Abstract
Archaea were detected in molecular diversity studies of the permanently frozen Lake Fryxell, Antarctica. Two clusters of methanogens were detected in the sediments, and another cluster of possibly methanotrophic Euryarchaeota was detected in the anoxic water column just above the sediments. One crenarchaeote was detected in water just below the oxycline. The Archaea present in Lake Fryxell are likely involved in the major biogeochemical cycles that occur there.
Several permanently frozen lakes exist in the Taylor Valley, McMurdo Dry Valleys, Antarctica. These lakes support an exclusively microbial biology and have waters that vary from strictly freshwater to hypersaline (33). Lake Fryxell is essentially a freshwater lake (18, 33) and is the most productive of the lakes in the Taylor Valley (20). However, Lake Fryxell also shows significant sulfur cycling (10); the water column has a strong gradient of sulfide, exceeding 1 mM just above the sediments (Fig. 1). Our previous work has shown that a diverse community of phototrophic purple bacteria (1, 12, 13), sulfur chemolithotrophic bacteria (W. M. Sattley, E. A. Karr, L. A. Achenbach, and M. T. Madigan, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. I-129, 2003), and sulfate-reducing bacteria (SRB) (14) exists in Lake Fryxell. However, besides sulfidogenesis, methanogenesis also occurs in Lake Fryxell (32). Methane is produced in the sediments and is consumed in the anoxic water column (Fig. 1). Thus, in addition to sulfur cycling, methane cycling also occurs in Lake Fryxell, implying that both methanogenic and methanotrophic prokaryotes are present.
FIG. 1.
Geochemistry of Lake Fryxell. Methane data were obtained from reference 32. Salinity in Lake Fryxell ranges from 0.1 to 0.66% NaCl from 6 to 18 m (18).
In this paper we explore the biodiversity of Archaea in Lake Fryxell based on 16S rRNA gene sequences. The results reveal that at least two phyla of Archaea inhabit this lake, including methanogenic and possibly methanotrophic species, as well as sulfur-metabolizing species.
Sample collection and processing.
Water and sediment samples were collected from Lake Fryxell during November 2001 and November 2003 at Global Positioning System coordinates 77°36.570S, 163°08.969E as previously described (13, 14). Water samples were collected at depths of 9, 11, 14, and 17 m, and surface sediment samples were collected with an Eckman dredge. Limnological parameters were measured directly in the field or in samples returned to Crary Lab as previously described (13, 14).
PCR and phylogenetic analyses.
Microbial biomass was concentrated and genomic DNA extracted as previously described (13, 14). Total community DNA was amplified using the archaeon-specific primers ARCH 344F (5′-ACGGGGTGCAGGCGCGA-3′) and ARCH 915R (5′-GTGCTCCCCCGCCAATTCCT-3′) (3). PCRs were established and processed as previously described (13, 14) with cycling parameters as follows: an initial denaturation for 15 s at 94°C followed by 30 cycles of 15 s at 94°C, 20 s at 54°C, and 54 s at 72°C; the reaction was concluded with a 1-minute extension at 72°C. Denaturing gradient gel electrophoresis (DGGE) was carried out on a 6% acrylamide gel with a 20 to 70% denaturant range and electrophoresis at 200 V for 3.5 h at 60°C (3). Selected DGGE bands were excised and sequenced as previously described (13, 14).
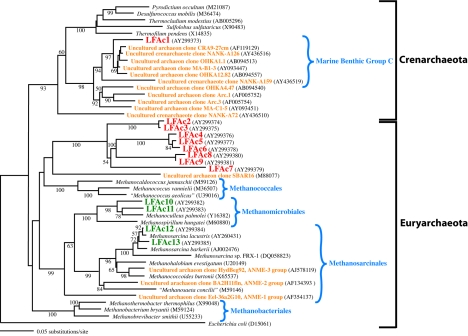
Details of phylogenetic tree construction are described in the legend to Fig. 2. GenBank accession numbers for the organisms used to build the tree are indicated in Fig. 2.
FIG. 2.
Phylogenetic tree of 16S rRNA genes obtained using archaeon-specific primers on bulk DNA from the water column and sediments of Lake Fryxell. The tree is based on 621 nucleotide positions of the 16S rRNA gene, using the Kimura two-parameter distance measure in a heuristic search. All designations beginning with LF are from Lake Fryxell. Relevant environmental clone sequences from other studies are included. GenBank accession numbers are in parentheses. Bootstrap values of >50% based on 100 replicates are represented. Red, water column clones; green, sediment clones; orange, other environmental sequences. Depths from which clones were obtained: LFAc1, 11 m; LFAc2, -3, -5, -8 and -9, 14 m; LFAc4, -6, and -7, 17 m.
Euryarchaeota in Lake Fryxell.
Genes encoding archaeal 16S rRNA were amplified from DNA obtained from either water or sediments of Lake Fryxell, and the amplification products were resolved by DGGE (data not shown). Selected DGGE bands were reamplified and sequenced. All of the reamplified bands were from water taken at 11, 14, or 17 m or from surface sediments.
Phylogenetic analysis of the 16S rRNA gene sequences obtained showed that at least four clusters of Archaea inhabited Lake Fryxell, i.e., three clusters of Euryarchaeota and one of Crenarchaeota (Fig. 2). Within the Euryarchaeota, at least two clusters of methanogens were detected. Phylotypes LFAc12 and -13 were closely related to 16S rRNA gene sequences from Methanosarcina species (Fig. 2). The presence of Methanosarcina-like methanogens in Lake Fryxell sediments has been confirmed by the recent isolation of strain FRX-1, a methylotrophic Methanosarcina species (31). The 16S rRNA gene sequence of strain FRX-1 is distantly related to those of our environmental clones LFAc12 and -13 (Fig. 2).
The other methanogens in Lake Fryxell sediments (phylotypes LFAc10 and -11) clustered with species of Methanoculleus. Unlike for Methanosarcina, no cultures of Methanoculleus species have been obtained from Lake Fryxell; however, a Methanoculleus-like 16S rRNA gene was previously detected in a molecular diversity study of the cyanobacterial mats that develop in the peripheral melt waters of this lake (2, 35). Unfortunately, no overlap existed between the mat sequence and our sequences, so they could not be compared. However, both mat and sediment sequences showed Methanoculleus palmolei to be the closest cultured relative (Fig. 2) (2), suggesting that the same species may reside in both of these habitats.
A large cluster of Euryarchaeota (phylotypes LFAc2 to -9) was found in the anoxic deep waters of Lake Fryxell between 14 and 17 m (Fig. 2). This cluster was related to marine Euryarchaeota (4) and to several orders of methanogens (36). This group will be discussed below in connection with the anoxic methanotrophy that occurs in Lake Fryxell.
Crenarchaeota in Lake Fryxell.
The sole crenarchaeote phylotype recovered from Lake Fryxell was obtained from water collected near the oxycline. This sequence showed significant similarity to the Crenarchaeota cluster Marine Benthic group C (4, 7) (Fig. 2). At a depth of 11 m in Lake Fryxell, both sulfide and oxygen are detectable (Fig. 1). Since both oxygen and sulfide are present and the 9- to 11-m oxygen zone is known to support sulfide- and thiosulfate-oxidizing chemolithotrophs (Sattley et al., Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., 2003), we speculate that LFAc1 may utilize sulfur as either an electron donor or electron acceptor.
Ecology of methanogenesis and sulfidogenesis in Lake Fryxell.
Lake Fryxell is unusual among Taylor Valley lakes in that it shares aspects of both freshwater and marine ecosystems (18). The relatively high concentration of sulfate in the water column of Lake Fryxell (>1 mM) is sufficient to support significant dissimilatory sulfate reduction (Fig. 1), a process typical of marine sediments (37). However, as a result of sulfidogenesis, sulfate is greatly depleted between 14 m and the sediments, with levels in Lake Fryxell surface sediments below 100 μM (Fig. 1). The latter is typical of freshwater sediments, where methanogenesis is the dominant terminal anaerobic metabolism (21, 27).
As is typical in freshwater lakes (21, 27), the competition for H2 between methanogens and SRB in Lake Fryxell sediments should favor methanogens; the sediments are sulfate limited (Fig. 1) and the lake supersaturated with CO2 (18, 24). Indeed, significant rates of H2-driven methanogenesis have been measured in Lake Fryxell sediments (32). Moreover, metabolism of acetoclastic and methylotrophic methanogens such as Methanosarcina, an organism known to exist in Lake Fryxell sediments (Fig. 2) (31), should funnel acetate and available methylated compounds into methane. Again, direct measurements of acetate conversion to methane in Lake Fryxell sediments support this (32).
In contrast to the case for methanogens, the major electron donors for sulfate reduction in Lake Fryxell are unknown; however, methane is likely to be one of them (see the next section). Interestingly, high concentrations of dissolved organic carbon, including fatty acids, are present in Lake Fryxell (22, 23, 32, 34). Since fatty acids are key substrates of marine SRB, this suggests that fatty acid-degrading SRB are either absent or minor components of this ecosystem. Nutritional studies of three strains of SRB isolated from Lake Fryxell support this. All three strains are unable to grow on fatty acids and lack a salt requirement (29; W. M. Sattley and M. T. Madigan, unpublished data), properties associated with freshwater species of SRB (37). Moreover, enrichment cultures for acetate-degrading SRB in Lake Fryxell have been negative (W. M. Sattley and M. T. Madigan, unpublished data). By contrast, our molecular biodiversity study of SRB in Lake Fryxell detected several distant relatives of marine species in the sediments and water column (14). Since both freshwater and marine SRB use H2 as an electron donor (38), any H2 produced in the Lake Fryxell water column should go to sulfate reduction.
Anoxic methanotrophy in Lake Fryxell.
The limnological profile of Lake Fryxell shows that methane, produced in the sediments (32), disappears in the anoxic zone between the sediments and a depth of about 12 m (Fig. 1). Direct measurements of anoxic methanotrophy in Lake Fryxell have confirmed this (J. Lawson, personal communication). Measurements of Eh in Lake Fryxell have shown that sulfate reduction is possible below a depth of 11 m (19). Between a depth of 12 m and the sediments, about 0.9 mM of CH4 is consumed and 1 mM sulfide produced (Fig. 1). Since alternative electron acceptors such as NO3−, Mn4+, and Fe3+ are undetectable in the anoxic waters of Lake Fryxell (19), sulfate is almost certainly the oxidant for the methane oxidation that occurs there, as it is in marine sediments (9) and other sulfidic lakes, such as Big Soda Lake, Nevada (11). Assuming that anoxic methanotrophy is sulfate driven in Lake Fryxell, what is the microbiology of this process?
We propose that anoxic methanotrophy in Lake Fryxell is catalyzed by the water column Euryarchaeota (phylotypes LFAc2 to -9) that we have identified in this study. It is exactly within the highly sulfidic and methanotrophic deep waters of Lake Fryxell that these organisms were restricted (Fig. 1 and 2). Anoxic methanotrophy in marine sediments is catalyzed by three groups of Archaea, i.e., ANME-1, ANME-2, and ANME-3 (8, 15), living in consortia with sulfate-reducing bacteria (9). Although phylotypes LFAc2 to -9 are only distantly related to the ANME groups (Fig. 2), this does not preclude the possibility that they are a new group of methanotrophs that partner with sulfate-reducing bacteria to oxidize methane. However, even if eventual isolation of cultures of phylotypes LFAc2 to -9 shows them not to be methanotrophs, their metabolism should be of considerable interest. This is because in contrast to their euryarchaeotal relatives that inhabit oxic marine waters, phylotypes LFAc2 to -9 inhabit strictly anoxic and highly sulfidic freshwater and are thus likely to employ novel forms of energy metabolism.
Final remarks.
Although the presence of Archaea in constantly cold marine waters is well known (4, 16, 17, 25, 26, 28, 30), our discovery of a diverse group of Archaea in Lake Fryxell is the first such report of their existence in the water column and sediments of a permanently frozen continental Antarctic lake (77°S). Besides strain FRX-1 (31), cultures of two other methanogens have been isolated from Antarctic lakes. A new species of the genus Methanococcoides, M. burtonii, was isolated from Ace Lake in eastern Antarctica (68°S) (6). A truly psychrophilic methanogen, Methanogenium frigidum, was also isolated from Ace Lake (5). Although no close relatives of these organisms were detected in our molecular survey of Lake Fryxell (Fig. 2), we state this with the caveat that not all of our DGGE bands were selected for sequence analysis. Thus, M. burtonii- and M. frigidum-like methanogens as well as other methanogens (and even other Crenarchaeota) could reside in Lake Fryxell. In addition to M. burtonii and M. frigidum, other methanogens have been detected in molecular diversity studies of freshwater lakes in the South Orkney Islands (62°S) (28), near the Antarctic continent.
Continued study of the Archaea in Lake Fryxell, the only lake in the Taylor Valley that supports extensive methanogenesis and sulfidogenesis (10, 18, 19, 32), may reveal new connections between the sulfur and methane cycles in cold environments. A major challenge is to culture the organisms responsible for these processes in Lake Fryxell, in particular those involved in anoxic methanotrophy. This will be required if we are to fully understand the terminal anaerobic processes that occur in the constantly cold waters and sediments of this permanently frozen Antarctic lake.
Nucleotide sequence accession numbers.
Environmental 16S rRNA gene sequences LFAc1 to -13 obtained from Lake Fryxell water or sediment have been deposited in GenBank under accession numbers AY299373 to AY299385, respectively.
Acknowledgments
This work was supported by NSF grants OPP0085481 and MCB0237576.
We thank Raytheon Polar Services, Petroleum Helicopters Inc., and John Priscu and the McMurdo LTER limno team for logistic support in the Antarctic. We also thank Melissa Kendall for the 16S rRNA gene sequence of strain FRX-1 and Deb Jung for graphics preparation.
REFERENCES
- 1.Achenbach, L. A., J. Carey, and M. T. Madigan. 2001. Photosynthetic and phylogenetic primers for detection of anoxygenic phototrophs in natural environments. Appl. Environ. Microbiol. 67:2922-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brambilla, E., H. Hippe, A. Hagelstein, B. J. Tindall, and E. Stackebrandt. 2001. 16S rDNA diversity of cultured and uncultured prokaryotes of a mat sample from Lake Fryxell, McMurdo Dry Valleys, Antarctica. Extremophiles 5:22-33. [DOI] [PubMed] [Google Scholar]
- 3.Casamayor, E. O., R. Massana, S. Benlloch, L. Øverås, B. Diez, V. J. Goddard, J. M. Gasol, I. Joint, F. Rodríguez-Valera, and C. Pedrós-Alió. 2002. Changes in archaeal, bacterial and eukaryal assemblages along a salinity gradient by comparison of genetic fingerprinting methods in a multipond solar saltern. Environ. Microbiol. 4:338-348. [DOI] [PubMed] [Google Scholar]
- 4.DeLong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franzmann, P. D., Y. Lui, D. L. Balkwill, H. C. Aldrich, E. Conway de Macario, and D. R. Boone. 1997. Methanogenium frigidum sp. nov., a psychrophilic H2-utilizing methanogen from Ace Lake, Antarctica. Int. J. Syst. Bacteriol. 47:1068-1072. [DOI] [PubMed] [Google Scholar]
- 6.Franzmann, P. D., N. Springer, W. Ludwig, E. Conway de Macario, and M. Rhode. 1993. A methanogenic archaeon from Ace Lake, Antarctica: Methanococcoides burtonii sp. nov. Syst. Appl. Microbiol. 15:573-581. [Google Scholar]
- 7.Fuhrman, J. A., K. McCallum, and A. A. Davis. 1992. Novel major archaebacterial group from marine plankton. Nature 356:148-149. [DOI] [PubMed] [Google Scholar]
- 8.Hallam, S. J., P. R. Girguis, C. M. Preston, P. M. Richardson, and E. F. DeLong. 2003. Identification of methyl coenzyme M reductase A (mcrA) genes associated with methane-oxidizing Archaea. Appl. Environ. Microbiol. 69:5483-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinrichs, K. U., and A. B. Boetius. 2002. The anaerobic oxidation of methane: new insights in microbial ecology and biogeochemistry, p 457-477. In G. Wefer, D. Billet, D. Hebbeln, B. B. Jørgensen, M. Schlüter, and T. van Weering (ed.), Ocean margin systems. Springer-Verlag, Heidelberg, Germany.
- 10.Howes, B. L., and R. L. Smith. 1990. Sulfur cycling in a permanently ice covered amictic Antarctic lake, Lake Fryxell. Ant. J. U.S. 25:230-233. [Google Scholar]
- 11.Iversen, N., R. S. Oremland, and M. J. Klug. 1987. Big Soda Lake (Nevada). 3. Pelagic methanogenesis and anaerobic methane oxidation. Limnol. Oceanog. 32:804-814. [Google Scholar]
- 12.Jung, D. O., L. A. Achenbach, E. A. Karr, S. Takaichi, and M. T. Madigan. 2004. A gas vesiculate planktonic strain of the purple nonsulfur bacterium Rhodoferax antarcticus isolated from Lake Fryxell, Dry Valleys, Antarctica. Arch. Microbiol. 182:236-243. [DOI] [PubMed] [Google Scholar]
- 13.Karr, E. A., W. M. Sattley, D. O. Jung, M. T. Madigan, and L. A. Achenbach. 2003. Remarkable diversity of phototrophic purple bacteria in a permanently frozen Antarctic lake. Appl. Environ. Microbiol. 69:4910-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karr, E. A. W. M. Sattley, M. R. Rice, D. O. Jung, M. T. Madigan, and L. A. Achenbach. 2005. Diversity and distribution of sulfate-reducing bacteria in the permanently frozen Lake Fryxell, McMurdo Dry Valleys, Antarctica. Appl. Environ. Microbiol. 71:6353-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knittel, K., T. Lösekann, A. Boetius, R. Kort, and R. Amann. 2005. Diversity and distribution of methanotrophic Archaea at cold seeps. Appl. Environ. Microbiol. 71:467-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotsyurbenko, O. R., A. N. Nozhevnikova, T. I. Soloviova, and G. A. Zavarzin. 1996. Methanogenesis at low temperatures by microflora of tundra wetland soil. Antonie Leeuwenhoek 69:75-86. [DOI] [PubMed] [Google Scholar]
- 17.Kotsyurbenko, O. R., K-J. Chin, M. V. Glagolev, S. Stubner, M. V. Simankova, A. N. Nozhevnikova, and R. Conrad. 2004. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 6:1159-1173. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence, M. J. F., and C. H. Hendy. 1985. Water column and sediment characteristics of Lake Fryxell, Taylor Valley, Antarctica. N. Zealand J. Geol. Geophys. 28:543-552. [Google Scholar]
- 19.Lee, P. A., J. A. Mickucki, C. M. Foreman, J. C. Priscu, G. R. DiTullio, S. F. Riseman, S. J. deMora, C. R. Wolf, and L. Kester. 2004. Thermodynamic constraints on microbially mediated processes in lakes of the McMurdo Dry Valleys, Antarctica. Geomicrobiol. J. 21:221-237. [Google Scholar]
- 20.Lizotte, M. P., and J. C. Priscu. 1998. Pigment analysis of the distribution, succession, and fate of phytoplankton in the McMurdo Dry Valley lakes of Antarctica, p. 229-239. In J. C. Priscu (ed.), Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. Antarctic Research Series, vol. 72. American Geophysical Union, Washington, D.C. [Google Scholar]
- 21.Lovley, D. R., and M. J. Klug. 1986. Model for the distribution of sulfate reduction and methanogenesis in freshwater sediments. Geochim. Cosmochim. Acta 50:11-18. [Google Scholar]
- 22.Matsumoto, G. I. 1989. Biogeochemical study of organic substances in Antarctic lakes. Hydrobiologia 172:265-289. [Google Scholar]
- 23.Matsumoto, G. I., K. Watanuki, and T. Torrii. 1989. Vertical distribution of organic constituents in an Antarctic lake: Lake Fryxell. Hydrobiologia 172:291-303. [Google Scholar]
- 24.Neumann, K., W. B. Lyons, and D. J. DesMarais. 1998. Inorganic carbon-isotope distribution and budget in the Lake Hoare and Lake Fryxell basins, Taylor Valley, Antarctica. Ann. Glaciol. 27:685-690. [Google Scholar]
- 25.Nozhevnikova, A. N., C. Holliger, A. Ammann, and A. J. B. Zehnder. 1997. Methanogenesis in sediments from deep lakes at different temperatures (2-70°C). Water Sci. Technol. 36:57-64. [Google Scholar]
- 26.Nozhevnikova, A. N., M. V. Simankova, S. N. Parshina, and O. R. Kotsyurbenko. 2001. Temperature characteristics of methanogenic archaea and acetogenic bacteria isolated from cold environments. Water Sci. Technol. 44:41-48. [PubMed] [Google Scholar]
- 27.Oremland, R. S. 1988. Biogeochemistry of methanogenic bacteria, p. 641-705. In A. J. B. Zehnder (ed.), Biology of anaerobic bacteria. John Wiley & Sons, New York, N.Y.
- 28.Purdy, K. J., D. B. Nedwell, and T. M. Embley. 2003. Analysis of the sulfate-reducing bacterial and methanogenic archaeal populations in contrasting Antarctic sediments. Appl. Environ. Microbiol. 69:3181-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees, G. N., P. H. Janssen, and C. G. Harfoot. 1986. An unusual strain of Desulfovibrio sp. from an Antarctic lake. FEMS Microbiol. Lett. 37:363-366. [Google Scholar]
- 30.Simankova, M. V., O. R. Kotsyurbenko, T. Lueders, A. N. Nozhevnikova, B. Wagner, R. Conrad, and M. W. Friedrich. 2003. Isolation and characterization of new strains of methanogens from cold terrestrial habitats. Syst. Appl. Microbiol. 26:312-318. [DOI] [PubMed] [Google Scholar]
- 31.Singh, N., M. M. Kendall, Y. Liu, and D. R. Boone. 2005. Isolation and characterization of methylotrophic methanogens from anoxic marine sediments in Skan Bay, Alaska, description of Methanococcoides alaskense sp. nov., and emendation of Methanosarcina baltica. Intl. J. Syst. Evol. Microbiol. 55:2531-2538. [DOI] [PubMed] [Google Scholar]
- 32.Smith, R. L., L. G. Miller, and B. L. Howes. 1993. The geochemistry of methane in Lake Fryxell, an amictic, permanently ice-covered, Antarctic lake. Biogeochemistry 21:95-155. [Google Scholar]
- 33.Spigel, R. H., and J. C. Priscu. 1998. Physical limnology of the McMurdo Dry Valleys Lakes, p. 153-187. In J. C. Priscu (ed.), Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. Antarctic Research Series, vol. 72. American Geophysical Union, Washington, D.C. [Google Scholar]
- 34.Takacs, C. D., J. C. Priscu, and D. M. McKnight. 2001. Bacterial dissolved organic carbon demand in McMurdo Dry Valley lakes, Antarctica. Limnol. Oceanogr. 46:1189-1194. [Google Scholar]
- 35.Taton, A., S. Grubisic, E. Brambilla, R. De Wit, and A. Wilmotte. 2003. Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): a morphological and molecular approach. Appl. Environ. Microbiol. 69:5157-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitman, W. B., D. R. Boone, Y. Koga, and J. Keswani. 2001. Taxonomy of methanogenic Archaea, p. 211-213. In D. R. Boone, R. W. Castenholz, and G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer, New York, N.Y. [Google Scholar]
- 37.Widdel, F. 1986. Microbiology and ecology of sulfate- and sulfur-reducing bacteria, p. 468-586. In A. J. B. Zehnder (ed.), Ecology of anaerobic microorganisms. John Wiley & Sons, New York, N.Y.
- 38.Widdel, F., and T. A. Hansen. 1992. The dissimilatory sulfate- and sulfur-reducing bacteria, p. 583-624. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer, New York, N.Y.