Abstract
The noncharacterized gene previously proposed as the d-tagatose 3-epimerase gene from Agrobacterium tumefaciens was cloned and expressed in Escherichia coli. The expressed enzyme was purified by three-step chromatography with a final specific activity of 8.89 U/mg. The molecular mass of the purified protein was estimated to be 132 kDa of four identical subunits. Mn2+ significantly increased the epimerization rate from d-fructose to d-psicose. The enzyme exhibited maximal activity at 50°C and pH 8.0 with Mn2+. The turnover number (kcat) and catalytic efficiency (kcat/Km) of the enzyme for d-psicose were markedly higher than those for d-tagatose, suggesting that the enzyme is not d-tagatose 3-epimerase but d-psicose 3-epimerase. The equilibrium ratio between d-psicose and d-fructose was 32:68 at 30°C. d-Psicose was produced at 230 g/liter from 700-g/liter d-fructose at 50°C after 100 min, corresponding to a conversion yield of 32.9%.
The International Society of Rare Sugars defines rare sugars as monosaccharides and their derivatives that rarely exist in nature. According to this definition, d-psicose is a rare sugar. Rare sugars have recently attracted much attention due to their many uses, including uses as low-calorie sweeteners and bulking agents (11, 12, 14, 17, 18), as immunosuppressants in allogeneic orthotopic liver transplantation in rats (6), as potential inhibitors of various glycosidases (20), in ischemia-reperfusion injury of the rat liver (5), and in segmented neutrophil production without other detrimental clinical effects (21). The rare sugars can be made by microbial and enzymatic reactions with ketose epimerase, aldose isomerase, aldose reductase, and oxidoreductase. Bioproduction strategies for all rare sugars have been illustrated with ring form structures (4).
d-Psicose, a carbon-3 epimer of d-fructose, is present in small quantities in commercial carbohydrate or agricultural products. This rare sugar is absorbed poorly in the digestive tract (19), has zero energy for growth, is a useful sweetener used as an aid for weight reduction (18), represses hepatic lipogenic enzyme activity (16), and is nontoxic (17). Even though few studies have investigated the ability of d-tagatose 3-epimerase to convert d-fructose into d-psicose (7-9, 22), only one enzyme source, d-tagatose 3-epimerase from Pseudomonascichorii, has been used for d-psicose production (8). The enzyme was purified and characterized (8), and the d-tagatose 3-epimerase gene was then cloned on the basis of the N-terminal amino acid sequence of the purified d-tagatose 3-epimerase. The gene was expressed in Escherichia coli and then characterized (7).
In this study, the noncharacterized gene previously proposed as the d-tagatose 3-epimerase gene from Agrobacterium tumefaciens was cloned and expressed in E. coli. Biochemical properties such as metal ions, pH, temperature, epimerization reactions, and kinetic parameters were investigated. Determination of the equilibrium ratio and bioconversion between d-fructose and d-psicose by the d-psicose 3-epimerase were also attempted. The genetic and biochemical properties of the enzyme were compared to those of P. cichorii d-tagatose 3-epimerase, which is the only enzyme reported to produce d-psicose (7, 8).
MATERIALS AND METHODS
Bacterial strains, plasmid, and culture conditions.
A. tumefaciens ATCC 33970, E. coli BL21(DE3) (Novagen, Darmstadt, Germany), and the pET-24 a (+) plasmid were used as a source of genomic DNA, host, and vector for expression, respectively. A. tumefaciens was shake cultured (200 rpm) at 30°C for 10 h in a 250-ml flask containing 50 ml of 3.0% trypticase soy broth. The recombinant E. coli for protein expression was cultivated with shaking (200 rpm) in 500 ml of Luria-Bertani (LB) medium in a 2,000 ml flask containing 20-μg/ml kanamycin at 37°C. When the absorbance of bacteria reached 0.5 at 600 nm, isopropyl-β-d-thiogalactopyranoside (IPTG) was added at a final concentration of 0.1 mM, and the d-psicose 3-epimerase was induced and expressed at 16°C overnight.
Gene cloning.
The genomic DNA was isolated from harvested cells of A. tumefaciens using a genomic DNA buffer set (Qiagen, Hilden, Germany). The gene encoding d-psicose 3-epimerase was amplified from the genomic DNA by PCR with Vent DNA polymerase (New England Biolabs, Beverly, MA). The sequence of the oligonucleotide primers used for gene cloning was based on the DNA sequence of the gene previously proposed as the d-tagatose 3-epimerase gene (3). Forward (PE-F, 5′-CGAAAGAGACACCCCATATGAAACACGGCATC-3′) and reverse (PE-R, 5′-GGATATTATCGCAAACTCGAGGCCACCAAGAACGAAG-3′) primers were designed to introduce the NdeI and XhoI restriction sites (underlined) and were synthesized by Bioneer Co. (Daejon, Korea). The amplified DNA fragment was extracted using the QIAquick gel extraction kit (Qiagen) and was digested with the endonucleases NdeI and XhoI (New England Biolabs). The digested DNA fragment was purified using the QIAquick PCR purification kit (Qiagen) and then ligated into the NdeI and XhoI sites of pET-24 a (+). E. coli was transformed with the ligation mixture and plated on LB agar containing kanamycin. A kanamycin-resistant colony was selected, and plasmid DNA from the transformant was isolated using a plasmid purification kit (Qiagen). The resulting expression vector for the A. tumefaciens d-psicose 3-epimerase gene was designated pETPE and confirmed by digestion with both NdeI and XhoI endonucleases. The recombinant E. coli cells containing pETPE were grown on LB medium. The expression of the gene encoding d-psicose 3-epimerase was determined by both sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the assay of enzyme activity.
DNA and protein sequence analysis.
DNA sequencing was performed at the DNA sequencing facility of Macrogen Co. (Seoul, Korea). The amino acid sequence of d-psicose 3-epimerase was aligned with other epimerases with the GenomeNet CLUSTAL W program.
Purification of d-psicose 3-epimerase.
The cells were harvested from culture broth by centrifugation at 6,000 × g for 30 min at 4°C, washed twice with 0.85% NaCl, and then resuspended in a lysis buffer (50 mM NaH2PO4-300 mM NaCl, pH 8.0) containing 1-mg/ml lysozyme. The resuspended cells were disrupted by a French press at 15,000 lb/in2. The cell debris was removed by centrifugation at 13,000 × g for 20 min at 4°C, the supernatant was filtered through a 0.45-μm filter, and the filtrate was used as a crude extract. All purification steps using columns were carried out in a cold room at 4°C with a fast protein liquid chromatography system (Amersham Biosciences, Uppsala, Sweden). The crude extract was applied to a 5-ml HisTrap HP affinity chromatography column (Amersham Biosciences) equilibrated with a 50 mM sodium phosphate buffer containing 300 mM NaCl and 10 mM imidazole (pH 8.0). The column was washed extensively with the same buffer, and the bound protein was eluted with a linear gradient between 10 to 200 mM imidazole with a flow rate of 1 ml/min. Highly active fractions were pooled then loaded onto a HiPrep 16/60 desalting chromatography column (Amersham Biosciences), previously equilibrated with 50 mM N-(2-hyroxyethyl)piperazine-N′-(3-propanesulfonic acid) (EPPS) buffer (pH 8.0); the loaded protein was eluted with a flow rate of 6 ml/min; and pooled enzyme fractions were then subjected to Sephacryl S-300 HR gel permeation chromatography (Amersham Biosciences) equilibrated with 50 mM EPPS containing 0.15 M NaCl (pH 8.0) and eluted with a flow rate of 0.66 ml/min. After dialysis against 50 mM EPPS buffer (pH 8.0), the resulting solution was used as a purified enzyme.
Determination of molecular mass.
The subunit molecular mass was analyzed using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS, AXIMA-LNR system; Shimadzu, Kyoto, Japan) with a +20-kV detector, a 3.2-mV/b digitizer, and a cinnamic acid matrix. MALDI-TOF MS was calibrated with apomyoglobin (16,952.3 Da) and aldolase (39,212.3 Da). The concentration of d-psicose 3-epimerase was 0.25 mg/ml.
To determine the molecular mass of the native enzyme, purified enzyme was applied to a Superdex 200 gel permeation chromatography column (HR 10/30; Amersham Pharmacia Biotech) and eluted with 50 mM Tris-HCl (pH 8.0) containing 0.1 M KCl at a flow rate of 0.7 ml/min. The column was calibrated with catalase (232 kDa), aldolase (158 kDa), bovine serum albumin (67 kDa), chymotrypsinogen A (25 kDa), and RNase A (13.7 kDa) as reference proteins (gel filtration calibration kit; Amersham Biosciences).
Enzyme assay.
d-Psicose 3-epimerase was used without EDTA treatment. Unless otherwise stated, enzyme was used after incubation at 20°C with 1 mM Mn2+ for 4 h and following by overnight dialysis at 4°C against 50 mM EPPS buffer (pH 8.0); all reactions were performed at 50°C for 5 min in 50 mM EPPS buffer (pH 8.0) containing 1.0% (wt/vol) d-fructose and 0.04-U/ml enzyme and stopped after 5 min by boiling the enzyme at 100°C. One unit of d-psicose 3-epimerase activity was defined as the amount of the enzyme producing 1 μmol of d-psicose per min at pH 8.0 and 50°C.
Metal ions, pH, and temperature effects on d-psicose 3-epimerase.
To evaluate the effect of metal ions on d-psicose 3-epimerase, enzymes were used after the treatment of EDTA with the addition of 1 mM each metal ions (such as Mn2+, Co2+, Fe2+, Mg2+, Ca2+, Ba2+, Mo2+, Zn2+, Cu2+, Fe3+, and Al3+) and after incubation at 20°C with 1 mM Mn2+ for 4 h and dialysis overnight at 4°C against 50 mM EPPS buffer (pH 8.0). The activity of d-psicose 3-epimerase was defined as the value relative to activity without the addition of metal ions. To examine the effect of pH on d-psicose 3-epimerase, the pH was varied between 6.5 and 8.5. Two different buffer systems were used: a 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer for pH values between 6.5 and 7.5 and a 50 mM EPPS buffer for pH values from 7.5 to 8.5. To investigate the effect of temperature, temperature was varied from 30 to 70°C.
The experimental enzyme deactivation data were fitted to a first-order curve, and the half-life (t1/2) of the enzyme was calculated using SigmaPlot 8.0 software (15). The influence of temperature on enzyme stability for d-psicose production was monitored for 240 min at temperatures from 45 to 60°C at pH 8.0.
Determination of epimerization activities and kinetic parameters for substrates.
The epimerization reaction for d-fructose, d-psicose, d-sorbose, d-tagatose, d-xylulose, d-ribulose, d-fructose 6-phosphate, or d-ribulose 5-phosphate as a substrate was determined with 10 mM substrate. Various concentrations ranging from 5 to 100 mM of d-fructose, d-psicose, d-sorbose, or d-tagatose were used to determine kinetic parameters of d-psicose 3-epimerase. The Km and kcat values for substrates were calculated using enzyme concentration and Lineweaver-Burk plots of the Michaelis-Menten equation (13).
Equilibrium ratio determination and bioconversion between d-psicose and d-fructose.
The equilibrium ratio of d-psicose and d-fructose was determined from the average value after the reactions were performed at temperatures at 30°C and 40°C for 24 h using five initial ratios (percentages) of 0:100, 25:75, 50:50, 75:25, and 100:0 without the addition of metal ions and with the addition of 1.0 mM Mn2+. The total concentration of d-psicose and d-fructose used was 0.1% (wt/vol), and 10 U/ml of enzyme was used.
The bioconversion of 1% d-fructose into d-psicose by the enzyme was performed for 60 min. For high production of d-psicose, the reaction buffer containing 15 U of enzyme and 700 g/liter d-fructose was incubated at 50°C and pH 8.0 for 100 min.
Analytical methods.
Protein concentrations were determined by the Bradford method using bovine serum albumin as a standard protein (1). The concentrations of d-fructose, d-psicose, d-sorbose, d-tagatose, d-xylulose, and d-ribulose were determined by high-performance liquid chromatograph (SCL-10A; Shimadzu, Kyoto, Japan) equipped with a Shimadzu RID-10A detector and a BP-100 Ca2+ carbohydrate column (Benson Polymeric Inc. Reno, NV). The column was eluted at 80°C with water at a flow rate of 0.5 ml/min.
RESULTS AND DISCUSSION
Cloning of the gene encoding A. tumefaciens d-psicose 3-epimerase.
The d-psicose 3-epimerase gene (0.87 kb) from the genomic DNA of A. tumefaciens ATCC 33970 was amplified using primers based on the DNA sequence of the noncharacterized gene previously reported as the d-tagatose 3-epimerase gene of A. tumefaciens C58 (GenBank accession number AE008210) (3), cloned into the pET-24 a (+) vector to produce pETPE. The gene encoding d-psicose 3-epimerase from A. tumefaciens ATCC 33970 was the same as the d-tagatose 3-epimerase gene from A. tumefaciens C58.
Amino acid sequence alignment of A. tumefaciens d-psicose 3-epimerase.
The expressed enzyme contained a hexahistidine tag at the carboxyl terminus, consisting of 289 amino acid residues, and showed epimerization activity from d-fructose to d-psicose. The epimerization for d-psicose has not been reported with other epimerases except P. cichorii d-tagatose 3-epimerase. The amino acid sequence of A. tumefaciens d-psicose 3-epimerase was linearly aligned with that of P. cichorii d-tagatose 3-epimerase (7) and had 38% similarity (Fig. 1). The other noncharacterized genes previously proposed as the d-tagatose 3-epimerase genes of Agrobacterium tumefaciens (GenBank accession number AE009403), Photorhabdus luminescens (subsp. laumondii), Sinorhizobium meliloti, Rhodopirellula baltica, and Mesorhizobium loti exhibited 35%, 33%, 29%, 27%, and 26% amino acid sequence similarities, respectively. The amino acid sequence alignments indicate that the d-psicose 3-epimerase has no significant similarities to d-tagatose 3-epimerases.
FIG. 1.
Alignment of the amino acid sequence of d-psicose 3-epimerase from A. tumefaciens (previously proposed as d-tagatose 3-epimerase) with that of d-tagatose 3-epimerase from P. cichorii. ATPE, A. tumefaciens d-psicose 3-epimerase (GenBank accession number AE008210); PCTE, P. cichorii d-tagatose 3-epimerase (GenBank accession number AB000361).
Previously cloned sugar epimerases such as d-ribulose 5-phosphate 3-epimerase, l-ribulose 5-phosphate 3-epimerase, UDP-glucose 4-epimerase, UDP-galactose 4-epimerase, UDP-N-acetylglucosamine epimerase, CDP-tyvelose epimerase, dTDT-4-keto-l-rhamnose epimerase, dTDT-6-deoxy-d-glucose 3,5-epimerase, and alginate-C5-mannuronan epimerase (7) exhibit no extensive homology with d-psicose 3-epimerase, suggesting that d-psicose 3-epimerase is genetically different from other sugar epimerases.
Purification of A. tumefaciens d-psicose 3-epimerase.
The crude enzyme was purified by HisTrap HP, HiPrep, and Sephacryl S-300 HR chromatography. The d-psicose 3-epimerase was purified 2.29 fold with a yield of 42.4% and a final specific activity of 8.89 U/mg.
By MALDI-TOF MS, the molecular mass of the protein was estimated as 33,071 ± 66 Da, which was consistent with the calculated value of 33,093 Da based on the 289-residue amino acid and six histidine sequences. The molecular mass of the native enzyme was estimated as 132 kDa by Superdex 200 chromatography to be a tetramer of 33-kDa subunits. However, P. cichorii d-tagatose 3-epimerase had a molecular mass of 68 kDa by gel filtration chromatography and consisted of two identical subunits (8).
Effects of metal ion, pH, and temperature on A. tumefaciens d-psicose 3-epimerase.
Among the metal ions tested at an addition of 1 mM to the substrate, Mn2+ and Co2+ strongly enhanced d-fructose epimerization by A. tumefaciens d-psicose 3-epimerase, giving 274% and 268% relative activity, respectively. Zn2+ and Cu2+ were strongly inhibitory for d-psicose 3-epimerase activity, giving 4.4% and 2.2% residual activity, respectively. The activity of d-psicose 3-epimerase was 20% after the removal of metal ions by treatment with EDTA. When the enzyme was used after Mn2+ incubation and dialysis, the relative activity was 385%. Mn2+ significantly increased the epimerization rate from d-fructose to d-psicose, and the addition of 1 mM Mn2+ to the substrate might have resulted in interference in substrate and enzyme due to excess Mn2+. Therefore, all further experiments were performed after Mn2+ incubation and dialysis. The sugar epimerase like l-ribulose 5-phosphate 4-epimerase requires Mn2+ for catalytic activity, and the metal ion increases the epimerization reaction (2, 10). However, the d-tagatose 3-epimerase from P. cichorii does not require any cofactor for its activity (8).
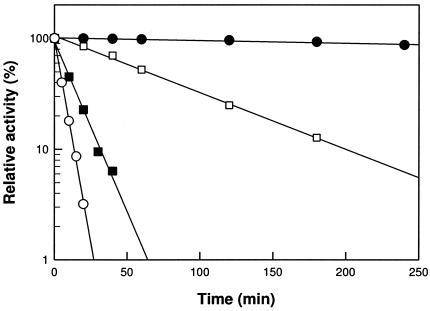
The pH and temperature were varied, and the maximum activity of d-psicose 3-epimerase exhibited pH 8.0 and 50°C. The maximum activity of P. cichorii d-tagatose 3-epimerase showed pH 7.5 and 60°C after a 10-min reaction (8). The activity of d-psicose 3-epimerase was very stable below 45°C but decreased above 50°C with increasing reaction time (Fig. 2). The half-lives (t1/2) for the enzyme were 1265, 63.5, 8.90, and 3.99 min at 45, 50, 55, and 60°C, respectively.
FIG. 2.
Thermal inactivation of A. tumefaciens d-psicose 3-epimerase. The reactions were performed at 45°C (•), 50°C (□), 55°C (▪), and 60°C (○).
Substrate specificity of A. tumefaciens d-psicose 3-epimerase.
The d-psicose 3-epimerase reversibly converted cis configuration ketoses at carbons 3 and 4 (d-psicose and d-tagatose, d-ribulose) to their carbon 3 epimers, trans ketoses (d-fructose, d-sorbose, and d-xylulose) and showed a higher level of activity for cis ketoses than for trans-ketoses (Table 1). The substrate specificity of the enzyme was greatest for d-psicose and decreased for other substrates in the following order: d-fructose, d-tagatose, d-ribulose, d-xylulose, and d-sorbose. There was no activity on d-fructose-6-phosphate or d-ribulose-5-phosphate. The activity of the enzyme on d-psicose was 2.7-fold higher than for d-tagatose. However, the epimerization activity of P. cichorii d-tagatose 3-epimerase for d-tagatose was 1.7-fold higher than for d-psicose. The activity of d-psicose 3-epimerase for d-ribulose was lower than for d-psicose, whereas that of d-tagatose 3-epimerase was higher (8).
TABLE 1.
Epimerization activities of A. tumefaciens d-psicose 3-epimerase and P. cichorii d-tagatose 3-epimerase for ketoses
| Ketose | % Relative activity
|
|
|---|---|---|
| d-Psicose 3-epimerase | d-Tagatose 3-epimerasea | |
| d-Psicose | 100 ± 1.2 | 100 |
| d-Tagatose | 33.7 ± 0.6 | 167 |
| d-Fructose | 50.7 ± 0.8 | 33.3 |
| d-Sorbose | 0.7 ± 0.1 | 33.3 |
| d-Ribulose | 5.6 ± 0.2 | 150 |
| d-Xylulose | 4.5 ± 0.1 | 66.7 |
| d-Fructose-6-phosphate | 0.0 | 0.0 |
| d-Ribulose-5-phosphate | 0.0 | 0.0 |
Cited from reference 8.
The A. tumefaciens d-psicose 3-epimerase showed lower Michaelis-Menten constant (Km), higher turnover number (kcat), and higher catalytic efficiency (kcat/Km) values for d-psicose than for d-fructose (Table 2). The kcat/Km for d-psicose was 586-fold higher than for d-tagatose, indicating that the enzyme was not d-tagatose 3-epimerase but d-psicose 3-epimerase, and therefore a new enzyme. The Km and Vmax values of P. cichorii d-tagatose 3-epimerase for d-tagatose were 55 mM and 30 U/mg, respectively, but the kcat value for d-tagatose and the kinetic parameters for the other substrates were not reported (8).
TABLE 2.
Kinetic parameters of A. tumefaciens d-psicose 3-epimerase for hexoketoses
| Ketose | kcat (min−1) | Km (mM) | kcat/Km (mM−1 min−1) |
|---|---|---|---|
| d-Psicose | 2,381 ± 22 | 12 ± 0.11 | 205 ± 2.67 |
| d-Tagatose | 270 ± 3 | 762 ± 4.3 | 0.35 ± 0.004 |
| d-Fructose | 2,068 ± 28 | 24 ± 0.15 | 85 ± 1.27 |
| d-Sorbose | 1.6 ± 0.1 | 65 ± 0.52 | 0.03 ± 0.002 |
Equilibrium ratio determination and bioconversion between d-psicose and d-fructose.
The equilibrium ratio between d-psicose and d-fructose of P. cichorii d-tagatose 3-epimerase was 20:80 at 30°C (8). The data were obtained from one point reaction with 1% d-fructose. In this study, to obtain an exact equilibrium ratio, a high-concentration enzyme, a low-concentration substrate, and five initial ratios (percentages) of 0:100, 25:75, 50:50, 75:25, and 100:0 were used. The equilibrium ratio of A. tumefaciens d-psicose 3-epimerase was 32:68 at 30°C and 33:67 at 40°C.
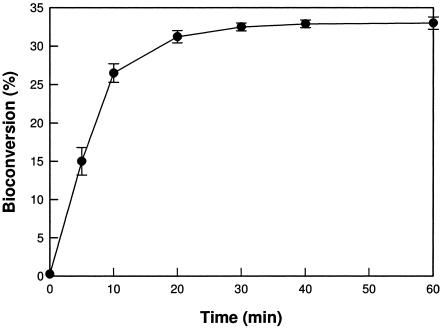
The bioconversion from d-fructose into d-psicose was a conversion yield of 33% at 50°C after 60 min (Fig. 3). For high levels of production of d-psicose, 15 U of enzyme and 700-g/liter d-fructose were used, and d-psicose at 230 g/liter was obtained after 100 min, corresponding to a conversion yield of 32.8%. This concentration was 1.53-fold higher than the d-psicose concentration (150 g/liter) obtained from P. cichorii d-tagatose 3-epimerase (22), suggesting that d-psicose 3-epimerase is a potential d-psicose producer. For a clear comparison with P. cichorii d-tagatose 3-epimerase, the biochemical properties are summarized in Table 3.
FIG. 3.
Bioconversion of d-fructose into d-psicose by A. tumefaciens d-psicose 3-epimerase.
TABLE 3.
Biochemical properties of A. tumefaciens d-psicose 3-epimerase and P. cichorii d-tagatose 3-epimerase
| Biochemical property | d-Psicose 3-epimerase | d-Tagatose 3-epimerasea |
|---|---|---|
| Molecular mass of native protein (kDa) | 132 (tetramer) | 68 (dimer) |
| Temperature for maximum activity (°C) | 50 | 60 |
| pH for maximum activity (buffer) | 8.0 (EPPS) | 7.5 (Tris-HCl) |
| Metal requirement | Mn2+ | No |
| Highest specificity | d-Psicose | d-Tagatose |
| Km for d-tagatose (mM) | 762 | 55 |
| Equilibrium ratio at 30°C between d-psicose and d-fructose | 32:68 | 20:80 |
| High production of d-psicose (g/liter) (temperature, pH) | 230 (50°C, 8.0) | 150b (45°C, 7.0) |
In conculsion, the noncharacterized gene previously proposed as the d-tagatose 3-epimerase gene from A. tumefaciens was cloned and expressed. The catalytic efficiency of the expressed enzyme for d-psicose was 586-fold higher than for d-tagatose, indicating that the enzyme was not d-tagatose 3-epimerase but d-psicose 3-epimerase, and therefore a new enzyme. Using the new enzyme, d-psicose was produced at 230 g/liter from 700-g/liter d-fructose, corresponding to a conversion yield of 32.9%. These findings have considerable importance for the possible commercial manufacture of d-psicose by an enzymatic process.
Acknowledgments
This study was supported by grant number R01-2004-000-10012-0 from the Basic Research Program of the Korea Science and Engineering Foundation (KOSEF).
REFERENCES
- 1.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 2.Deupree, J. D., and W. A. Wood. 1972. l-Ribulose 5-phosphate 4-epimerase from Aerobacter aerogenes. Evidence for a role of divalent metal ions in the epimerization reaction. J. Biol. Chem. 247:3093-3097. [PubMed] [Google Scholar]
- 3.Goodner, B., S. G. G. Hinkle, N. Miller, M. Blanchard, B. Qurollo, B. S. Goldman, Y. Cao, M. Askenazi, C. Halling, L. Mullin, K. Houmiel, J. Gordon, M. Vaudin, O. Iartchouk, A. Epp, F. Liu, C. Wollam, M. Allinger, D. Doughty, C. Scott, C. Lappas, B. Markelz, C. Flanagan, C. Crowell, J. Gurson, C. Lomo, C. Sear, G. Strub, C. Cielo, and S. Slater. 2001. Genome sequence of the plant pathogen and biotechnology agent Agrobacterium tumefaciens C58. Science 294:2323-2328. [DOI] [PubMed] [Google Scholar]
- 4.Granstrom, T. B., G. Takata, M. Tokuda, and K. Izumori. 2004. Izumoring: a novel and complete strategy for bioproduction of rare sugars. J. Biosci. Bioeng. 97:89-94. [DOI] [PubMed] [Google Scholar]
- 5.Hossain, M. A., K. Izuishi, M. Tokuda, K. Izumori, and H. Maeta. 2004. d-Allose has a strong suppressive effect against ischemia/reperfusion injury: a comparative study with allopurinol and superoxide dismutase. J. Hepatobiliary Pancreat. Surg. 11:181-189. [DOI] [PubMed] [Google Scholar]
- 6.Hossain, M. A., H. Wakabayashi, F. Goda, S. Kobayashi, T. Maeba, and H. Maeta. 2000. Effect of the immunosuppressants FK506 and d-allose on allogenic orthotopic liver transplantation in rats. Transplant Proc. 32:2021-2023. [DOI] [PubMed] [Google Scholar]
- 7.Ishida, Y., T. Kamiya, H. Itoh, Y. Kimura, and K. Izumori. 1997. Cloning and characterization of the d-tagatose 3-epimerase gene from Pseudomonas cichorii ST-24. J. Ferment. Bioeng. 83:529-534. [Google Scholar]
- 8.Itoh, H., H. Okaya, A. R. Khan, S. Tajima, S. Hayakawa, and K. Izumori. 1994. Purification and characterization of d-tagatose 3-epimerase from Pseudomonas sp. ST-24. Biosci. Biotechnol. Biochem. 58:2168-2171. [Google Scholar]
- 9.Itoh, H., T. Sato, and K. Izumori. 1995. Preparation of d-psicose from d-fructose by immobilized d-tagatose 3-epimerase. J. Ferment. Bioeng. 80:101-103. [Google Scholar]
- 10.Lee, L. V., R. R. Poyner, M. V. Vu, and W. W. Cleland. 2000. Role of metal ions in the reaction catalyzed by l-ribulose-5-phosphate 4-epimerase. Biochemistry 39:4821-4830. [DOI] [PubMed] [Google Scholar]
- 11.Levin, G. V. 2002. Tagatose, the new GRAS sweetener and health product. J. Med. Food 5:23-36. [DOI] [PubMed] [Google Scholar]
- 12.Levin, G. V., L. R. Zehner, J. P. Saunders, and J. R. Beadle. 1995. Sugar substitutes: their energy values, bulk characteristics and potential health benefits. Am. J. Clin. Nutr. 62(Suppl.):1161S-1168S. [DOI] [PubMed] [Google Scholar]
- 13.Lineweaver, H., and D. Burk. 1934. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56:658-666. [Google Scholar]
- 14.Livesey, G., and J. C. Brown. 1996. d-Tagatose is a bulk sweetener with zero energy determined in rats. J. Nutr. 126:1601-1609. [DOI] [PubMed] [Google Scholar]
- 15.Longo, M. A., and D. Combes. 1999. Thermostability of modified enzymes: a detailed study. Chem. Technol. Biotechnol. 74:25-32. [Google Scholar]
- 16.Matsuo, T., Y. Baba, M. Hashiguchi, K. Takeshita, K. Izumori, and H. Suzuki. 2001. Dietary d-psicose, a C-3 epimer of d-fructose, suppresses the activity of hepatic lipogenic enzymes in rats. Asia Pac. J. Clin. Nutr. 10:233-237. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo, T., and K. Izumori. 2004. d-Psicose, a rare sugar that provides no energy and additionally beneficial effects for clinical nutrition. Asia Pac. J. Clin. Nutr. 13:S127. [Google Scholar]
- 18.Matsuo, T., H. Suzuki, M. Hashiguchi, and K. Izumori. 2002. d-Psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. (Tokyo) 48:77-80. [DOI] [PubMed] [Google Scholar]
- 19.Matsuo, T., T. Tanaka, M. Hashiguchi, K. Izumori, and H. Suzuki. 2003. Metabolic effects of d-psicose in rats: studies on faecal and urinary excretion and caecal fermentation. Asia Pac. J. Clin. Nutr. 12:225-231. [PubMed] [Google Scholar]
- 20.Muniruzzaman, S., Y. T. Pan, Y. Zeng, B. Atkins, K. Izumori, and A. D. Elbein. 1996. Inhibition of glycoprotein processing by l-fructose and l-xylulose. Glycobiology 6:795-803. [DOI] [PubMed] [Google Scholar]
- 21.Murata, A., K. Sekiya, Y. Watanabe, F. Yamaguchi, N. Hatano, K. Izumori, and M. Tokuda. 2003. A novel inhibitory effect of d-allose on production of reactive oxygen species from neutrophils. J. Biosci. Bioeng. 96:89-91. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita, K., A. Suga, G. Takada, and K. Izumori. 2000. Mass production of d-psicose from d-fructose by a continuous bioreactor system using immobilized d-tagatose 3-epimerase. J. Biosci. Bioeng. 90:453-455. [DOI] [PubMed] [Google Scholar]