digested genomic DNA with nahAc probes; M, molecular size marker, λ-DNA digested with HindIII.
Sequence analysis of the naphthalene degradation genes.
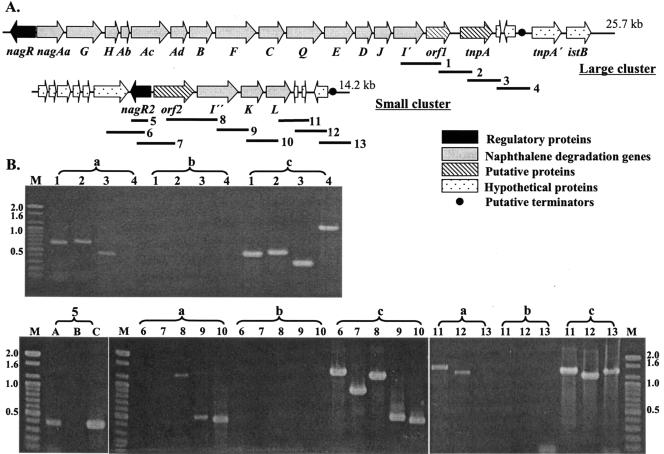
The nucleotide sequence of the gene clusters encoding the naphthalene-degradation pathway was determined by using a genome walking strategy. Twenty-one open reading frames (ORFs) related to the naphthalene catabolic genes were deduced; these were divided into two clusters nagRAaGHAbAcAdBFCQEDJI′ORF1tnpA and nagR2ORF2I"KL (large cluster; 25,748 bp, 56.64% G+C content; small cluster, 14,217 bp, 64.37% G+C content) (Fig. 3A). For each gene, the putative function, position, predicted product size, and significant matches to the predicted gene product are summarized (Table 2).
FIG. 3.
Physical maps of naphthalene degradation genes from P. naphthalenivorans strain CJ2 and of RT-PCR analysis of expressed genes. (A) Gene order in large and small naphthalene catabolic clusters. Bold solid lines show locations of 13 primer pairs used in the RT-PCR assays. (B) Agarose gel electrophoresis of 13 RT-PCR products amplified from strain CJ2 grown on naphthalene. Numbers refer to the locations of PCR fragments shown in Fig. 3A. Lowercase letters refer to amplification conditions: a, RT-PCR products from total RNA; b, PCR products from total RNA without RT; c, PCR products from genomic DNA. M, molecular size marker (100-bp ladder).
Analysis of the DNA sequences showed that the naphthalene catabolic operon structure of strain CJ2 was similar to that of Ralstonia sp. strain U2, which metabolizes naphthalene via the gentisate pathway, encoded by the nag operon. The putative two-component salicylate 5-hydroxylase of the large cluster (nagG [with the Rieske-type iron-sulfur center] and small subunit, nagH) is homologous to that of strain U2, which function together with nagAaAb gene products (14). Surprisingly, strain CJ2's naphthalene catabolic genes were not contiguous; in fact, efforts at long PCR were unable to span the gap between the two clusters. Furthermore, in the large cluster three ORFs (nagY [putatively involved in chemotaxis], nagM, and nagN), present in Ralstonia sp. strain U2, were absent.
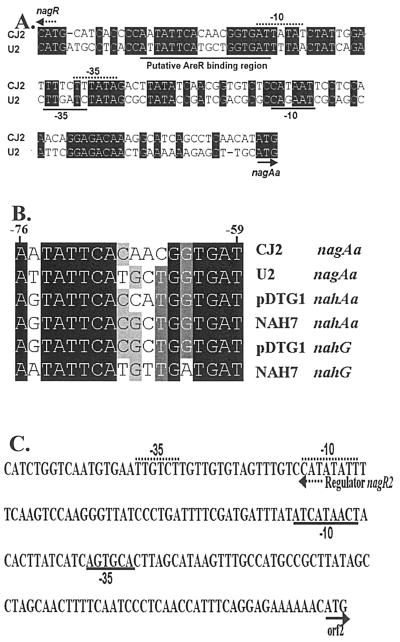
Examination of the DNA sequence between nagR and nagAa revealed that the promoter of strain CJ2 had a high degree of identity to that of Ralstonia sp. strain U2, especially near the putative AreR binding region and the putative −35 and −10 boxes (Fig. 4A). Upstream of this promoter between bases −76 and −59 is a symmetrical dyad motif TTCAN6TGAT (Fig. 4B) characteristic of the LysR family which has been identified as important for NahR and NagR function (23, 39). BLAST searches examining the downstream region of the large gene cluster revealed two putative transposase-related ORFs, tnpA′ and istB (Fig. 3A).
FIG. 4.
Comparative sequence analysis of regulatory promoters. (A) Promoters and NagR binding regions of the nag promoter region from strains U2 and CJ2. The arrows indicate the start of translation. The putative −35 and −10 motifs and the putative NagR binding motif are underlined. (B) Alignment of the conserved upstream regions controlled by NahR and NagR regulators. (C) The putative promoter region of nagR2 ORF2 in the small cluster of naphthalene degradation genes in strain CJ2. Conserved regions are enclosed in boxes.
The small cluster of P. naphthalenivorans CJ2 featured a MarR-type transcriptional regulator (nagR2), a putative gentisate 1,2-dioxygenase (nagI"), and a putative salicylate 5-hydroxylase (ORF2). The gentisate dioxygenases (nagI′ and nagI") of strain CJ2 exhibited 77.4% DNA sequence identity to one another and 82% amino acid identity to the analogous protein of Ralstonia sp. strain U2 (Table 2). However, the ORF2 gene product of the small gene cluster was most closely related to the putative salicylate 5-hydroxylase of Azoarcus sp. strain EbN1 with 89% amino acid identity (Table 2). This salicylate 5-hydroxylaase, unlike nagGH of strain U2, is a single-component enzyme without a Rieske iron-sulfur center. Surprisingly, the MarR-type transcriptional regulator (nagR2), divergently transcribed from ORF2 of the small cluster, was closely related to a Bradyrhizobium regulatory gene and not to typical catabolic regulators of pseudomonads (Table 2).
Expression of nag genes.
RT-PCR was used to amplify mRNA purified from strain CJ2 cells grown on MSB agar with naphthalene vapor. The primer sets used were specific for the downstream end (nagI′ and beyond) in the large nag cluster and spanned the entire small nag cluster (Fig. 3A). Because the large operon closely resembled that of Ralstonia sp. strain U2, we presumed that expression began at nagAa and extended into nagI′. The amplified products were analyzed by agarose gel electrophoresis (Fig. 3B). The presence of amplified DNA fragments obtained with each primer pair suggests that contiguous genes in each cluster were transcribed on the same message. No amplification product was obtained when RT was omitted from the reaction mixture. The data in Fig. 3 show that transcription extended two ORFs beyond nagI′ (fragments 1 to 3) but not beyond to fragment 4. In the second gene cluster, transcripts were found from “nagR2 through ORF2 to nagI”KL (fragments 5, 8, 9, 10, 11, and 12; Fig. 3B). Thus, we infer that there were termination sequences at the end of each cluster: regions beyond the termination sequences were not transcribed.
Genetic and phenotypic characterization of the nagR and nagR2 genes.
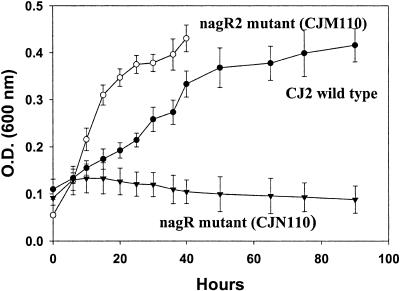
To verify the putative role of nagR (LysR-type) and R2 (MarR-type) regulators in P. naphthalenivorans CJ2, these regulatory genes were disrupted by using Campbell-type single-crossover homologous recombination. The sought genotypes of transconjugants were verified by using outer primer pairs, onrc-F/lacZ-R, omrc-F/lacZ-R (data not shown). When fewer than 3% (vol/vol) of cells were inoculated, very poor growth was observed from CJ2, as well as the two mutants. However, a heavier inoculum (8% [vol/vol]) allowed growth. The cell density of mutant CJN110, mutant CJM110, and wild-type CJ2, grown on 0.5% naphthalene crystals, was monitored by measuring the optical density at 600 nm of the cultures (Fig. 5). The nagR mutant strain, CJN110, showed a serious growth defect: this result was consistent with the previous reports (23, 38, 55). However, the second regulatory (nagR2) mutant strain, CJM110, grew faster than the wild type (Fig. 5). This is consistent with the known role of marR genes to function as repressors. Although a light brown color from the medium of the wild-type CJ2 was observed after 30 h, the color of the nagR2 mutant medium (CJM110) became dark black after 45 h. Therefore, the growth (optical density) of strain CJM110 could not be measured beyond 45 h.
FIG. 5.
Characterization of regulatory mutants. (A) Growth of wild-type strain CJ2, the nagR mutant CJN110, and nagR2 mutant CJM110 in MSB-N liquid culture. The growth of each strain was monitored by measuring the optical density (O.D.) at 600 nm of the cultures. The mutant strain CJM110 caused the medium to darken after 45 h. For location of nagR and nagR2, see Fig. 3.
Northern blot analysis was conducted to confirm the transcription of the second gene cluster in wild-type strain CJ2 and the mutant strain CJM110. Consistent with the growth of the strains, Northern blot data confirmed that transcription of the second cluster gene expression in strain CJM110 was higher than that of the wild type (date not shown).
Expression of R2-ORF2::lacZ report construct in strain CJ2.
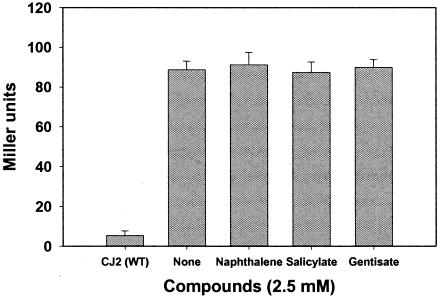
The LysR-type regulatory genes associated with naphthalene catabolism are known to be induced by naphthalene, salicylate, and gentisate. To examine the influence of these compounds on expression of nagR2, we constructed nagR2-ORF2::lacZ fusion in strain CJ2. Strain CJM112 was incubated in the presence of naphthalene, salicylate, or gentisase and assayed for β-galactosidase expression (Fig. 6). None of the compounds tested caused detectable induction of β-galactosidase. Given the function of nagR2 as a repressor, we would expect this gene's physiological cues to be distinctive from LysR-type activators.
FIG. 6.
Reporter assays examining transcriptional activation of nagR2 in the small gene cluster of P. naphthalenivorans strain CJ2. Shown are β-galactosidase activities (Miller units) from the nagR2-ORF2::lacZ transcriptional fusion in cells grown in MSB liquid media containing naphthalene crystals, salicylate, or gentisate (2.5 mM). Mean values for three independent cultures are shown with standard deviation.
DISCUSSION
Although metabolism of naphthalene via gentisate has long been biochemically described (see, for example, references 13 and 18), Ralstonia sp. strain U2 was the first genetically characterized bacterium utilizing the gentisate pathway from naphthalene to central metabolites (14, 56). In strain U2, 18 structural genes occur in a single operon under regulatory control of the LysR-type gene, nagR.
In the present investigation, the nucleotide sequences in P. naphthalenivorans CJ2 were determined for a new complete naphthalene metabolic cluster of genes homologous to those of Ralstonia sp. strain U2. Four observations suggest very similar biochemical reactions for naphthalene metabolism in strains U2 and CJ2: (i) the high degree of similarity observed between homologous catabolic genes (Fig. 3 and Table 2); (ii) similar organization of the nag promoter region (Fig. 4); (iii) the phenotype of a nagR mutant (strain CJN110; Fig. 5) matches that of other naphthalene degraders with mutations in LysR-type regulatory genes (33, 38); and (iv) the expression pattern of a portion of the genes in strain CJ2 resembles that of other naphthalene degraders (Fig. 3).
However, the naphthalene catabolic operons in strains U2 and CJ2 also show striking contrasts. The naphthalene catabolic genes of P. naphthalenivorans CJ2 were split into large and small clusters (nagRAaGHAbAcAdBFCQEDJI′ORF1tnpA and nagR2ORF2I"KL). Accompanying this operon division was duplicated functionality of two genes [salicylate-5-hydroxylase (nagGH and ORF2) and gentisate 1,2-dioxygenase (nag I′ and nag I")] and added MarR-type regulation to the small gene cluster. Furthermore, strain U2's three ORFs (nagY [putatively involved in chemotaxis] and nagM and nagN [the latter two have no known function) (56) were absent in strain CJ2. Operon rearrangements and regulation are discussed below.
The presence of two additional genes (ORF2 and nagI) and two regulators may be physiologically advantageous to the host. It has been suggested that routine gene regulatory mechanisms allow cells to adjust their metabolism within a modest range of conditions. When extreme conditions (such as competition for resources) cannot be accommodated by existing genetic systems, adaptation may be manifest as an increase in gene copy number or alterations in regulatory systems (15, 47). P. naphthalenivorans strain CJ2 was discovered by using a field-based stable isotope probing (SIP) procedure in naphthalene-contaminated freshwater sediment (21). Because ecological fitness is implicit in SIP-based identification of active microorganisms, it would be expected that the genetic basis of the likely fitness determinant (metabolizing naphthalene in situ in sediments at a coal tar contaminated site) might be novel. Thus, a distinctive naphthalene operon structure for ecologically fit strain CJ2 was not unexpected. However, it is not yet possible to attribute ecological fitness to a specific constellation of genetic traits because contaminated field sediments feature unknown selective pressures (complex populations and uncharacterized physiological conditions). Recent reports of abundant nag-related genes being expressed in contaminated freshwater sediments (10, 21, 52) do provide a strong suggestion that microorganisms carrying nag genes may be broadly distributed and ecologically important.
Transcriptional activation plays a prominent role in regulatory control of catabolic pathways in pseudomonads. As summarized by van der Meer (47), XyIS (a member of the AraC family of activator proteins) activates transcription in the TOL system after binding to the effector molecule, benzoate. Furthermore, all LysR-type regulators (]e.g., NahR [naphthalene], CatR/CatM [catechol/benzoate], TfdS/TfdR [2,4-dichlorophenoxyacetic acid], TcbR [trichlorobenzene], ClcR [chlorocatechol], and PcpR [pentachlorophenol]) exert positive control. In contrast, the MarR family of transcriptional regulators comprises a subset of winged helix DNA-binding proteins that act as repressors (50). In E. coli, the marRAB operon is a regulatory locus that controls multiple antibiotic resistance (46). However, in other bacteria (e.g., Deinococcus radiodurans, Acinetobacter spp., and Pseudomonas spp.) marR-type regulators, or related homologous domains, control catabolic functions such as dissimilation of hydroxycinnamic acid (D. Parke and N. Ornston, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol. 2003, abstr. K-112, p. 103, 2003) and nitrobenzene (32). Indeed, the MarR family is prominent for its “phenolic-sensing capabilities” involved in “environmental, surveillance of aromatic compounds” (50). Here we reported finding the MarR-type regulator, nagR2, in the small gene cluster of strain CJ2. The phenotypic impact of nagR2 was demonstrated when a nagR2 mutant showed accelerated growth on naphthalene, confirming that the NagR2 acts as a negative regulator (Fig. 5). Moreover, a nagR2-ORF2::lacZ fusion experiment showed that the expression of second cluster was not induced by LysR-type inducers (naphthalene, salicylate, or gentisate) (Fig. 6). Thus, it can be concluded that the regulation of the small gene cluster in strain CJ2 is novel: the archetypal positive LysR-type control has been replaced by negative MarR-type control. Additional experiments are required to explain potential physiological advantages (if any) of the replacement.
The “modular” or “mosaic” nature of catabolic operons is well recognized (5, 28, 31, 47, 48, 51). It is clear that variations in catabolic capabilities in prokaryotes have evolved and continue to evolve via a series of acquisitions and rearrangements at DNA scales, ranging from a few nucleotides (e.g., transition or inversion) to many kilobases (e.g., gene transfer, duplication, or deletion) (47). Documenting the mechanism of operon development is not facile. We must rely upon retrospective inspection of nucleotide sequences for traits that include G+C content, codon usage, identity of noncoding homologous DNA regions, and remnants of transposons or IS elements (see, for example, reference 5). These sometimes allow historical steps in the development of operons and entire plasmids to be inferred (9, 17, 26). As demonstrated by Fuenmayor et al. (14), cloning and expression assays can also be insightful and have led to the suggestion that the nagGH (salicylate mono-oxygenase) functionality in strain U2 developed by insertion of nagGH within the otherwise continuous functional cluster nagAaAbAcAd.
Several clues about the potential origin of strain CJ2's peculiar operon structure have emerged. Three observations (below) support the hypothesis that horizontal gene transfer occurred from Azoarcus to strain CJ2.
(i) Gene insertion has occurred.
In Ralstonia sp. strain U2 (which carries a contiguous complete nag operon), the order ofgenes is nagAaGHAbAcAdBFCQEDJIKLMN. Relative to strain U2, strain CJ2's small operon features a two-gene insertion (nagR2 and ORF2) at the 5′ end of a duplicated nagI′.
(ii) Gene sequence similarities suggest transferred genes originated in Azoarcus.
BLAST searching and nucleotide analyses of three loci show Azoarcus sp. strain EbN1 to be the closest match to genes in strain CJ2's catabolic operons. The ORF2 gene (putative salicylate 5-hydroxylase) in the second gene cluster exhibited 89% amino acid identity to its homologue in Azoarcus sp. strain EbN1. Also, in the region just beyond the putative terminator in the large gene cluster (Fig. 4A), putative transposase-related ORFs, tnpA′ and istB, exhibited 56 and 65% amino acid identity, respectively, with their homologues in Azoarcus sp. strain EbN1.
(iii) MarR in Azoarcus.
Schuehle et al. (40) have shown that the genes encoding aerobic metabolism of 2-aminobenzoate by Azoarcus evansii include a MarR-type regulator. Thus, there is precedent for MarR control of catabolism in Azoarcus.
The circumstantial evidence presented above suggests that that the two-gene insert, nagR2ORF2, and other operon rearrangements in strain CJ2 may have originated in a Azoarcus-like host. The mechanism of horizontal gene transfer between the two cell lines and the specific mobile genetic elements involved remain unclear. Such horizontal transfer of aromatic degradation and regulatory genes (3, 19, 53) may have been enhanced in the naphthalene-contaminated environment from which strain CJ2 was isolated.
Acknowledgments
This study was supported by grant ES012834 from the National Institute of Environmental Health Sciences and by grants from MOST/KOSEF to the Environmental Biotechnology National Core Research Center (grant R15-2003-012-02002-0) and to the 21C Frontier Microbial Genomics and Application Center Program (grant MG05-0104-4-0), Ministry of Science and Technology, Korea.
We thank Stephen C. Winans (Department of Microbiology, Cornell University) for providing vectors pVIK112 and pVIK110.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Assinder, S. J., and P. A. Williams. 1988. Comparison of the meta-pathway operons on NAH plasmid pWW60-22 and TOL plasmid pWW53-4 and its evolutionary significance. J. Gen. Microbiol. 234:2769-2778. [DOI] [PubMed] [Google Scholar]
- 3.Basta, T., A. Keck, J. Klein, and A. Stolz. 2004. Detection and characterization of conjugative degradative plasmids in xenobiotic-degrading Sphingomonas strains. J. Bacteriol. 186:3862-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch, R., E. Garcia-Valdes, and E. R. B. Moore. 1999. Genetic characterization and evolutionary implication of a chromosomally encoded naphthalene-degradation upper pathway from Pseudomonas stutzeri AN10. Gene 236:149-157. [DOI] [PubMed] [Google Scholar]
- 5.Bosch, R., E. Garcia-Valdes, and E. R. B. Moore. 2000. Complete nucleotide sequence and evolutionary significance of a chromosomally encoded naphthalene-degradation lower pathway from Pseudomonas stutzeri AN10. Gene 245:65-74. [DOI] [PubMed] [Google Scholar]
- 6.Cane, P. A., and P. A. Williams. 1986. A restriction map of the catabolic plasmid pWW60-1 and the location of some of its catabolic genes. J. Gen. Microbiol. 132:2919-2929. [Google Scholar]
- 7.Crosa, J. H., M. E. Tolmasky, L. A. Actis, and S. Falkow. 1994. Plasmids, p. 365-386. In P. K. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 8.Demaneche, S., C. Meyer, J. Micoud, M. Louwagie, J. C. Willison, and Y. Jouanneau. 2004. Identification and functional analysis of two aromatic-ring-hydroxylating dioxygenases from a Sphingomonas strain that degrades various polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 70:6714-6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis, J. J., and G. J. Zylstra. 2004. Complete sequence and genetic organization of pDTG1, the 83-kilobase naphthalene degradation plasmid from Pseudomonas putida strain NCIB 9816-4. J. Mol. Biol. 341:753-768. [DOI] [PubMed] [Google Scholar]
- 10.Dionisi, H. M., C. S. Chewning, K. H. Morgan, F. M. Menn, J. P. Easter, and G. S. Sayler. 2004. Abundance of dioxygenase genes similar to Ralstonia sp. strain U2 nagAc is correlated with naphthalene concentrations in coal tar-contaminated freshwater sediments. Appl. Environ. Microbiol. 70:3988-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn, N. W., and I. C. Gunsalus. 1973. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J. Bacteriol. 114:974-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenstadt, E., B. C. Carlton, and B. J. Brown. 1994. Gene mutation, p. 297-316. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 13.Fuenmayor, S. L., and V. Rodriguez Lemoine. 1992. Characterization of polycyclic aromatic hydrocarbons degradative soil Pseudomonas. Acta Cient. Venez. 43:349-354. [PubMed] [Google Scholar]
- 14.Fuenmayor, S. L., M. Wild, A. L. Boyes, and P. A. Williams. 1998. A gene cluster encoding steps in conversion of naphthalene to gentisate in Pseudomonas sp. strain U2. J. Bacteriol. 180:2522-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gevers, D., K. Vandepoele, C. Simillion, C., and Y. Van dePeer. 2004. Gene duplication and biased functional retention of paralogs in bacterial genomes. Trends Microbiol. 12:148-154. [DOI] [PubMed] [Google Scholar]
- 16.Goyal, A. K., and G. J. Zylstra. 1996. Molecular cloning of novel genes for polycyclic aromatic hydrocarbon degradation from Comamonas testosteroni GZ39. Appl. Environ. Microbiol. 62:230-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greated, A., L. Lambertsen, P. A. Williams, and C. M. Thomas. 2002. Complete sequence of the IncP-9 TOL plasmid pWWO from Pseudomonas putida. Environ. Microbiol. 4:856-871. [DOI] [PubMed] [Google Scholar]
- 18.Grund, E., B. Denecke, and R. Eichenlaub. 1992. Naphthalene degradation via salicylate and gentisate by Rhodococcus sp. strain B4. Appl. Environ. Microbiol. 58:1874-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrick, J. B., K. G. Stuart-Keil, W. C. Ghiorse, and E. L. Madsen. 1997. Natural horizontal transfer of a naphthalene dioxygenase gene. Appl. Environ. Microbiol. 63:2330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohnstock, A. M., K. G. Stuart-Keil, E. Kull, and E. L. Madsen. 2000. Naphthalene and donor cell density influence field conjugation of naphthalene catabolism plasmids. Appl. Environ. Microbiol. 66:3088-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon, C. O., W. Park, P. Padmanabhan, C. DeRito, J. R. Snape, and E. L. Madsen. 2003. Discovery of a previously undescribed bacterium with distinctive dioxygenase that is responsible for in situ biodegradation in contaminated sediment. Proc. Natl. Acad. Sci. USA 100:13591-13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon, C. O., W. Park, W. C. Ghiorse, and E. L. Madsen. 2004. Polaromonas naphthalenivorans sp. nov., a naphthalene-degrading bacterium from naphthalene-contaminated sediment. Int. J. Syst. Evol. Microbiol. 54:93-97. [DOI] [PubMed] [Google Scholar]
- 23.Jones, R. M., B. Britt-Compton, and P. A. Williams. 2003. The naphthalene catabolic genes (nag) genes of Ralstonia sp. strain U2 are an operon that is regulated by nagR, a LysR-type transcriptional regulator. J. Bacteriol. 185:5847-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalogeraki, V. S., and S. C. Winans. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188:69-75. [DOI] [PubMed] [Google Scholar]
- 25.Kasai, Y., K. Shindo, S. Harayama, and N. Misawa. 2003. Molecular characterization and substrate preference of a polycyclic aromatic hydrocarbon dioxygenase from Cycloclasticus sp. strain A5. Appl. Environ. Microbiol. 69:6688-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulakov, L. A., S. Chen, C. C. R. Allen, and M. J. Larkin. 2005. Web-type evolution of Rhodococcus gene clusters associated with utilization of naphthalene. Appl. Environ. Microbiol. 71:1754-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurie, A. D., and G. Lloyd-Jones. 1999. The phn genes of Burkholderia sp. strain RP007 constitute a divergent gene cluster for polycyclic aromatic hydrocarbon catabolism. J. Bacteriol. 181:531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence, J. G., and J. R. Roth. 1996. Selfish operons: horizontal transfer may drive evolution of gene clusters. Genetics 143:1843-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lessner, D. J., G. R. Johnson, R. E. Parales, J. C. Spain, and D. T. Gibson. 2002. Molecular characterization and substrate specificity of nitrobenzene dioxygenase from Comamonas sp. strain JS765. Appl. Environ. Microbiol. 68:634-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, J. H. 1972. Experiments in Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Osborn, A. M., and D. Böltner. 2002. When phage, plasmids, and transposons collide: genomic islands, and conjugative - and mobilizable-transposons as a mosaic continuum. Plasmid 48:202-212. [DOI] [PubMed] [Google Scholar]
- 32.Park, H. S., and H.-S. Kim. 2001. Genetic and structural organization of the aminophenol catabolic operon and its implication for evolutionary process. J. Bacteriol. 183:5074-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, W., and E. L. Madsen. 2004. Characterization of nah in Pseudomonas putida Cg1 and its role in bacterial survival in soil. Appl. Microbiol. Biotechnol. 66:209-216. [DOI] [PubMed] [Google Scholar]
- 34.Park, W., P. Padmanabhan, S. Padmanabhan, G. J. Zylstra, and E. L. Madsen. 2002. nahR, encoding a LysR-type transcriptional regulator, is highly conserved among naphthalene-degrading bacteria isolated from a coal tar waste-contaminated site and in extracted community DNA. Microbiology 148:2319-2329. [DOI] [PubMed] [Google Scholar]
- 35.Park, W., C. O. Jeon, A. M. Hohnstock-Ashe, S. C. Winans, G. J. Zylstra, and E. L. Madsen. 2003. Identification and characterization of the conjugal transfer region of the pCg1 plasmid from naphthalene-degrading Pseudomonas putida Cg1. Appl. Environ. Microbiol. 69:3263-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., and K. J. Janssen. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulator. Annu. Rev. Microbiol. 47:597-629. [DOI] [PubMed] [Google Scholar]
- 38.Schell, M. A., and E. F. Poser. 1989. Demonstration, characterization and mutational analysis of NahR protein binding to nah and sal promoters. J. Bacteriol. 171:837-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schell, M. A., and P. Wender. 1986. Identification of the nahR gene product and nucleotide sequences required for its activation of the sal operon. J. Bacteriol. 166:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuehle, K., M. Jahn, S. Ghisla, and G. Fuchs. 2001. Two similar gene clusters coding for enzymes of a new type of aerobic 2-aminobenzoate (anthranilate) metabolism in the bacterium Azoarcus evansii. J. Bacteriol. 183:5268-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serdar, C. M., and D. T. Gibson. 1989. Isolation and characterization of altered plasmids in mutant strains of Pseudomonas putida NCIB9816. Biochem. Biophys. Res. Commun. 164:764-771. [DOI] [PubMed] [Google Scholar]
- 42.Simon, M. J., T. D. Osslund, R. Saunders, B. D. Ensley, S. Suggs, A. Harcourt, W.-C. Suen, D. L. Cruden, D. T. Gibson, and G. J. Zylstra. 1993. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB9816-4. Gene 127:31-37. [DOI] [PubMed] [Google Scholar]
- 43.Stanier, R. Y., N. J. Palleroni, and M. Doudorhoff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 44.Stuart-Keil, K. G., A. M. Hohnstock, K. P. Drees, J. B. Herrick, and E. L. Madsen. 1998. Plasmid responsible for horizontal transfer of naphthalene catabolism genes between bacteria at a coal tar-contaminated site are homologous to pDTG1 from Pseudomonas putida NCIB9816-4. Appl. Environ. Microbiol. 64:3633-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suen, W. C., B. E. Haigler, and J. C. Spain. 1996. 2,4-Dinitrotoluene dioxygenase from Burkholderia sp. strain DNT: similarity to naphthalene dioxygenase. J. Bacteriol. 178:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sulavik, M. C., M. Dazer, and P. F. Miller. 1979. The Salmonella typhimurium mar locus: molecular and genetic analyses and assessment of its role in virulence. J. Bacteriol. 179:1857-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Meer, J. 1997. Evolution of novel metabolic pathways for the degradation of chloroaromatic compounds. Antonie Leeuwenhoek 71:159-178. [DOI] [PubMed] [Google Scholar]
- 48.van der Meer, J. R., W. M. deVos, S. Harayama, and A. J. B. Zehnder. 1992. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol. Rev. 56:677-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wigmore, G. J., and D. W. Ribbons. 1981. Selective enrichment of Pseudomonas spp. defective in catabolism after exposure to halogenated substrates. J. Bacteriol. 146:920-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkinson, S. P., and A. Grove. 2004. HucR, a novel uric acid-responsive member of the MarR family of transcriptional regulators from Deinococcus radiodurans. J. Biol. Chem. 279:51442-51450. [DOI] [PubMed] [Google Scholar]
- 51.Williams, P. A., and J. R. Sayers. 1994. The evolution of pathways for aromatic hydrocarbon oxidation in Pseudomonas. Biodegradation 5:195-217. [DOI] [PubMed] [Google Scholar]
- 52.Wilson, M. S., C. Bakermans, and E. L. Madsen. 1999. In situ, real-time catabolic gene expression: extraction and characterization of naphthalene dioxygenase mRNA transcripts from groundwater. Appl. Environ. Microbiol. 65:80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson, M. S., J. B. Herrick, C. O. Jeon, D. E. Hinman, and E. L. Madsen. 2003. Horizontal transfer of phnAc dioxygenase genes within one of two phenotypically and genotypically distinctive naphthalene-degrading guilds from adjacent soil environments. Appl. Environ. Microbiol. 69:2172-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yen, K.-M., and I. C. Gunsalus. 1982. Plasmid gene organization: naphthalene/salicylate oxidation. Proc. Natl. Acad. Sci. USA 79:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yen, K.-M., and I. C. Gunsalus. 1985. Regulation of naphthalene catabolic genes of plasmid NAH7. J. Bacteriol. 162:1008-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou, N.-Y., S. L. Fuenmayor, and P. A. Williams. 2001. nag genes of Ralstonia (formerly Pseudomonas) sp. strain U2 encoding enzymes for gentisate catabolism. J. Bacteriol. 183:700-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, N. Y., J. Al-Dulayymi, M. S. Baird, and P. A. Williams. 2002. Salicylate 5-hydroxylase from Ralstonia sp. strain U2: a monooxygenase with close relationships to and shared electron transport proteins with naphthalene dioxygenase. J. Bacteriol. 184:1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]