Abstract
We describe, in Ethiopia, a third successful pattern of human adaptation to high-altitude hypoxia that contrasts with both the Andean “classic” (erythrocytosis with arterial hypoxemia) and the more recently identified Tibetan (normal venous hemoglobin concentration with arterial hypoxemia) patterns. A field survey of 236 Ethiopian native residents at 3,530 m (11,650 feet), 14–86 years of age, without evidence of iron deficiency, hemoglobinopathy, or chronic inflammation, found an average hemoglobin concentration of 15.9 and 15.0 g/dl for males and females, respectively, and an average oxygen saturation of hemoglobin of 95.3%. Thus, Ethiopian highlanders maintain venous hemoglobin concentrations and arterial oxygen saturation within the ranges of sea level populations, despite the unavoidable, universal decrease in the ambient oxygen tension at high altitude.
The demonstration in the past 20 years that the “Andean man” model of high-altitude human adaptation does not generalize to natives of the Tibetan Plateau changed scientific understanding of human adaptation to high-altitude hypoxia. Comparisons of Andean and Tibetan high-altitude natives residing at the same altitudes [usually in the range of 3,500–4,000 m, or 11,600–13,200 feet, where partial pressure of inspired oxygen (PIO2) is 64–60% that of sea level] have revealed quantitative differences in traits associated classically with offsetting ambient hypobaric hypoxia. For example, a hematocrit or hemoglobin concentration elevated over normal sea level values was long considered a hallmark of lifelong adaptation to high-altitude hypoxia (1); however, studies of Tibetans have demonstrated that it is not a necessary response to ambient hypoxia or arterial hypoxemia. The population contrast extends to other traits as well: a comparative study reported that Andean high-altitude natives at 4,000 m had hemoglobin concentration and oxygen saturation of hemoglobin more than 1 standard deviation higher than Tibetans at the same altitude (2). The mean hemoglobin concentration of Tibetans was not elevated above sea level values despite very low oxygen saturation. The third major high-altitude population, natives of the Semien Plateau of Ethiopia, has not been studied for these traits. Here we present the results of a field study designed to examine hematological aspects of high-altitude adaptation of Ethiopians from 14 to 86 years of age, native residents at 3,530 m (11,650 feet) in the Ambaras Region of the Semien Mountains National Park, North Gondar, Ethiopia. The results illustrate, in Ethiopia, a third pattern of adaptation to high-altitude hypoxia (Table 1).
Table 1.
Three patterns of adaptation to high-altitude hypoxia are identified by comparing the presence (+) or absence (−) of erythrocytosis and arterial hypoxemia
| Partial pressure of inspired oxygen, % of sea level | Erythrocytosis | Arterial hypoxemia | |
|---|---|---|---|
| Sea level | 100 | − | − |
| Ethiopian | 64 | − | − |
| Tibetan | 60 | − | + |
| Andean | 60 | + | + |
Methods
Population and Sample.
Three hundred and thirteen native residents of the Ambaras Region of the Semien Mountains National Park, North Gondar, Ethiopia, mostly of Amharic ethnicity, 14 years of age and older volunteered for a field study in December–January of 1995–1996. Eligibility was determined on the basis of birth and residence in one of the study communities or a nearby community at a similar altitude. Ambaras is a traditional rural community, where residents farm barley and herd sheep, goats, and cattle for consumption and sale. The sample represents ≈18% of the resident population in the relevant age range in the two Peasant Associations from which the study sample was drawn.
Measurements.
An Ethiopian physician (A.G.) collected demographic information and a brief health history from each of the 313 participants during a single visit to a field laboratory established in a central location in the community during December 1995. Hemoglobin concentration, percent of oxygen saturation of arterial hemoglobin, height, and weight were measured on site. Hemoglobin concentration was measured in duplicate (using the cyanmethemoglobin technique, Hemocue Hemoglobinometer, Hemocue, Angelholm, Sweden) immediately after drawing a 3-ml venipuncture blood sample. The reported hemoglobin concentrations are the average of two readings each of two cuvettes. The Hemocue Hemoglobinometer was validated by comparison with a Coulter Model T660 at Metrohealth Hospital, Cleveland. The average difference between the Hemocue and the laboratory reference machine was 0.02 g/dl (n = 19) before fieldwork and 0.20 g/dl (n = 21) afterward. The remaining sample was allowed to separate, and the samples were frozen in liquid nitrogen for transport to and subsequent analyses in our laboratories in Cleveland. All participants had normal hemoglobin A as evaluated by hemoglobin electrophoresis performed using cellulose acetate (Helena Laboratories), and none had an elevated percent of Hemoglobin A2 indicative of thalassemia (as quantified by minicolumn chromatography, Isolab Quik-SEP A1 Columns, Isolab). Erythropoietin concentration was measured by ELISA immunoassay (R & D Systems). The Case Western Reserve University and Addis Ababa University Faculty of Medicine institutional review boards approved the research protocol.
Percent oxygen saturation of arterial hemoglobin was measured noninvasively with finger pulse oximetry (Criticare model 501+, Criticare Systems, Waukesha, WI). Measurements from this model pulse oximeter have been compared with simultaneous measurements obtained from arterial blood samples, and average ≈2% higher at saturations >80% (3, 4). Skin color does not affect the accuracy of finger pulse oximetry (refs. 5–7 and J. W. Severinghaus, personal communication). The reported oxygen saturation is the average of six readings taken 10 seconds apart (8). Height and weight were measured without shoes and with light clothing.
Analyses.
Data points more than 3 standard deviations from the sample mean are excluded from analysis (n = 5 oxygen saturation measurements). To avoid several potential sources of confounding, data from people with poor health, current pregnancy, or recent childbirth were excluded from analyses (n = 28). None of the participants smoked. People with iron deficiency were excluded to reduce the possibility that iron lack was limiting an erythropoietic response. An absence of iron deficiency was defined by normal values of zinc erythrocyte protoporphyrin, plasma ferritin, and transferrin receptor concentration (9). Plasma ferritin was measured with RIA (Ramco, Houston), zinc erythrocyte protoporphyrin was measured with hematofluoremetry (Aviv model 206D, AP hematofluoremeter, Aviv Associates, Lakewood, NJ) and transferrin receptor concentration was measured with enzyme immunoassay (Ramco). Iron deficiency was identified on the bases of zinc erythrocyte protoporphyrin ≥70 μg/dl (n = 6), or plasma ferritin concentration <12 ng/ml (n = 4) or transferrin receptor concentration >8.3 μg/liter (n = 1) (10, 11) or a lack of all three iron status measures (n = 32). Data from 236 healthy people without iron deficiency are presented here. The average zinc erythrocyte protoporphyrin concentration of the reported sample was 30 ± 0.7 μg/dl, the average plasma ferritin concentration was 117 ± 4.8 ng/ml, and the average transferrin receptor concentration was 3.4 ± 0.07 μg/ml (n = 236).
Adults were defined as individuals 21 years of age and older because these cross-sectional data indicated that height growth was complete by then. Body mass index (BMI) was calculated as weight in kg divided by the square of height in m. The 20 males under the age of 21 years had an average age of 18 ± 0.5 years, an average weight of 37 ± 1.6 kg, an average height of 150 ± 1.9 cm, and an average BMI of 16.2 ± 0.5 kg/m2. The 26 females under the age of 21 years had an average age of 17 ± 0.5 years, an average weight of 39 ± 1.1 kg, an average height of 148 ± 1.4 cm, and an average BMI of 17.5 ± 0.3 kg/m2. The 108 males 21 years of age and older had an average age of 44 ± 1.6 years, an average weight of 51 ± 0.5 kg, an average height of 165 ± 0.5 cm, and an average BMI of 19.0 ± 0.2 kg/m2. The 81 females 21 years of age and older had an average age of 41 ± 1.5 years, an average weight of 44 ± 0.6 kg, an average height of 152 ± 0.6 cm, and an average BMI of 19.1 ± 0.2 kg/m2 .
The normality of the distribution of the analyzed variables was evaluated by inspecting probability plots displaying the observed cumulative proportions of each variable plotted on the y axis against the expected cumulative proportions under the assumption of a normal distribution of that variable plotted on the x axis. Those analyses were performed separately for the four age–sex groups. The data points clustered about the straight line y = x, and no values deviated markedly from that line. Therefore, it was concluded that they were normally distributed. Means and SEMs are reported, and a significance level of P < 0.05 was accepted.
Results
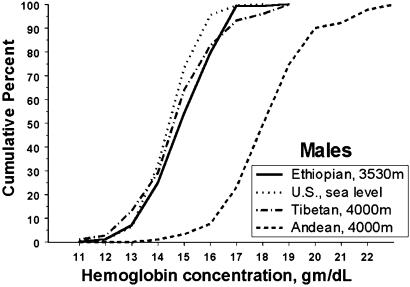
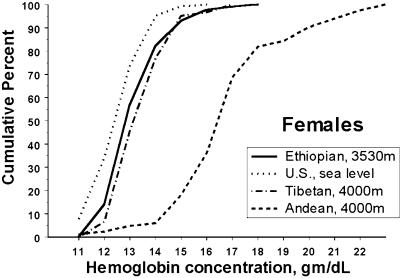
The average hemoglobin concentration of Ambaras males was 15.9 ± 0.1 g/dl, with a range from 12.7 to 18.9 g/dl (n = 128). The average hemoglobin concentration of Ambaras females was 15.0 ± 0.1 with a range from 12.0 to 18.2 (n = 108) g/dl. The Ambaras males' mean hemoglobin concentration was just 0.3 g/dl (2%) higher than the U.S. sea level mean of 15.3 ± 0.02, with a range of 10.4–18.7 g/dl calculated from the published NHANES (National Health and Nutrition Examination Survey) III data set (12, 13, ††). The Ethiopian females' mean hemoglobin concentration was 1.6 g/dl (12%) above the U.S. sea level mean of 13.4 ± 0.02, with a range of 5.2–16.9 g/dl. There was no significant difference between the mean hemoglobin concentrations of adults 21+ years of age and the younger Ethiopians. Figs. 1 and 2 are cumulative frequency distributions of hemoglobin concentration for Ambaras males and females as compared with the U.S. sea level sample and Tibetan and Andean samples at 4,000 m. The hemoglobin distributions of U.S. sea level males, Ambaras males, and Tibetan males coincide nearly completely throughout the range, and all three contrast markedly with the Andean male distribution, which is shifted ≈3.5 g/dl higher. The distribution of U.S. sea level female hemoglobin concentration is slightly lower than that of Ambaras and Tibetan females, whereas all three curves contrast markedly with the Andean female distribution, which is also shifted ≈3.5 g/dl higher.
Fig. 1.
Hemoglobin concentration distributions of U.S. sea level and Ethiopian and Tibetan high-altitude males coincide and contrast with the higher hemoglobin concentrations of Andean males. Cumulative frequency distribution of hemoglobin concentration of Ethiopian high-altitude, U.S. sea level, and Tibetan and Andean high-altitude males.
Fig. 2.
Hemoglobin concentration distributions of U.S. sea level and Ethiopian and Tibetan high-altitude females coincide and contrast with the higher hemoglobin concentrations of Andean females. Shown is the cumulative frequency distribution of hemoglobin concentration of Ethiopian high-altitude, U.S. sea level, and Tibetan and Andean high-altitude females.
Consistent with the lack of elevated hemoglobin concentration in the Ambaras sample, reflecting a lack of increased production of red blood cells, the mean erythropoietin concentration of the sample was 6.6 ± 0.3 milliunits/ml with a range from 0.5 to 24.5 (n = 215). There were no sex or age differences in mean erythropoietin concentration. That mean was just within the sea level normal range of 5–30 milliunits/ml (14). To exclude the possibility of the presence of the anemia of chronic disease (a low hemoglobin concentration caused by infection or inflammation and characterized by low erythropoietin production), we determined serum concentrations of C-reactive protein, an acute phase protein produced during inflammatory states (15). Only 15 of the 235 Ethiopians for whom C-reactive protein was measured had clinically significant elevation (more than 10 mg/liter). That is, just 6% of the sample may have had an anemia of chronic disease. Excluding those 15 did not change the mean hemoglobin concentration and slightly lowered the mean erythropoietin concentration by 0.1 milliunits/ml. Those results are the opposite of the expected elevation of both means if the anemia of chronic disease were the cause of the observed hemoglobin and erythropoietin distributions. These results are consistent with others describing erythropoietin concentration at high altitude in the sense that Ethiopian mean hemoglobin and erythropoietin concentrations are both lower than means of other samples at comparable altitudes (16).
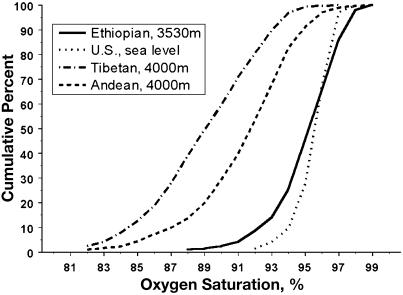
These data indicate that neither iron deficiency, infection (including infection by parasites), nor inflammation were preventing an increase in hemoglobin concentration that would otherwise have occurred. To quantify the physiologic stimulus to erythropoiesis, arterial hypoxemia was measured as the percent of oxygen saturation of arterial hemoglobin. The Ambaras highlanders had an average oxygen saturation of 95.3 ± 0.2% with a range from 88 to 99 (n = 209) that was slightly lower than that among Ethiopian migrants residing near sea level (207 m in Cleveland) who had an average oxygen saturation of 96.7 ± 0.2% (n = 48) (t = 4.1, df = 255, P < 0.05). That is, the Ambaras Ethiopian sample exhibited clinically minimal but statistically significant arterial hypoxemia despite residing at 3,530 m (11,650 feet), where the partial pressure of inspired oxygen is one-third lower than sea level. The cumulative frequency distribution of oxygen saturation in Fig. 3 demonstrates the nearly complete coincidence of the distribution of the Ambaras and a U.S. sea level sample, the lower oxygen saturation of an Andean sample and much lower oxygen saturation of a Tibetan sample. [The Tibetan and Andean oxygen saturation data were reported in refs. 17 and 18. The sea level data are not previously published (n = 78). Oxygen saturation measurement protocols were the same at all four sites.]
Fig. 3.
Oxygen saturation distributions of U.S. sea level residents and high-altitude Ethiopians coincide and contrast with lower oxygen saturations of high-altitude Tibetan and Andean residents. Cumulative frequency distribution of oxygen saturation of Ethiopian high-altitude, U.S. sea level, and Tibetan and Andean high-altitude samples.
Discussion
This sample of high-altitude native residents from the Semien Plateau of Ethiopia had hemoglobin concentrations that differed little from sea level values. The similar findings of erythropoietin concentration and oxygen saturation in the normal sea level range were consistent. The findings would not be remarkable if this were a sea level sample, but it is a sample of people exposed to chronic, lifelong hypobaric hypoxia. Ethiopians must have unique adaptations of oxygen uptake or delivery that result in the absence of an hypoxemic stimulus to increase red blood cell production and hemoglobin concentration despite their high-altitude residence.
The Tibetan and Andean hemoglobin concentration data plotted in Figs. 1 and 2 were reported previously (12). That publication used zinc erythrocyte protoporphyrin and plasma ferritin to detect iron deficiency. Subsequent analyses of transferrin receptor concentration in those two samples indicated that an elevated transferrin receptor concentration was present in 15 of 130 Tibetans (11.5%) and 3 of 162 Andean (2%) highlanders previously identified as not having iron deficiency. The sample mean hemoglobin concentrations were not affected by excluding those with elevated transferrin receptor concentration. Thus differences in iron status do not account for the lower Ethiopian and Tibetan hemoglobin concentrations. The hypothetical possibility that smoking accounts for the high Andean hemoglobin concentrations may be discounted because both males and females exhibited the pattern, and females were nonsmokers in all three samples.
Figs. 1 and 2 present a U.S. “white” population as a sea level comparison because there are no hemoglobin data from sea Ethiopian level samples screened for potential confounders. A U.S. “black” sample might also be considered to compare populations with very broadly similar geographic origins. A subsample of U.S. blacks from the published NHANES III dataset (defined as described in the footnote) had a mean hemoglobin concentration of 14.7 g/dl ± 0.3 (n = 984) and 12.7 ± 0.3 (n = 1,226) for males and females, respectively. The explanation (genetic and/or environmental) for the lower mean hemoglobin concentration of U.S. blacks as compared with whites has been debated over the years (19, 20) without resolution. In addition, the ancestors of the U.S. black population are from West, rather than East, Africa (21), and the Ethiopian Amharic and Oromo populations have allele frequencies at many loci that are intermediate between Europeans and sub-Saharan Africans (reviewed in ref. 22). Thus, the U.S. “black” population is not an ideal comparison. However, there is an Ethiopian intermediate-altitude comparison. A sample from 2,400 m, screened for good health, has essentially the same hemoglobin concentration as the present sample: 16.1 and 14.3 g/dl for adult males and females (23). The absence of an altitude-related difference in hemoglobin concentration in the published Ethiopian sample from 2,400 m and the present sample from 3,530 m (U.S. and Andean samples show a hemoglobin response across this altitude range) supports the inference that this populations remains within the sea-level range despite exposure to high-altitude hypoxia.
The mechanism for achieving the high oxygen saturation is unknown. The flow of gas along the oxygen transport chain is proportional to pressure differences and conductance between the links and thus suggests there is high conductance from the lung to the blood. Hypothetically, this could result from higher diffusion from alveoli to pulmonary blood and/or higher oxygen affinity for hemoglobin. The latter could potentially result from a mutant form of hemoglobin, lower 2,3-diphosphoglycerate or respiratory alkalosis secondary to marked hyperventilation. Because all had normal hemoglobin A by electrophoresis, the possibility of a high oxygen affinity variant of hemoglobin can be excluded (24). Future study of Ethiopian samples spanning the inhabited altitude range from sea level to ≈4,000 m are necessary to confirm and explain these findings.
The results of this study suggest that Ethiopian high-altitude natives respond to hypobaric hypoxia differently than Andean or Tibetan highlanders. This Ethiopian sample resembles samples from the Tibetan Plateau in exhibiting little or no elevation of hemoglobin concentration in this altitude range, but differs from Tibetans who have very low oxygen saturation. It differs from Andean high-altitude natives who exhibited both substantial elevation of hemoglobin concentration and low oxygen saturation. These findings suggest there are three patterns of adaptation to high-altitude hypoxia among indigenous populations (Table 1). Learning why the three populations differ will require two lines of future investigation. One is understanding the biological mechanisms and the underlying genetics that allow successful high-altitude adaptation with quantitatively different suites of traits for oxygen sensing, response, and delivery. The other is understanding the evolutionary processes that produced these patterns to explain how and why several successful human adaptations to high altitude evolved.
Acknowledgments
We thank Mr. Abebaw Derso and Mr. Daniel Tessema for providing technical assistance in the field. We thank Dr. Seleshi Ayalew, Representative of the Ministry of Health in North Gondar, Mr. Wolde Gabriel, Director of the Semien Mountains National Park, Mr. Wubie Moges, and Mr. Berhanu Mogosho for their assistance with logistics and data collection in the field. We are grateful to the residents of Ambaras for their hospitality and cooperation. We are also grateful to the members of the Northeast Ohio Ethiopian Community Organization and to the other low-altitude residents and their families and friends for their cooperation in oxygen saturation measurements at sea level. L. Bowman, J. Minium, and D. Resnicki performed the laboratory analyses in Cleveland and Z. Berkowitz prepared the figures. The field work was supported by National Geographic Society Grant 5520-95 (to C.M.B.).
The values calculated from NHANES III are from a subsample of “whites” 20–49 years of age residing in the U.S. outside the “West” (to exclude the high-altitude residents of the Mountain States) whose self-reported health was good, very good, or excellent and who were not iron deficient as defined by having plasma ferritin concentrations >12 ng/ml and zinc erythrocyte protoporphyrin levels <70 μg/dl.
References
- 1.Winslow R. M. & Monge, C., (1987) Hypoxia, Polycythemia, and Chronic Mountain Sickness (Johns Hopkins Univ. Press, Baltimore).
- 2.Beall C. M. (2000) in Oxygen Sensing: Molecule to Man, ed. Forster, R. E., II (Kluwer Academic, New York), pp. 63–74.
- 3.Severinghaus J. W. & Naifeh, K. H. (1987) Anesthesiology 67, 551-558. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson B. G., Sarkisian, C. & Tremper, K. (1988) Chest 93, 515-517. [DOI] [PubMed] [Google Scholar]
- 5.Adler J. N., Hugher, L. A., Vivilecchia, R. & Camargo, C. A. (1998) Acad. Emer. Med. 5, 965-970. [DOI] [PubMed] [Google Scholar]
- 6.Bothma P., Joynt, G., Lipman, J., Hon, H., Mathala, B., Scribante, J. & Kromberg, J. (1996) S. Afr. Med. J. 86, 594-596. [PubMed] [Google Scholar]
- 7.Cui W., Ostrander, L. & Lee, B. (1990) IEEE Trans. Biomed. Eng. 37, 632-639. [DOI] [PubMed] [Google Scholar]
- 8.Beall C. M., Blangero, J., Williams-Blangero, S. & Goldstein, M. C. (1994) Am. J. Phys. Anthropol. 95, 271-276. [DOI] [PubMed] [Google Scholar]
- 9.Cook J. D. (1999) Proc. Nutr. Soc. 58, 489-495. [DOI] [PubMed] [Google Scholar]
- 10.Expert Scientific Working Group (1985) Am. J. Clin. Nutr. 42, 1318-1330. [DOI] [PubMed] [Google Scholar]
- 11.Ramco Laboratories, (1994) TfR: An in Vitro Enzyme Immunoassay for Quantifying Human Transferring Receptor (Ramco Laboratories, Houston).
- 12.Beall C. M., Brittenham, G. M., Strohl, K. P., Blangero, J., Williams-Blangero, S., Goldstein, M. C., Decker, M. J., Vargas, E., Villena, M., Soria, R., et al. (1998) Am. J. Phys. Anthropol. 106, 385-400. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services National Center for Health Statistics, (1996) Third National Health and Nutrition Examination Survey, 1988–1994, NHANES III Adult, Exam and Laboratory Files (CD-ROM). Public Use Data File Documentation Number 76200 (Centers for Disease Control and Prevention, Hyattsville, MD).
- 14.Ratcliffe P. J., Eckardt, K.-U. & Bauer, C. (1996) in Handbook of Physiology Section 4: Environmental Physiology, eds. Fredley, M. J. & Blatteis, C. M. (Oxford Univ. Press, New York), Vol. II, pp. 1125–1154. [Google Scholar]
- 15.Gabay C. & Kushner, I. (1999) New Engl. J. Med. 340, 448-454. [DOI] [PubMed] [Google Scholar]
- 16.Winslow R. M. (1991) in Response and Adaptation to Hypoxia: Organ to Organelle, ed. Fitzgerald, R. S. (Oxford Univ. Press, New York), pp. 143–153.
- 17.Beall C. M., Almasy, L. A., Blangero, J., Williams-Blangero, S., Brittenham, G. M., Strohl, K. P., Decker, M., Vargas, E., Villena, M., Soria, R., et al. (1999) Am. J. Phys. Anthropol. 108, 41-51. [DOI] [PubMed] [Google Scholar]
- 18.Beall C. M., Strohl, K., Blangero, J., Williams-Blangero, S., Brittenham, G. M. & Goldstein, M. C. (1997) Hum. Biol. 69, 597-604. [PubMed] [Google Scholar]
- 19.Kent S. (1997) Ethn. Dis. 7, 79-90. [PubMed] [Google Scholar]
- 20.Perry G. S., Byers, T., Yip, R. & Margen, S. (1992) J. Nutr. 122, 1417-1424. [DOI] [PubMed] [Google Scholar]
- 21.Curtin P. D., (1969) The Atlantic Slave Trade; A Census (University of Wisconsin Press, Madison).
- 22.Ciminelli B. M., Pompei, F., Relucenti, M., Lum, J. K., Simpore, J., Spedini, G., Martinez-Labarga, C. & Pardo, M. G. (2001) Hum. Biol. 74, 243-252. [DOI] [PubMed] [Google Scholar]
- 23.Tsegaye A., Messele, T., Tilahun, T., Hailu, E., Sahlu, T., Doorly, R., Fontanet, A. L. & Rinke de Wit, T. (1999) Clin. Diagn. Lab. Immunol. 6, 410-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molchanova T. P., Postnikov, Y., Gu, L. H., Prior, J. F., Raven, J. L., Bennett, J. A. & Huisman, T. H. (1993) Hemoglobin 17, 247-250. [DOI] [PubMed] [Google Scholar]