Abstract
Wax esters are esters of long-chain fatty acids and long-chain fatty alcohols which are of considerable commercial importance and are produced on a scale of 3 million tons per year. The oil from the jojoba plant (Simmondsia chinensis) is the main biological source of wax esters. Although it has a multitude of potential applications, the use of jojoba oil is restricted, due to its high price. In this study, we describe the establishment of heterologous wax ester biosynthesis in a recombinant Escherichia coli strain by coexpression of a fatty alcohol-producing bifunctional acyl-coenzyme A reductase from the jojoba plant and a bacterial wax ester synthase from Acinetobacter baylyi strain ADP1, catalyzing the esterification of fatty alcohols and coenzyme A thioesters of fatty acids. In the presence of oleate, jojoba oil-like wax esters such as palmityl oleate, palmityl palmitoleate, and oleyl oleate were produced, amounting to up to ca. 1% of the cellular dry weight. In addition to wax esters, fatty acid butyl esters were unexpectedly observed in the presence of oleate. The latter could be attributed to solvent residues of 1-butanol present in the medium component, Bacto tryptone. Neutral lipids produced in recombinant E. coli were accumulated as intracytoplasmic inclusions, demonstrating that the formation and structural integrity of bacterial lipid bodies do not require specific structural proteins. This is the first report on substantial biosynthesis and accumulation of neutral lipids in E. coli, which might open new perspectives for the biotechnological production of cheap jojoba oil equivalents from inexpensive resources employing recombinant microorganisms.
Wax esters are oxoesters of long-chain fatty acids esterified with long-chain fatty alcohols. They are widespread in nature and can be found in plants, animals, and microorganisms. Wax esters fulfill a variety of quite diverse and important biological functions (for an overview, see reference 9). These functions include protection from desiccation, UV light, and pathogens (e.g., plant epicuticular waxes); structural fuctions (e.g., beeswax), regulation of buoyancy and/or sound transmission (e.g., spermaceti oil of sperm whales), and energy storage (e.g., seeds of the jojoba plant Simmondsia chinensis and other members of the family Euphorbiaceae). Also, mammals produce wax esters as a significant constituent of the oils secreted by the sebaceous and meibomian glands onto the surfaces of the skin and eye, respectively (4, 17).
Wax esters have a multitude of important technical applications in a variety of areas, including medicine, cosmetics, and food industries, as well as their more traditional usage as lubricants. The liquid wax esters from the jojoba plant (jojoba oil), which have a carbon chain length of C38 to C44 and which are composed mainly of C20:1 fatty acids and C20:1 and C22:1 fatty alcohols (16), have been the main natural source of wax esters for commercial applications since the global ban on whale hunting. However, the high price of jojoba oil has limited its use, which is currently restricted to medical and cosmetics applications as a basis for ointments, creams, and other care products. Today, wax esters are mainly produced chemically or by biotechnological processes employing immobilized lipases (10). However, even the lipase-based biotechnological wax ester production depends on fatty alcohols as substrates, which at present must be synthesized chemically. Thus, there is still a strong demand for the production of cheap jojoba-like wax esters completely produced from inexpensive renewable resources like fatty acids.
In bacteria, the accumulation of wax esters as intracytoplasmic lipophilic storage compounds has been described sporadically for members of the genera Moraxella (1) and Micrococcus (20). However, wax ester seems to be a common storage lipid within the genus Acinetobacter (6, 7, 8, 11). The wax esters produced by Acinetobacter species comprise C30 to C36 wax esters composed of saturated and monounsaturated fatty acid and fatty alcohol moieties (6, 8). The composition of the synthesized wax esters, as well as their degree of saturation, can be varied by the use of the carbon source and by the cultivation temperature (3, 5). Thereby, a chemical composition very similar to the wax esters produced by the jojoba plant can be revealed. Thus, Acinetobacter might be an alternative source for the production of jojoba-like wax esters. However, the wax ester content of Acinetobacter species never exceeds 14% of the cell dry weight (6). Therefore, high-level production of wax esters in bacteria will probably not be achieved without metabolic engineering employing recombinant strains.
The pathway for de novo biosynthesis of wax esters in bacteria has been studied in most detail with Acinetobacter baylyi strain ADP1 (formerly Acinetobacter sp. strain ADP1). It utilizes long-chain acyl-coenzyme A (acyl-CoA), which is reduced in a NADPH-dependent reaction to fatty aldehyde catalyzed by the acyl-CoA reductase Acr1 (19). The subsequent reduction of the fatty aldehyde to the corresponding fatty alcohol is catalyzed by an NADPH-dependent fatty aldehyde reductase. However, the gene encoding this fatty aldehyde reductase has not yet been identified. Finally, the resulting fatty alcohol is esterified with long-chain acyl-CoA producing the wax ester catalyzed by the wax ester synthase/acyl-CoA-diacylglycerol acyltransferase (WS/DGAT). This novel, highly unspecific type of acyltransferase not only synthesizes wax esters but also is responsible for the by far major part of triacylglycerol production in A. baylyi strain ADP1 (12, 22).
Since the gene encoding a bacterial fatty aldehyde reductase remains unidentified, heterologous reconstitution of the entire pathway for bacterial wax ester biosynthesis from simple carbon sources is currently not feasible. Recombinant wax ester biosynthesis in a bacterial host can only be established in Pseudomonas citronellolis utilizing fatty alcohols as direct precursor substrates (12). In contrast to bacteria, fatty alcohols are synthesized in the jojoba plant by a bifunctional fatty acyl-CoA reductase from acyl-CoA in coupled NADPH-dependent reactions without release of the fatty aldehyde intermediate (18). Functional expression of this plant acyl-CoA reductase gene and fatty alcohol biosynthesis were recently demonstrated with Escherichia coli (15). Thus, the aim of the present study was the establishment of recombinant wax ester biosynthesis in E. coli by heterologous coexpression of a plant acyl-CoA reductase gene and a bacterial WS/DGAT gene. This should provide a basis for the biotechnological production of these industrial relevant compounds from inexpensive and renewable substrates.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
E. coli BL21(DE3) (Novagen, Madison, Wis.) was used in this study. The plasmids used were pCGN7800, harboring the jojoba acyl-CoA reductase gene cloned in pET3a colinear to the T7 promoter (15), and pBBR1MCS-2::atfA, harboring the WS/DGAT gene from A. baylyi strain ADP1 colinear to the lacZ promoter (23).
Recombinant strains of Escherichia coli were cultivated for 40 h in LB medium (0.5% [wt/vol], yeast extract [BD Diagnostic, Sparks, MD], 1% [wt/vol] Bacto tryptone [BD Diagnostic, Sparks, MD], and 1% [wt/vol] NaCl) containing 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C in the presence of ampicillin (75 mg liter−1) and kanamycin (50 mg liter−1) for selection of pCGN7800 and pBBR1MCS-2::atfA, respectively. Cells were grown aerobically in 300-ml baffled Erlenmeyer flasks containing 50 ml medium on an orbital shaker (130 rpm). Modified LB (mLB) medium contained 1% (wt/vol) yeast extract and 1% (wt/vol) NaCl but no tryptone. Where indicated, sodium oleate was added from a 10% (wt/vol) stock solution in H2O to a final concentration of 0.2% (wt/vol).
For electron microscopic studies, A. baylyi strain ADP1 (ATCC 33305) was cultivated under conditions promoting storage lipid accumulation as described previously (12).
Thin-layer chromatography (TLC).
TLC analysis of lipid extracts from whole cells was done as described previously (12) using the solvent system hexane:diethylether:acetic acid (90:7.5:1 [vol/vol/vol]) for wax ester and fatty acid butyl ester (FABE) analysis. Lipids were visualized by being sprayed with 40% (vol/vol) sulfuric acid and charred. Oleyl oleate and butyl oleate were purchased from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany) and used as reference substances for wax esters or FABEs, respectively.
Fatty acid analysis.
Fatty acid analysis of whole cells was done by gas liquid chromatography (GC) according to reference 13 after derivatization to fatty acid methyl esters by sulfuric acid-catalyzed methanolysis. Fatty acid methyl esters were analyzed by GC on an Agilent 6850 GC (Agilent Technologies, Waldbronn, Germany) equipped with a BP21 capillary column (50 m by 0.22 mm; film thickness, 250 nm; SGE, Darmstadt, Germany) and a flame ionization detector (Agilent Technologies, Waldbronn, Germany). A 2-μl portion of the organic phase was analyzed after split injection (1:5); hydrogen (constant flow, 0.6 ml min−1) was used as carrier gas. The temperatures of the injector and detector were 250°C and 275°C, respectively. The following temperature program was applied: 120°C for 5 min, increase of 3°C min−1 to 180°C, increase of 10°C min−1 to 220°C, and 220°C for 31 min. Substances were identified by comparison of their retention times to those of standard fatty acid methyl esters.
GC/MS analysis of isolated wax esters.
The putative wax esters were purified by preparative TLC, and their structures were determined by coupled GC-mass spectrometry (GC/MS) on a HP6890 GC (Hewlett Packard, Waldbronn, Germany) coupled to a mass spectrometer (GCT; Waters-Micromass, Manchester, United Kingdom) with electron impact ionization. A 1-μl portion of the purified wax esters dissolved in chloroform was analyzed after split injection (1:10) employing an HP5 capillary column (30 m by 0.32 mm; film thickness, 250 nm; Hewlett Packard, Waldbronn, Germany). Helium (constant flow, 1.5 ml min−1) was used as a carrier gas. The temperatures of the injector and detector were 280°C. The following temperature program was applied: 40°C for 2 min, increase of 20°C min−1 to 300°C, and 300°C for 15 min.
GC and GC/MS analysis of FABEs.
For quantification of FABEs, 20 mg of lyophilized whole cells was extracted with 1.2 ml chloroform-methanol (2:1; vol/vol). The FABE content in the total lipid extract was determined by GC under conditions essentially as described above for fatty acid methyl ester analysis by application of the same temperature program. Quantification was done by employing authentic standards.
For GC/MS analysis, FABEs were purified by preparative TLC. GC/MS analysis of FABEs dissolved in chloroform was done on a series 6890 GC system equipped with a series 5973 EI MSD mass-selective detector (Hewlett Packard, Waldbronn, Germany). A 3-μl portion of the organic phase was analyzed after splitless injection employing a BP21 capillary column (50 m by 0.22 mm; film thickness, 250 nm; SGE, Darmstadt, Germany). Helium (constant flow, 0.6 ml min−1) was used as a carrier gas. The temperatures of the injector and detector were 250°C and 240°C, respectively. The same temperature program as described for GC analysis was applied. Data were evaluated using the NIST Mass Spectral Search program (21).
GC/MS analysis of 1-butanol.
The presence of butanol in cell-free culture supernatants was determined by GC/MS on a series 6890 GC system equipped with a series 5973 EI MSD mass-selective detector (Hewlett Packard, Waldbronn, Germany). A 5-μl portion of the aqueous phase was analyzed after splitless injection employing a BP21 capillary column (50 m by 0.22 mm; film thickness, 250 nm; SGE, Darmstadt, Germany). Helium (constant flow, 0.6 ml min−1) was used as a carrier gas. The temperatures of the injector and detector were 250°C and 240°C, respectively. The following temperature program was applied: 45°C for 20 min, increase of 10°C min−1 to 220°C, and 220°C for 20 min. Data were evaluated using the NIST Mass Spectral Search program (21).
Transmission electron microscopy (TEM) and immune electron microscopy.
Cells of E. coli BL21(DE3) harboring pCGN7800 and pBBR1MCS-2::atfA cultivated in LB medium with or without the addition of 0.2% (wt/vol) sodium oleate and cells of A. baylyi strain ADP1 cultivated under conditions promoting storage lipid accumulation as described previously (22) were fixed in 6% (wt/vol) paraformylaldehyde, embedded in Lowicryl K4M (Polyscience, Germany), and polymerized at −35°C. Ultrathin sections were cut with an ultramicrotome Ultracut S (Leica Mikroskopie und Systeme GmbH, Wetzlar, Germany). To avoid nonspecific binding of antibodies, the sections were kept for 15 min in a solution of 5% (wt/vol) bovine serum albumin (BSA) in phosphate-buffered saline. Afterwards, the sections were labeled with polyclonal anti-WS/DGAT immunoglobulin Gs (IgGs) from rabbit for 2 h (22). After being washed five times in phosphate-buffered saline-BSA solution for 5 min each time and washed in Tris/BSA solution for 5 min, the specimens were labeled for 1 h with goat anti-rabbit IgGs coupled to 10-nm colloidal gold particles (Sigma-Aldrich, St. Louis, Mo.) binding to the primary antibodies. Subsequently, the immunogold-labeled sections were washed five times in Tris/BSA solution for 5 min each time; after this, they were washed once in H2O and then stained with uranyl acetate and lead citrate. The sections were analyzed in a H-500 transmission electron microscope (Hitachi, Ltd., Tokyo, Japan) operated at 75 kV. Photographs were taken in the bright-field mode with Agfa-Gevaert 23D56 film. Negative controls were carried out with cells from the same preparation but omitting the labeling with the primary antibodies (anti-WS/DGAT IgGs).
RESULTS
Heterologous wax ester biosynthesis in metabolically engineered E. coli.
Heterologous expression of WS/DGAT alone did not result in the biosynthesis of detectable amounts of wax esters or other lipids in E. coli BL21(DE3) harboring plasmid pBBR1MCS-2::atfA during cultivation on LB medium as revealed by TLC analysis (Fig. 1, lane 1), since the metabolism of E. coli does not provide the required fatty alcohols as substrates. Low levels of fatty alcohol synthesis could be achieved with E. coli by expression of the bifunctional acyl-CoA reductase from the jojoba plant, as was shown recently (15). However, this alone did again not result in wax ester biosynthesis (Fig. 1, lane 2). Only coexpression of the plant acyl-CoA reductase gene provided on plasmid pCGN7800, as well as the bacterial WS/DGAT localized on pBBR1MCS-2::atfA, resulted in the de novo formation of detectable amounts of wax esters in recombinant E. coli BL21(DE3) during cultivation in LB medium, as shown by TLC analysis (Fig. 1, lane 3).
FIG. 1.
Neutral lipid biosynthesis in recombinant E. coli BL21(DE3) strains. Cells were cultivated for 40 h at 37°C in LB medium (lanes 1 to 3) or LB medium supplemented with 0.2% (wt/vol) sodium oleate (lanes 4 to 6) and analyzed by TLC. Total lipid extracts obtained from 3 mg lyophilized cells each were applied to lanes 1 to 6. Lanes: A, butyl oleate (40 μg); B, oleyl oleate (40 μg); 1 and 4, E. coli BL21(DE3) (pBBR1MCS-2::atfA); 2 and 5, E. coli BL21(DE3) (pCGN7800); laes 3 and 6, E. coli BL21(DE3) (pCGN7800 plus pBBR1MCS-2::atfA).
Wax ester biosynthesis was not reflected by an increased fatty acid content in the E. coli strain harboring pCGN7800 plus pBBR1MCS-2::atfA (Table 1), indicating that only trace amounts of lipids were produced. By the addition of 0.2% (wt/vol) sodium oleate to the cultivation medium, an approximately threefold increase in wax ester formation was observed, amounting to up to ca. 1% of the cellular dry weight (CDW), as could be roughly estimated by TLC analysis by comparison with the reference substance oleyl oleate (Fig. 1).
TABLE 1.
Fatty acid content and fatty acid composition of recombinant E. coli BL21 (DE3) strainsa
| Plasmid(s) | Oleate addition | Total fatty acids (% CDW) | Fatty acid composition (mol%)
|
||||
|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | |||
| pCGN7800 | − | 2.0 | 15.8 | 77.0 | 2.8 | 1.6 | 2.8 |
| pBBR1MCS-2::atfA | − | 1.9 | 17.2 | 75.7 | 4.2 | 1.2 | 1.7 |
| pCGN7800 + pBBR1MCS-2::atfA | − | 2.1 | 14.5 | 77.2 | 3.9 | 1.6 | 2.8 |
| pCGN7800 | + | 2.5 | 10.9 | 56.1 | 8.9 | 1.5 | 22.6 |
| pBBR1MCS-2::atfA | + | 4.3 | 7.2 | 47.0 | 10.1 | 1.1 | 34.6 |
| pCGN7800 + pBBR1MCS-2::atfA | + | 5.2 | 6.1 | 48.8 | 10.6 | 1.1 | 33.4 |
Cells were cultivated for 40 h in LB medium with or without addition of 0.2% (wt/vol) sodium oleate. Lyophilized cells were analyzed by GC.
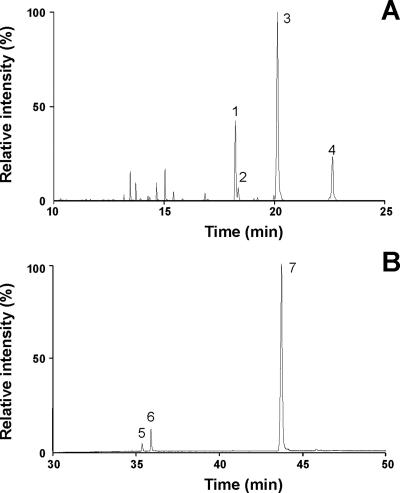
GC/MS analysis of purified wax esters produced by E. coli BL21(DE3) (pCGN7800 plus pBBR1MCS-2::atfA) during cultivation in LB medium containing 0.2% (wt/vol) sodium oleate revealed a mixture of various wax ester species, predominantly consisting of palmityl oleate (C34:1), palmityl palmitoleate (C32:1), and oleyl oleate (C36:2) (Fig. 2A). In contrast, palmityl palmitate (C32:0) and palmityl oleate (C34:1) were the most abundant wax ester species produced in the absence of sodium oleate (data not shown). Thus, palmityl alcohol (1-hexadecanol; C16:0) was the main fatty alcohol constituent of wax esters synthesized in recombinant E. coli, even during the cultivation on oleate. In contrast, oleic acid (C18:1) and its degradation product palmitoleic acid (C16:1) were the most prominent fatty acid moieties of wax esters. Since palmitic acid (C16:0) always represented the predominant fatty acid of total lipids in recombinant E. coli strains (Table 1), this indicates a preferred incorporation of unsaturated fatty acids into wax esters.
FIG. 2.
GC/MS analyses of wax esters and FABEs purified from E. coli BL21(DE3) (pCGN7800 plus pBBR1MCS-2::atfA). Cells were cultivated in LB medium supplemented with 0.2% (wt/vol) sodium oleate. Neutral lipids were purified from total lipid extracts of lyophilized cells by preparative TLC and subjected to GC/MS analyses. (A) Total ion profile of GC/MS analysis of purified wax esters. Identified substances: 1, palmityl palmitoleate (m/z [C32H62O2]+ = 478.4); 2, palmityl palmitate (m/z [C32H64O2]+ = 480.4); 3, palmityl oleate (m/z [C34H66O2]+ = 506.5); 4, oleyl oleate (m/z [C36H68O2]+ = 532.6). (B) Total ion profile of GC/MS analysis of purified FABEs. Identified substances: 5, butyl palmitate (m/z [C20H40O2]+ = 312.3); 6, butyl palmitoleate (m/z [C20H38O2]+ = 310.3); 7, butyl oleate (m/z [C22H44O2]+ = 340.3).
FABE biosynthesis in E. coli heterologously expressing WS/DGAT.
Unexpectedly, in addition to wax esters, the significant formation of another lipophilic compound was observed with E. coli BL21(DE3) (pCGN7800 plus pBBR1MCS-2::atfA) during cultivation on oleate, as revealed by TLC analysis (Fig. 1, lane 6). This substance also occurred in the E. coli strain harboring only the WS/DGAT gene on plasmid pBBR1MCS-2::atfA under these conditions (Fig. 1, lane 4). GC/MS analysis identified this compound as a mixture of various FABEs, mainly consisting of butyl oleate (C22:1) (Fig. 2B). Quantification by GC revealed a FABE content of 10.3 and 15.1 mg g CDW−1 in E. coli BL21(DE3) harboring pBBR1MCS-2::atfA or pBBR1MCS-2::atfA plus pCGN7800, respectively, in the presence of oleate, whereas no FABEs could be detected in the absence of oleate.
1-Butanol is not known to occur as intermediate of the metabolism in E. coli; thus, contamination of medium components was the most likely source of butanol for FABE production in strains harboring WS/DGAT. Systematic analysis of all medium components identified Bacto tryptone as the only source of contamination containing ca. 0.5 mM 1-butanol in a 1% (wt/vol) solution (data not shown). Accordingly, no FABEs were synthesized by E. coli BL21(DE3) harboring pBBR1MCS-2::atfA in the presence of oleate during the cultivation in a mLB medium containing no tryptone (Fig. 3).
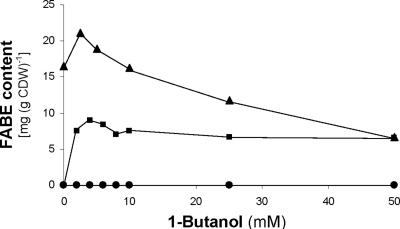
FIG. 3.
1-Butanol dependency of FABE biosynthesis in recombinant E. coli. E. coli BL21(DE3) (pBBR1MCS-2::atfA) was cultivated for 40 h at 37°C in LB medium (▴), mLB medium (•), or mLB medium + 0.2% (wt/vol) sodium oleate (▪). The FABE content of lyophilized cells was determined by GC analysis.
Attempts to increase the FABE content of E. coli BL21(DE3) harboring pBBR1MCS-2::atfA by the addition of 1-butanol to the medium revealed a saturation occurring even at very low 1-butanol concentrations (Fig. 3). Maximum FABE contents were 21.1 and 9.0 mg g CDW−1 during growth in LB or mLB medium, respectively, when 0.2% (wt/vol) sodium oleate was added. No FABEs were produced in mLB medium in the absence of oleate (Fig. 3).
Structure of neutral lipid inclusions in recombinant E. coli.
Biosynthesis of small amounts of wax esters in E. coli BL21(DE3) harboring pCGN7800 plus pBBR1MCS-2::atfA during growth in LB medium did not lead to the formation of obvious intracellular lipid inclusions, as shown by TEM (Fig. 4B). In contrast, significant amounts of wax esters and FABEs were produced in this strain in the presence of oleate, resulting in an enhanced cellular fatty acid content (5.2% of CDW compared to 2.5% of CDW in the strain harboring only the plasmid pCGN7800) (Table 1). These neutral lipids were accumulated as insoluble lipid bodies in the cytoplasm (Fig. 4C to F). Typically, only one large lipid inclusion could be observed per cell, occasionally accompanied by one or few smaller droplets (Fig. 4D). The lipid bodies exhibited a diameter of 100 to 200 nm and seemed to be surrounded by a boundary layer. Therefore, they resembled intracellular wax ester inclusions in the natural WS/DGAT host A. baylyi strain ADP1, in both size and structure (Fig. 4A).
FIG. 4.
Structure of neutral lipid inclusions and localization of WS/DGAT in recombinant E. coli BL21(DE3). Ultrathin sections of cells were analyzed by TEM (A) or by immune TEM employing anti-WS/DGAT IgGs (B to F). Arrows indicate gold particles. Scale bars, 200 nm. (A) A. baylyi strain ADP1 cultivated under conditions promoting storage lipid accumulation. (B) E. coli BL21(DE3) (pCGN7800 plus pBBR1MCS-2::atfA) grown in LB medium. (C to F) E. coli BL21(DE3) (pCGN7800 plus pBBR1MCS-2::atfA) grown in LB medium plus 0.2% (wt/vol) sodium oleate.
By employing specific polyclonal antibodies raised against WS/DGAT, this acyltransferase was found predominantly either in soluble form in the cytoplasm or associated with the boundary layer surrounding the lipid inclusions. A smaller portion was also found to be associated with the cytoplasm membrane (Fig. 4B to F).
DISCUSSION
Despite a broad range of potential applications in many different fields, the commercial use of wax esters from the jojoba plant, termed jojoba oil, is currently strongly restricted due to its high price. Therefore, there is a great demand for alternative sources of jojoba oil-like wax esters. Here, we describe the establishment of heterologous wax ester biosynthesis in a recombinant E. coli strain. Cultivation in the presence of oleate led to the formation of C32:1, C34:1, and C36:2 wax esters (Fig. 2A), which are chemically very similar to the wax esters produced by the jojoba plant (C38:2 to C44:2). Although the amounts of wax esters were relatively small, this nevertheless clearly demonstrated that the biotechnological production of jojoba oil-like lipids from inexpensive and renewable resources like fatty acids employing recombinant microorganisms is feasible in the future. Furthermore, to our knowledge this is the first report of substantial neutral lipid biosynthesis in E. coli and the first example of de novo biosynthesis of wax esters in a recombinant microorganism.
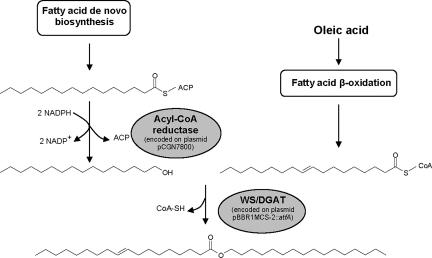
Since no gene encoding a bacterial fatty aldehyde reductase mediating the second step in wax ester biosynthesis could be identified so far, heterologous reconstitution of the entire bacterial pathway in a recombinant organism is not possible at present. However, wax ester synthesis in this study was achieved by a novel approach, combining a plant bifunctional acyl-CoA reductase and a bacterial WS/DGAT. For various reasons, jojoba acyl-CoA reductase is not ideal for use in E. coli and is probably the factor limiting wax ester production. The enzyme is specific for very-long-chain acyl-CoAs (C24:1), which do not occur in this host. Its activity towards shorter-chain-length acyl-CoAs typically present in E. coli (C16:0 and C18:1) is >25-fold lower. However, in contrast to palmitoyl-CoA, the jojoba acyl-CoA reductase exhibits a somewhat higher specificity towards the corresponding acyl carrier protein (ACP) thioester (palmitoyl-ACP) (14). This explains why palmityl alcohol, synthesized from palmitoyl-ACP derived from fatty acid de novo biosynthesis, constitutes the predominant fatty alcohol moiety of wax ester produced in E. coli, even during cultivation on oleate (Fig. 5). Recently, other fatty alcohol-producing acyl-CoA reductases were identified in mice and humans which are specific for shorter-chain-length acyl-CoAs (C16:0 to C18:1) (2). By combining those enzymes with the bacterial WS/DGAT, higher wax ester levels might be obtained. However, it is not known if these acyl-CoA reductases can be expressed as functional active enzymes in E. coli.
FIG. 5.
Pathway for wax ester biosynthesis in recombinant E. coli. Shown is the formation of palmityl oleate, the predominant wax ester species produced during cultivation in LB medium containing 0.2% (wt/vol) oleate.
Surprisingly, the formation of FABEs was observed in WS/DGAT-expressing E. coli strains during cultivation on oleate, which could be attributed to the presence of trace amounts of 1-butanol in the medium component Bacto tryptone. On request, the supplier (BD Diagnostic, Sparks, MD) confirmed that 1-butanol is used during the manufacturing process explaining the presence of solvent residues. Obviously, the trace amounts of 1-butanol were taken up by the cells and used as an alternative substrate by WS/DGAT. This is another example of the extraordinary low substrate specificity of this promiscuous acyltransferase, capable of utilizing a broad range of very-short-chain-length to very-long-chain-length alcohols (C2 to C30) (22). FABE formation was dependent on the presence of oleate in the medium, indicating that fatty acid biosynthesis, in contrast to fatty acid β-oxidation, did not provide sufficient substrates for FABE biosynthesis. In addition, the specificity of WS/DGAT for 1-butanol is rather low compared to that of longer-chain-length fatty alcohols (22).
In lipid-producing cells of E. coli, reaching a total fatty acid content of 5.2% of the CDW during cultivation on oleate, intracytoplasmic lipid inclusions were formed (Fig. 4). The size and form of these lipid bodies corresponded well with those of wax ester inclusions occurring in the natural WS/DGAT host A. baylyi strain ADP1. Thus, this unambigiously proves that the formation and structural integrity of bacterial lipid bodies do not require any specific structural proteins, as was recently already speculated by Wältermann et al. (24).
According to a recently proposed model (25), lipid bodies in wax ester- and triacylglycerol-accumulating bacteria are formed by WS/DGAT proteins associated with the cytoplasm membrane. Lipid body biogenesis then proceeds in a first phase by forming an emulsion-like oleogenous layer attached to the cytoplasm membrane, followed by formation of progressively coalescing lipid prebodies in later stages. Finally, the mature lipid bodies, surrounded by a monolayer of phospholipids, are then released into the cytoplasm when a critical size is reached. However, structures interpreted by Wältermann et al. (24) as oleaginous layers and lipid prebodies could not be detected in recombinant E. coli in our study. This is in contrast to the fact that wax ester-accumulating cells of recombinant E. coli grown in LB medium, which exhibited a very low lipid content (Table 1), are virtually arrested in a stage similar to the early phases of lipid body biogenesis. Therefore, they were actually expected to be rich in these early structures. Furthermore, Wältermann and Steinbüchel (25) postulated that bacterial lipid biosynthesis exclusively takes place at the cytoplasm membrane but not at lipid bodies in the cytoplasm. In the case of FABE production in recombinant E. coli, however, a lipid compound is synthesized from two water-soluble substrates (1-butanol and acyl-CoA). Considering that a high portion of WS/DGAT is associated with the boundary layer of lipid inclusions in recombinant E. coli (Fig. 4), it is conceivable that FABE biosynthesis also takes place in the cytoplasm at the surface of lipid bodies, thereby refuting the dogma that bacterial lipid biosynthesis does not occur on lipid bodies by definition (25). In essence, neutral lipid biosynthesis in recombinant E. coli seems to differ in several aspects from the current model of lipid body biogenesis (25). Thus not least, studying wax ester and FABE biosynthesis in recombinant E. coli also might offer a new experimental approach to refine or even redefine our current understanding of lipid body biogenesis and structure in bacteria.
Acknowledgments
We gratefully thank Monsanto (St. Louis, MO) for the provision of plasmid pCGN7800.
REFERENCES
- 1.Bryn, K., E. Jantzen, and K. Bovre. 1977. Occurrence and patterns of waxes in Neisseriaceae. J. Gen. Microbiol. 102:33-43. [DOI] [PubMed] [Google Scholar]
- 2.Cheng, J. B., and D. W. Russell. 2004. Mammalian wax biosynthesis. I. Identification of two fatty acyl-coenzyme A reductases with different substrate specificities and tissue distributions. J. Biol. Chem. 279:37789-37797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeWitt, S., J. L. Ervin, D. Howes-Orchinson, D. Dalietos, and S. L. Neidleman. 1982. Saturated and unsaturated wax ester production by Acinetobacter sp. H01-N grown on C16-C20 n-alkanes. J. Am. Oil Chem. Soc. 59:69-74. [Google Scholar]
- 4.Downing, D. T., J. S. Strauss, and P. E. Pochi. 1969. Variability in the chemical composition of human skin surface lipids. J. Investig. Dermatol. 53:322-327. [DOI] [PubMed] [Google Scholar]
- 5.Ervin, J. L., J. Geigert, S. L. Neidleman, and J. Wadsworth. 1984. Substrate-dependent and growth temperature-dependent changes in the wax ester compositions produced by Acinetobacter sp. H01-N, p. 217-222. In C. Ratledge, P. Dawson, and L. Rattray (ed.), Biotechnology for the oil and fats industry. American Oil Chemists Society, Champaign, Ill.
- 6.Fixter, L. M., M. N. Nagi, J. G. McCormack, and C. A. Fewson. 1986. Structure, distribution and function of wax esters in Acinetobacter calcoaceticus. J. Gen. Microbiol. 132:3147-3157. [Google Scholar]
- 7.Fixter, L. M., and M. K. Sherwani. 1991. Energy reserves in Acinetobacter, p. 273-294. In K. J. Towner, E. Bergogne-Bérézin, and C. A. Fewson (ed.), The biology of Acinetobacter: taxonomy, clinical importance, molecular biology, physiology, industrial relevance. Plenum Press, New York, N.Y.
- 8.Gallagher, I. H. C. 1971. Occurrence of waxes in Acinetobacter. J. Gen. Microbiol. 68:245-247. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton, R. J. (ed.). 1995. Waxes: chemistry, molecular biology and functions. The Oily Press, Dundee, United Kingdom.
- 10.Hills, G. 2003. Industrial use of lipases to produce fatty acid esters. Eur. J. Lipid Sci. Technol. 105:601-607. [Google Scholar]
- 11.Ishige, T., A. Tani, Y. Sakai, and N. Kato. 2003. Wax ester production by bacteria. Curr. Opin. Microbiol. 6:244-250. [DOI] [PubMed] [Google Scholar]
- 12.Kalscheuer, R., and A. Steinbüchel. 2003. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 278:8075-8082. [DOI] [PubMed] [Google Scholar]
- 13.Kalscheuer, R., H. Luftmann, and A. Steinbüchel. 2004. Synthesis of novel lipids in Saccharomyces cerevisiae by heterologous expression of an unspecific bacterial acyltransferase. Appl. Environ. Microbiol. 70:7119-7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metz, J. G. April 1995. Fatty acyl reductase. U.S. patent 5,403,918.
- 15.Metz, J. G., M. R. Pollard, L. Anderson, T. R. Hayes, and M. Lassner. 2000. Purification of a jojoba embryo fatty acyl-coenzyme A reductase and expression of its cDNA in high erucic acid rape seed. Plant Physiol. 122:635-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miwa, T. K. 1971. Jojoba oil wax esters and derived fatty acids and alcohols: gas chromatographic analyses. J. Am. Oil Chem. Soc. 48:259-264. [Google Scholar]
- 17.Nikkari, T. 1974. Comparative chemistry of sebum. J. Investig. Dermatol. 62:257-267. [DOI] [PubMed] [Google Scholar]
- 18.Pollard, M. R., T. McKeon, L. M. Gupta, and P. K. Stumpf. 1979. Studies on biosynthesis of waxes by developing jojoba seed. II. The demonstration of wax biosynthesis by cell-free homogenates. Lipids 14:651-662. [Google Scholar]
- 19.Reiser, S., and C. Somerville. 1997. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J. Bacteriol. 179:2969-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell, N. J., and J. K. Volkman. 1980. The effect of growth temperature on wax ester composition in the psychrophilic bacterium Micrococcus cryophilus ATCC 15174. J. Gen. Microbiol. 118:131-141. [Google Scholar]
- 21.Stein, S., A. Levitsky, O. Fateev, and G. Mallard. 1998. The NIST Mass Spectral Search Program. Windows software, version 1.6d.
- 22.Stöveken, T., R. Kalscheuer, U. Malkus, R. Reichelt, and A. Steinbüchel. 2005. The wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferase from Acinetobacter sp. strain ADP1: characterization of a novel type of acyltransferase. J. Bacteriol. 187:1369-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uthoff, S., T. Stöveken, N. Weber, K. Vosmann, E. Klein, R. Kalscheuer, and A. Steinbüchel. 2005. Thio wax ester biosynthesis utilizing the unspecific bifunctional wax ester synthase/diacylglycerol acyltransferase of Acinetobacter sp. strain ADP1. Appl. Environ. Microbiol. 71:790-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wältermann, M., A. Hinz, H. Robenek, D. Troyer, R. Reichelt, U. Malkus, H. J. Galla, R. Kalscheuer, T. Stöveken, P. von Landenberg, and A. Steinbüchel. 2005. Mechanism of lipid-body formation in prokaryotes: how bacteria fatten up. Mol. Microbiol. 55:750-763. [DOI] [PubMed] [Google Scholar]
- 25.Wältermann, M., and A. Steinbüchel. 2005. Neutral lipid bodies in prokaryotes: recent insights into structure, formation, and relationship to eukaryotic lipid depots. J. Bacteriol. 187:3607-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]