Abstract
Biotransformation plays an increasingly important role in the industrial production of fine chemicals due to its high product specificity and low energy requirement. One challenge in biotransformation is the toxicity of substrates and/or products to biocatalytic microorganisms and enzymes. Biofilms are known for their enhanced tolerance of hostile environments compared to planktonic free-living cells. Zymomonas mobilis was used in this study as a model organism to examine the potential of surface-associated biofilms for biotransformation of chemicals into value-added products. Z. mobilis formed a biofilm with a complex three-dimensional architecture comprised of microcolonies with an average thickness of 20 μm, interspersed with water channels. Microscopic analysis and metabolic activity studies revealed that Z. mobilis biofilm cells were more tolerant to the toxic substrate benzaldehyde than planktonic cells were. When exposed to 50 mM benzaldehyde for 1 h, biofilm cells exhibited an average of 45% residual metabolic activity, while planktonic cells were completely inactivated. Three hours of exposure to 30 mM benzaldehyde resulted in sixfold-higher residual metabolic activity in biofilm cells than in planktonic cells. Cells inactivated by benzaldehyde were evenly distributed throughout the biofilm, indicating that the resistance mechanism was different from mass transfer limitation. We also found that enhanced tolerance to benzaldehyde was not due to the conversion of benzaldehyde into less toxic compounds. In the presence of glucose, Z. mobilis biofilms in continuous cultures transformed 10 mM benzaldehyde into benzyl alcohol at a steady rate of 8.11 g (g dry weight)−1 day−1 with a 90% molar yield over a 45-h production period.
Microbes in nature are commonly in surface-associated, matrix-enclosed structures referred to as biofilms (7, 28). Bacteria in biofilms can differ profoundly in terms of phenotypic characteristics (31, 33) or adaptive responses to stress (17, 38) from their planktonic counterparts, which can provide survival advantages and protection in a range of environmental conditions (16, 23). Biofilms are also commonly found on medical implants and are responsible for many persistent infections due to their increased resistance to antimicrobial agents (13).
While deleterious in clinical settings, biofilms are of great interest in biotechnology. For example, biofilms have been used as biocatalysts in bioremediation and wastewater treatment processes (21, 23, 27) and in fermentative vinegar (9) and ethanol (20) production from agricultural materials, as well as in biosynthesis of polymers (42). To our knowledge, the use of biofilms for transformation of fine chemicals into value-added products has not been reported previously.
Biotransformation using viable cells capable of cofactor regeneration is an important approach to fine-chemical production (30, 34). A common obstacle in biocatalytic fine-chemical production is the potential toxic effects of substrates and/or products on cells during the process. Several strategies, including cell immobilization, have been developed to reduce the toxicity (18, 34). Cell immobilization allows continuous operation with a reduced substrate concentration and continuous product removal. However, forced immobilization via cell entrapment may limit mass transfer (24), and forced immobilization via chemical bonding to a substratum surface may result in compromised cell viability (18). Alternatively, biofilms may be regarded as a natural form of surface immobilization with additional potential for enhanced stress resistance. We hypothesize that viable cells in biofilms may be more tolerant to toxic substrates and/or products and thus act as efficient biocatalysts for fine-chemical production.
The aim of this study was to illustrate the potential for the use of biofilms in fine-chemical production. We investigated biofilm resistance and biotransformation capacity in a model system, using Zymomonas mobilis for the transformation of benzaldehyde. This system was selected based on key criteria, including (i) the ability of Z. mobilis to form biofilms (20), (ii) the suggestion that benzaldehyde is highly toxic to cells due to interference with cell membranes and intracellular enzymes (22), and (iii) the facts that Z. mobilis has useful catalytic activities and is of industrial interest and that its genome has been sequenced (36). Bringer-Meyer and Sahm also reported that planktonic Z. mobilis cells, pulse fed with 10 mmol benzaldehyde per liter 10 times at 45-min intervals, transformed benzaldehyde into benzyl alcohol (37%) and R-phenylacetylcarbinol (3%) (5).
MATERIALS AND METHODS
Bacterial strain and cultivation.
Z. mobilis strain ATCC 31821 was used for this study. Planktonic cultures were grown in Erlenmeyer flasks with seed medium (35 to 40% of the volume of the flask), which contained (per liter) 20 g glucose, 5 g yeast extract, 5 g (NH4)2SO4, 0.6 g KH2PO4, 0.4 g Na2HPO4 · 12H2O, 0.2 g MgSO4 · 7H2O, and 0.01 g CaCl2 at pH 6.4. Cultures were agitated at 150 rpm at 30°C for approximately 24 h, after which time the stationary phase was reached and the cultures had an optical density at 600 nm of 2.9 to 3.2. The resulting cell suspensions were used for the study of planktonic cells and for inoculation of flow cells and packed-bed column biofilm reactors. The growth medium used in flow cells consisted of 10-fold-diluted seed medium, and the growth medium used for column reactors contained (per liter) 5 g glucose, 1 g yeast extract, 1 g (NH4)2SO4, 0.06 g KH2PO4, 0.04 g Na2HPO4 · 12H2O, 0.02 g MgSO4 · 7H2O, and 0.001 g CaCl2 at pH 6.4. 2-(N-Morpholino)ethanesulfonic acid (MES)-buffered benzaldehyde solution consisted of (per liter) 3.9 g MES, 9 g NaCl, and up to 5.3 g benzaldehyde (50 mM) (Sigma-Aldrich). The pH was adjusted to 6.4 using 7.5 M NaOH. For biotransformation, the medium for column reactors was supplemented with (per liter) 1.06 g benzaldehyde (10 mM) and 0.976 g MES (pH 6.4).
Biofilm establishment.
For microscopic analysis, biofilms were grown on glass surfaces in flow cells (channel dimensions, 1 by 4 by 40 mm) at room temperature, as previously described (26). Glass coverslips (Marienfeld, Germany) were treated with dimethylchlorosilane to make them hydrophobic (10) because Z. mobilis exhibited greater attachment and biofilm formation on hydrophobically treated glass than on nontreated glass (data not shown). The flow cell channels were inoculated with stationary-phase seed cultures and incubated with no flow for 1 h to facilitate initial cell attachment. After this attachment period, medium flow was restored, and the flow rate was maintained at 0.15 ml min−1. Estimated as described by Bakker et al. (2), the Reynolds number was 0.5 (indicative of laminar flow) and the theoretical shear rate was approximately 4 s−1. Biofilm development was visualized and photographed using a phase-contrast microscope equipped with a digital camera (Leica, Germany). Congo red (Sigma-Aldrich) was utilized to stain extracellular polysaccharide (EPS) as described by Allison and Sutherland (1).
Biofilms were also cultivated in continuous packed-bed column reactors to generate biomass for metabolic activity studies. The reactors were modified cotton-stoppered glass test tubes (inside diameter, 16 mm; height, 130 mm) into which a glass inlet was inserted at the bottom and an outlet was inserted at a height which defined a packing volume of 20 ml. Acid-washed, hydrophobically treated 3-mm glass beads (Ajax, Australia) were used as the packing material, and the measured void volume was 50%, with a ratio of surface area to packing volume of 29.6 cm2 cm−3. Packed reactors were filled with a seed culture for a 1-h attachment period. Medium flow was restored, and the flow rate was maintained at 0.15 ml min−1 at 30°C. This corresponded to a Reynolds number of approximately 0.012 (8), suggesting that there was laminar flow. We observed a biomass concentration gradient within the packed bed. Hence, for sampling, all of the glass beads in each biofilm reactor were harvested as a single unit.
Toxicity study.
Benzaldehyde toxicity for biofilms and planktonic cultures was studied in the absence of glucose in order to prevent benzaldehyde conversion. Biofilms were grown for 3 days in flow cell chambers for microscopic analysis and in packed-bed column reactors for metabolic activity studies. Planktonic cultures were grown for 12 h (exponential phase) and 24 h (stationary phase). To study benzaldehyde toxicity for biofilms, the growth medium was carefully replaced by MES-buffered benzaldehyde saline solutions. For planktonic cultures, medium replacement was performed by centrifugation and resuspension. Controls were exposed to MES-buffered saline without benzaldehyde for the same times.
Microscopic analysis.
After incubation in the presence of benzaldehyde for various times at room temperature, bacterial viability was examined qualitatively in flow cells using a fluorescent BacLight LIVE/DEAD bacterial viability staining kit (Molecular Probe Inc., Eugene, Oreg.) and confocal laser scanning microscopy (CLSM) as described by Webb et al. (40). Planktonic cultures in benzaldehyde solutions were centrifuged, resuspended in saline (0.9% [wt/vol] NaCl), and diluted 10-fold using the viability stain solution before CLSM visualization on glass slides.
Metabolic activity studies.
Benzaldehyde toxicity was assessed quantitatively by determining the residual metabolic activities of biofilms in packed-bed column reactors and of planktonic cultures in test tubes. The specific glucose consumption rate was used as an indicator of metabolic activity. This rate was defined as the number of millimoles of glucose consumed by cells equivalent to 1 mg of total protein per hour at 30°C, as calculated from the glucose consumption rate and the total protein content of the sample. After incubation with benzaldehyde at 30°C, the biofilms, as well as the planktonic cultures, were washed with 40 mM saline to remove residual benzaldehyde. To determine the glucose consumption rate, 10 ml of a glucose solution (MES buffered) was added to each reactor, and 1 ml of glucose solution was added to the saline-washed cell pellet from 1 ml of a planktonic culture. After 1 h of incubation at 30°C and clarification by centrifugation, the glucose concentrations in the solutions were analyzed using an enzymatic glucose analyzer (model 27 Stat Plus; Yellow Springs Instrument, United States). All of the glass beads from each reactor were collected in separate 50-ml test tubes, and 10 ml of saline was added to each tube. The attached biofilm biomass was measured by quantification of the total protein.
Determination of total protein and biomass.
For quantification of the biofilm biomass, total protein concentrations in packed-bed biofilm reactors were determined by the Bradford method (4), using the Coomassie Plus protein assay reagent (Pierce, United States). Bovine serum albumin was used as a standard and to spike biomass samples for verification of the method. To release cell proteins, 1.2 ml of 30% (wt/vol) NaOH was added to 10 ml of saline which contained packing materials with attached biofilm. After incubation at 30°C for 30 min, test tubes containing samples were heated in boiling water for 15 min. After cooling, 0.86 ml of 32% (wt/vol) HCl was added to each tube to neutralize the pH. Samples were then centrifuged at 17,400 × g for 5 min, and 0.05 ml of each supernatant was mixed with 1 ml of protein assay reagent. After 10 min the absorbance at 595 nm was determined. The total protein contents of planktonic cultures were determined by the same procedure and were approximately 43% (wt/wt) of the dry biomass (error, ±3%). The dry biomass of biofilms was therefore calculated by dividing the measured protein content by 0.43.
Biotransformation of benzaldehyde.
Biotransformation was carried out using 3-day-old biofilms grown in packed-bed column reactors at 30°C with a flow rate of 0.15 ml min−1. The medium used for cultivation of the cells was switched to the biotransformation medium containing benzaldehyde without flow disruption using nonporous Teflon tubing (Alltech, Australia). The reactor effluent was collected to measure the optical density at 600 nm. After removal of cells and proteins by precipitation with 10% (wt/vol) trichloroacetic acid and centrifugation (17,400 × g, 5 min) at 4°C, supernatants were analyzed for the substrate benzaldehyde and the products benzyl alcohol and R-phenylacetylcarbinol by high-performance liquid chromatography (HPLC) as previously described (32).
RESULTS
Biofilm establishment.
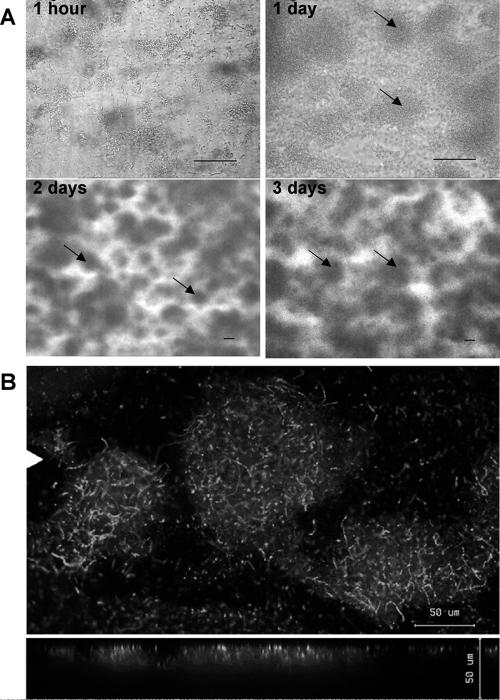
As shown by phase-contrast microscopy (Fig. 1A), Z. mobilis cells were attached to glass predominantly as single cells and small clusters 1 h after the initial attachment phase. After 1 day, attached cells had divided and formed a biofilm comprised of microcolonies. On day 2, the volume of Z. mobilis microcolonies had increased so that the biofilm became visible with the unaided eye. At a magnification of ×100, complex three-dimensional structured biofilms were observed, and water channels occurred throughout the biofilms. These biofilm structures were maintained for days 3 to 5. Congo red staining revealed the presence of an EPS-containing matrix surrounding cells in Z. mobilis biofilms (data not shown). Using CLSM and examining approximately 20 microcolonies, we estimated a mean thickness of 20 μm for the microcolonies after 3 days of incubation (Fig. 1B). Cells were densely packed, and formation of cell chains within microcolonies was observed. In packed-bed column reactors, quantification of total protein revealed an average of 54 μg (dry weight) cm−2 on hydrophobically treated glass beads, equivalent to 32 mg per 20-ml reactor.
FIG. 1.
Z. mobilis biofilm morphology in flow cells. (A) Phase-contrast images showing initial attachment and subsequent biofilm development during 3 days of Z. mobilis growth at a constant flow rate of 0.15 ml min−1 at room temperature. The arrows indicate microcolonies. (B) CLSM images of 3-day-old Z. mobilis biofilms stained with the BacLight LIVE/DEAD viability stain. The top and bottom images are topical and vertical views of Z. mobilis microcolonies. The white triangle in the top image indicates the position where the vertical section of the biofilm was taken. Bars, 50 μm.
Effect of benzaldehyde on cell viability.
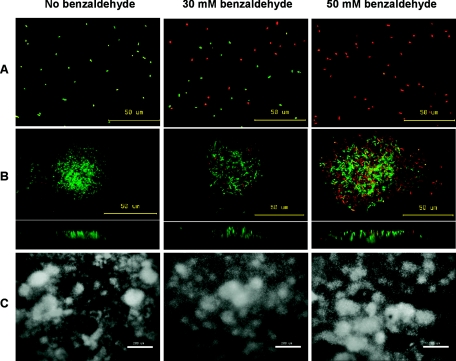
To examine whether biofilm cells of Z. mobilis display increased tolerance to benzaldehyde compared to planktonic cells, 3-day-old biofilms grown in flow cells and 24-h stationary-phase planktonic cultures were subjected to treatment with benzaldehyde concentrations of 10 to 50 mM for 1 h in the absence of glucose. Live and dead cells were assessed using CLSM and the BacLight LIVE/DEAD viability stain (Fig. 2A and B). As shown in Fig. 2A, 24-h planktonic Z. mobilis cultures were 100% viable in the absence of benzaldehyde. However, after exposure to 50 mM benzaldehyde for 1 h, all Z. mobilis planktonic cells that were stained with the LIVE/DEAD viability dye appeared red under fluorescent light, indicating that there was a strong toxic effect. Z. mobilis biofilm viability was also reduced upon exposure to benzaldehyde. Nevertheless, many cells in the biofilm remained viable after exposure to 50 mM benzaldehyde (Fig. 2B). Examining the vertical (side) view of the microcolonies, we observed that red cells in the colonies were distributed throughout the microcolony. Figure 2C shows that exposure to benzaldehyde did not influence the overall biofilm morphology during the timeframe of the experiment.
FIG. 2.
Qualitative analysis of benzaldehyde toxicity: CLSM images of 24-h planktonic cultures and 3-day-old Z. mobilis biofilms stained with the BacLight LIVE/DEAD viability stain. Planktonic (A) and biofilm (B and C) cultures were examined after 1 h of exposure in the absence or presence of benzaldehyde. Live cells fluoresced green, and dead cells fluoresced red. The top and bottom images in panel B are topical and vertical views of Z. mobilis microcolonies. (C) Overall biofilm morphology. (A and B) Bars, 50 μm. (C) Bars, 200 μm.
Effect of benzaldehyde on metabolic activity.
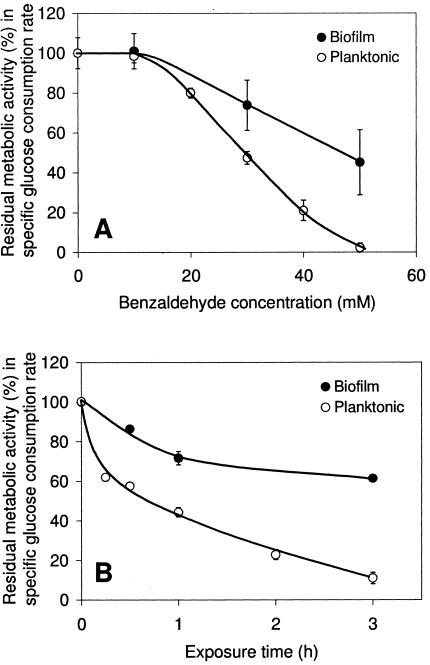
While the results observed in flow cells were qualitative, the benzaldehyde effect on Z. mobilis biofilms was quantified in packed-bed column reactors. Figure 3A shows the residual metabolic activities of biofilms and planktonic cells following a 1-h exposure to benzaldehyde. When subjected to treatment with 30 mM benzaldehyde, Z. mobilis biofilms retained approximately 75% of their initial activity, compared to 50% observed in planktonic cultures. A two-sample t test indicated that the residual metabolic activity in biofilm cells was significantly higher than that in planktonic cells (t = 5.22, df = 5, P < 0.0025, as determined by a one-tailed test). After exposure to 50 mM benzaldehyde, Z. mobilis biofilms maintained an average of 45% their initial activity, while planktonic cultures were completely inactivated. A two-sample t test again indicated that there was a significant difference between the residual metabolic activities (t = 5.13, df = 3, P < 0.01, as determined by a one-tailed test). These results strongly suggest that the formation of a biofilm enhanced cell tolerance to benzaldehyde. HPLC data confirmed that the benzaldehyde concentrations did not change during exposure of the biofilms.
FIG. 3.
Quantitative analysis of benzaldehyde toxicity. Residual metabolic activity was measured by the specific glucose consumption rates of 3-day-old Z. mobilis biofilms and 24-h planktonic cultures after 1 h of exposure to different benzaldehyde concentrations (A) and after exposure to 30 mM benzaldehyde for up to 3 h at 30°C (B). Samples exposed to benzaldehyde for less than 3 h were preincubated in MES-buffered saline before benzaldehyde exposure to provide a total incubation time of 3 h. One hundred percent residual activity corresponded to the specific glucose consumption rate of control cultures exposed to MES-buffered saline without benzaldehyde, which was on average 30 mmol glucose mg total protein−1 h−1. The error bars indicate the sample standard deviations of results from independent cultures (n ≥ 4 for panel A and n ≥ 2 for panel B).
Biofilm cultures are known to contain a mixture of growing and stationary-phase cells, while the planktonic cells used in the experiment were in the stationary phase. The growth phase could have influenced the sensitivity to benzaldehyde. Therefore, three 12-h planktonic Z. mobilis cultures in the exponential growth phase were also exposed to 50 mM benzaldehyde for 1 h. The results were similar to those obtained for the 24-h stationary-phase cultures, and there was a nearly complete loss of activity.
Based on the results obtained in the benzaldehyde concentration study, a concentration of 30 mM was selected to examine the effect of the length of benzaldehyde exposure on the inactivation of Z. mobilis biofilms and planktonic cultures. Figure 3B shows that biofilms were able to maintain 65% of their initial activity after 3 h of incubation with benzaldehyde. In contrast, the metabolic activity of planktonic cultures decreased to 70% of the initial activity in as little as 15 min, and no more than 10% of the initial activity was left at the end of the 3-h incubation period. Hence, exposure to 30 mM benzaldehyde for 3 h resulted in sixfold-higher residual metabolic activity in biofilm cells than in planktonic cells.
Continuous biotransformation of benzaldehyde by established biofilms.
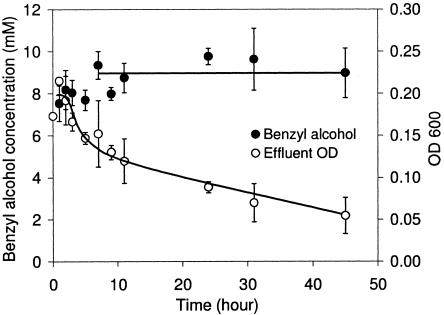
Figure 4 shows the biotransformation profile of 3-day-old Z. mobilis biofilms in the presence of glucose-supplemented medium and 10 mM benzaldehyde over a 45-h period. After 6 h, at a dilution rate of 0.9 h−1, Z. mobilis biofilms produced benzyl alcohol at an average concentration of 9 mM, which corresponded to a theoretical molar yield of 90% and volumetric productivity of 10 mM h−1 or 25.9 g liter−1 day−1. With 32 mg (dry weight) per 10 ml of void volume, the specific productivity was 8.11 g (g biomass)−1 day−1. The average concentration of benzaldehyde in the effluent was 1 mM. The concentrations of R-phenylacetylcarbinol were less than 0.2 mM, and other aromatic products were not detected. While the effluent optical density remained constant (approximately 0.2) during the 72 h prior to benzaldehyde addition, the optical density of the effluent decreased as much as 75% during the biotransformation. Microscopic examination confirmed that the decrease in optical density was related to the decrease in Z. mobilis cell concentration in the effluent. In contrast, the biofilm protein mass (measured at the start and at the end of the biotransformation), as well as the benzyl alcohol productivity, remained constant.
FIG. 4.
Continuous biotransformation determined by using 3-day-old Z. mobilis biofilms in the presence of 10 mM benzaldehyde and 5 g liter−1 glucose (27.8 mM) with a constant flow rate of 0.15 ml min−1 at 30°C. Benzaldehyde was added at time zero. The error bars indicate the sample standard deviations of results from independent reactors (n = 3). OD 600, optical density at 600 nm.
DISCUSSION
Over the past decade, microbial biofilms have attracted much attention from biotechnologists, who have demonstrated that biofilms can be used in bioremediation, wastewater treatment, fermentation, and biosynthesis (9, 20, 21, 23, 27, 42). In addition, the current study highlights the potential application of biofilms in the biotransformation of fine chemicals into value-added products. Formation of biofilms enhanced the tolerance of Z. mobilis cells to benzaldehyde compared to that of planktonic cells. Moreover, biotransformation using biofilms resulted in steady productivity during continuous operation, supporting the hypothesis that biofilms may act as efficient biocatalysts for fine-chemical production.
We also describe the biofilm development and morphology of Z. mobilis, an industrially relevant ethanologenic bacterium. Previously, Kundura and Pometto demonstrated that Z. mobilis biofilms could be used in ethanol fermentation (20). These authors reported increased ethanol productivity in continuous Z. mobilis biofilm reactors compared to the productivity of planktonic controls. However, neither the specific productivity nor the formation and morphology of the Z. mobilis biofilms were characterized. In the current study, we found that Z. mobilis cells were capable of forming a biofilm comprised of microcolonies embedded in EPS and interspersed with open water channels (Fig. 1). This architecture resembles the structures of many well-studied microbial biofilms (11, 39). From the strain of Z. mobilis used in the current study, Barrow and coworkers isolated an EPS and suggested a role for it in cell aggregation (3). This EPS may be involved in establishing and maintaining the complex three-dimensional structure of Z. mobilis biofilms reported in this paper.
By using microscopic analysis and metabolic activity studies, Z. mobilis biofilms were shown to be more tolerant of not only higher benzaldehyde concentrations but also prolonged benzaldehyde exposure than their planktonic counterparts (Fig. 2 and 3). The mechanisms underlying the enhanced benzaldehyde resistance in Z. mobilis biofilms are not fully understood yet. One possibility is that a benzaldehyde concentration gradient is formed within the three-dimensional structure of a biofilm, which might dilute the lethal concentration to a sublethal level in a deeper layer of the biofilm. However, viability staining and CLSM revealed that dead cells were located throughout the benzaldehyde-treated biofilms (Fig. 2B). Therefore, the resistance was probably not due to limited benzaldehyde penetration of biofilms. In a previous study, Campanac et al. suggested that the toxic aromatic compound benzalkonium chloride was able to penetrate biofilms of Staphylococcus aureus; nevertheless, the cells in the biofilms were more resistant than planktonic cells (6). Furthermore, the resistance of biofilms in the present study was not due to conversion of benzaldehyde into less toxic products since HPLC data confirmed that the benzaldehyde concentrations remained constant during the resistance study. It is well known that cells in different growth phases can have different sensitivities to toxins. Studies have suggested that biofilms predominately consist of stationary-phase cells (17), although rapidly dividing cells are also present (41). Our results show that exponential- and stationary-phase planktonic cells of Z. mobilis were equally susceptible to benzaldehyde, indicating that benzaldehyde resistance was not growth rate dependent for planktonic cells. In general, increased resistance in biofilms may be a result of altered gene expression which leads to physiological and/or structural changes during biofilm formation (17, 31, 33, 38). It is possible that the benzaldehyde resistance of Z. mobilis biofilms is conferred by, for example, benzaldehyde excretion or altered membrane composition to counteract any benzaldehyde effect on membrane fluidity. To identify the protective mechanisms, a detailed molecular comparison of biofilms and planktonic cells is necessary.
Continuous biotransformation of 10 mM benzaldehyde by Z. mobilis biofilms resulted in values for the benzyl alcohol molar yield, productivity, and specific productivity that were more than twofold higher than the previously reported values (37% molar yield, 4.9 mM h−1, and 3.41 g [g dry weight]−1 day−1, assuming a cell water content of 85%) for a fed-batch biotransformation by planktonic Z. mobilis (5). The specific productivity observed in the current biofilm study (8.11 g [g dry weight]−1 day−1) is in the range of values reported previously for various whole-cell processes with planktonic cultures, as shown in Table 1. Our data suggested that the biotransformation was catalyzed predominately in attached biofilms rather than in planktonic cells, as indicated by the 75% reduction in the effluent optical density during biotransformation, while the productivity and biofilm protein mass were unchanged (Fig. 4). Luke and Burton (23) showed in a phenol bioremediation study that a fungal biofilm maintained its catalytic capacity in a membrane reactor without inactivation for a period that was at least eight times longer than the period observed for free biomass in batch cultures. This enhancement was attributed to a continuing nutrient supply and biomass sustenance in the continuous system.
TABLE 1.
Whole-cell biocatalytic production processes and their specific productivities
The use of biofilms in biocatalytic fine-chemical production may have many advantages, including self-immobilization of the biocatalysts, increased resistance to toxic substrates and/or products, and potential long-term stability for continuous operation. Hence, surface-associated biofilms may function as “factories” for continuous fine-chemical production. Several issues will play a role in the success of “biofilm factories,” including the selection of suitable biofilm-forming biocatalysts, minimization of biofilm detachment, and reactor design and scale-up for optimal mass transfer and productivity. Indeed, many reported fine-chemical biotransformation processes use suspension cultures of microbes which can form biofilms, including Escherichia coli and Pseudomonas species (14, 19, 29, 35, 37). Full-scale biofilm reactors have already been used in bioremediation and wastewater treatment, including various reactors for optimal gas-liquid contact (25, 27). Furthermore, the present results did not reveal any mass transfer limitation since the specific glucose consumption rates for Z. mobilis biofilms were not lower than those for planktonic cells. Loss of biofilms during biotransformation was not an issue as the biofilm biomass remained constant over the 45-h biotransformation period.
In conclusion, we suggest that biofilm formation offers increased substrate tolerance and could allow continuous operation for the biotransformation of fine chemicals. Previous studies along with the present work show that there is good potential for future applications of “biofilm factories” in biocatalytic fine-chemical production processes.
Acknowledgments
We thank Mark Tanaka, Chris Marquis, Russell Cail, Martin Zarka, and Paul Halasz for their valuable technical advice. B.R. thanks William Costerton (Center for Biofilm Engineering, Montana) and Staffan Kjelleberg for organizing a free 1-week biofilm workshop at the University of New South Wales in 2002, which led to this study.
REFERENCES
- 1.Allison, D. G., and I. W. Sutherland. 1984. A staining technique for attached bacteria and its correlation to extracellular carbohydrate production. J. Microbiol. Methods 2:93-99. [Google Scholar]
- 2.Bakker, D. P., A. van der Plaats, G. J. Verkerke, H. J. Busscher, and H. C. van der Mei. 2003. Comparison of velocity profiles for different flow chamber designs used in studies of microbial adhesion to surfaces. Appl. Environ. Microbiol. 69:6280-6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow, K. D., J. G. Collins, P. L. Rogers, and G. M. Smith. 1984. The structure of a novel polysaccharide isolated from Zymomonas mobilis determined by nuclear magnetic resonance spectroscopy. Eur. J. Biochem. 145:173-179. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Bringer-Meyer, S., and H. Sahm. 1988. Acetoin and phenylacetylcarbinol formation by the pyruvate decarboxylases of Zymomonas mobilis and Saccharomyces carlsbergensis. Biocatalysis 1:321-331. [Google Scholar]
- 6.Campanac, C., L. Pineau, A. Payard, G. Baziard-Mouysset, and C. Roques. 2002. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob. Agents Chemother. 46:1469-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lapping-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 8.Coulson, J. M., and J. F. Richardson. 2002. Flow of fluids through granular beds and packed columns, p. 191-236. In J. R. Richardson, J. H. Harker, and J. R. Backhurst (ed.), Chemical engineering, 5th ed., vol. 2. Butterworth-Heinemann, Burlington, MA. [Google Scholar]
- 9.Crueger, W., and C. Crueger. 1989. Organic acids, p. 143-147. In W. Crueger, C. Creuger, and T. D. Brock (ed.), Biotechnology: a textbook of industrial microbiology. Sinauer Associates, Inc., Sunderland. MA.
- 10.Dalton, H. M., L. K. Poulsen, P. Halasz, M. L. Angles, A. E. Goodman, and K. C. Marshall. 1994. Substratum-induced morphological changes in marine bacterium and their relevance to biofilm structure. J. Bacteriol. 176:6900-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis, D. G., M. R. Parsek, J. P. Pearson, B. H. Inglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of bacteria biofilms. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 12.De Wulf, P., W. Soetaert, and E. J. Vandamme. 2000. Optimized synthesis of l-sorbose by C5-dehydrogenation of d-Sorbitol with Gluconobacter oxydans. Biotechnol. Bioeng. 69:339-343. [DOI] [PubMed] [Google Scholar]
- 13.Donlan, R. M., and J. W. Costerton. 2002. Biofilm: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutiérrez, M. C., V. Alphand, and R. Furstoss. 2003. Microbiological transformations. 52. Biocatalysed Baeyer-Villiger oxidation of 1-indanone derivatives. J. Mol. Catal. B Enzym. 21:231-238. [Google Scholar]
- 15.Haberland, J., W. Hummel, T. Daussmann, and A. Liese. 2002. New continuous production process for enantiopure (2R,5R)-hexanediol. Org. Process Res. Dev. 6:458-462. [Google Scholar]
- 16.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev. Microbiol. 2:95-108. [DOI] [PubMed] [Google Scholar]
- 17.Hentzer, M., L. Eberl, and M. Givskov. 2005. Transcriptome analysis of Pseudomonas aeruginosa biofilm development: anaerobic respiration and iron limitation. Biofilms 2:37-61. [Google Scholar]
- 18.Junter, G. A., and T. Jouenne. 2004. Immobilized viable microbial cells: from the process to the proteome… or the cart before the horse. Biotechnol. Adv. 22:633-658. [DOI] [PubMed] [Google Scholar]
- 19.Kaup, B., S. Bringer-Meyer, and H. Sahm. 2004. Metabolic engineering of Escherichia coli: construction of an efficient biocatalyst for d-mannitol formation in a whole-cell biotransformation. Appl. Microbiol. Biotechnol. 64:333-339. [DOI] [PubMed] [Google Scholar]
- 20.Kuduru, M. D., and A. L. Pometto. 1996. Continuous ethanol production by Zymomonas mobilis and Saccharomyces cerevisiae in biofilm reactors. J. Ind. Microbiol. 16:249-256. [DOI] [PubMed] [Google Scholar]
- 21.Lendenmann, U., J. C. Spain, and B. F. Smets. 1998. Simultaneous biodegradation of 2,4-dinitrotoluene and 2,6-dinitrotoluene in an aerobic fluidized-bed biofilm reactor. Environ. Sci. Technol. 32:82-87. [DOI] [PubMed] [Google Scholar]
- 22.Long, A., and O. P. Ward. 1989. Biotransformation of benzaldehyde by Saccharomyces cerevisiae: characterization of the fermentation and toxicity effects of substrates and products. Biotechnol. Bioeng. 34:933-941. [DOI] [PubMed] [Google Scholar]
- 23.Luke, A. K., and S. G. Burton. 2001. A novel application for Neurospora crassa: progress from batch culture to a membrane bioreactor for the bioremediation of phenols. Enzyme Microb. Technol. 29:348-356. [Google Scholar]
- 24.Mahmoud, W. M., A. M. M. El-Sayed, and R. W. Coughlin. 1990. Production of l-phenylacetyl carbinol by immobilized yeast cells. I. Batch fermentation. Biotechnol. Bioeng. 36:47-54. [DOI] [PubMed] [Google Scholar]
- 25.Metcalf, L., and H. Eddy. 2003. Attached growth and combined biological treatment processes, p. 887-981. In G. Tchobanoglous, F. L. Burton, and H. D. Stensel (ed.), Wastewater engineering: treatment and reuse. McGraw-Hill, New York, N.Y.
- 26.Moller, S., C. Sternberg, J. B. Andersen, B. B. Christensen, J. L. Ramos, M. Givskov, and S. Molin. 1998. In situ gene expression in mixed-culture biofilms: evidence of metabolic interactions between community members. Appl. Environ. Microbiol. 64:721-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolella, C., M. C. M van Loosdrecht, and J. J. Heijnen. 2000. Wastewater treatment with particulate biofilm reactors. J. Biotechnol. 80:1-33. [DOI] [PubMed] [Google Scholar]
- 28.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 29.Panke, S., M. Held, M. G. Wubbolts, B. Witholt, and A. Schmid. 2002. Pilot-scale production of (S)-styrene oxide from styrene by recombinant Escherichia coli synthesizing styrene monooxygenase. Biotechnol. Bioeng. 80:33-41. [DOI] [PubMed] [Google Scholar]
- 30.Panke, S., M. Held, and M. G. Wubbolts. 2004. Trends and innovations in industrial biocatalysis for the production of fine chemicals. Curr. Opin. Biotechnol. 15:272-279. [DOI] [PubMed] [Google Scholar]
- 31.Prigent-Combaret, C., O. Vidal, C. Dorel, and P. Lejeune. 1999. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J. Bacteriol. 181:5993-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosche, B., V. Sandford, M. Breuer, B. Hauer, and P. L. Rogers. 2001. Biotransformation of benzaldehyde into (R)-phenylacetylcarbinol by filamentous fungi or their extracts. Appl. Microbiol. Biotechnol. 57:309-315. [DOI] [PubMed] [Google Scholar]
- 33.Sauer, K., A. K. Camper, G. D. Ehrilich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis: today and tomorrow. Nature 409:258-268. [DOI] [PubMed] [Google Scholar]
- 35.Schneider, S., M. G. Wubbolts, G. Oesterhelt, D. Sanglard, and B. Witholt. 1999. Controlled regioselectivity of fatty acid oxidation by whole cells producing cytochrome P450BM-3 monooxygenase under varied dissolved oxygen concentrations. Biotechnol. Bioeng. 64:333-341. [DOI] [PubMed] [Google Scholar]
- 36.Seo, J. S., H. Chong, H. S. Park, K. O. Yoon, C. Jung, J. J. Kim, J. H. Hong, H. Kim, J. H. Kim, J. I. Kil, C. J. Park, H. M. Oh, J. S. Lee, S. J. Jin, H. W. Um, H. J. Lee, S. J. Oh, J. Y. Kim, H. L. Kang, S. Y. Lee, K. J. Lee, and H. S. Kang. 2005. The genome sequence of the ethanologenic bacterium Zymomonas mobilis ZM4. Nat. Biotechnol. 23:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skwarczynski, M., B. Lejczak, and P. Kafarski. 1999. Enantioselective hydrolysis of 1-butyryloxyalkylphosphonates by lipolytic microorganisms: Pseudomonas fluorescens and Penicillium citrinum. Chirality 11:109-114. [Google Scholar]
- 38.Szomolay, B., I. Klapper, J. Dockery, and P. S. Stewart. 2005. Adaptive responses to antimicrobial agents in biofilms. Environ. Microbiol. 7:1186-1191. [DOI] [PubMed] [Google Scholar]
- 39.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae EI Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb, J. S., L. S. Thompson, S. James, T. Charlton, T. Tolker-Nielsen, B. Koch, M. Givskov, and S. Kjelleberg. 2003. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 185:4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wentland, E. J., P. S. Stewart, C. T. Huang, and G. A. McFeters. 1996. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnol. Prog. 12:316-321. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, S. P., O. Norrlow, J. Wawrzynczyk, and E. S. Dey. 2004. Poly(3-hydroxybutyrate) biosynthesis in the biofilm of Alcaligenes eutrophus, using glucose enzymatically released from pulp fiber sludge. Appl. Environ. Microbiol. 70:6776-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]