Abstract
The effect of Cry proteins of Bacillus thuringiensis on the green lacewing (Chrysoperla carnea) was studied by using a holistic approach which consisted of independent, complementary experimental strategies. Tritrophic experiments were performed, in which lacewing larvae were fed Helicoverpa armigera larvae reared on Cry1Ac, Cry1Ab, or Cry2Ab toxins. In complementary experiments, a predetermined amount of purified Cry1Ac was directly fed to lacewing larvae. In both experiments no effects on prey utilization or fitness parameters were found. Since binding to the midgut is an indispensable step for toxicity of Cry proteins to known target insects, we hypothesized that specific binding of the Cry1A proteins should be found if the proteins were toxic to the green lacewing. In control experiments, Cry1Ac was detected bound to the midgut epithelium of intoxicated H. armigera larvae, and cell damage was observed. However, no binding or histopathological effects of the toxin were found in tissue sections of lacewing larvae. Similarly, Cry1Ab or Cry1Ac bound in a specific manner to brush border membrane vesicles from Spodoptera exigua but not to similar fractions from green lacewing larvae. The in vivo and in vitro binding results strongly suggest that the lacewing larval midgut lacks specific receptors for Cry1Ab or Cry1Ac. These results agree with those obtained in bioassays, and we concluded that the Cry toxins tested, even at concentrations higher than those expected in real-life situations, do not have a detrimental effect on the green lacewing when they are ingested either directly or through the prey.
Bacillus thuringiensis is a gram-positive bacterium that produces insecticidal crystal proteins (called Cry proteins, Cry toxins, or Bt toxins) known to be highly specific for several orders of insects, nematodes, mites, or protozoans. Briefly, the activity of these entomopathogenic proteins begins after ingestion, when the crystal dissolves and the protoxin is processed by the gut proteases to an activated form. After binding to specific receptors in the brush border membrane of midgut epithelial cells, insertion and pore formation lead to an osmotic imbalance, cell lysis, and insect death (6, 7, 37).
B. thuringiensis-based biopesticides, which make up 90% of the global biopesticide market, are widely used and are considered a valuable alternative to chemical insecticides in terms of safety to nontarget organisms and when resistance to chemical insecticides has developed. However, the most widespread use of Cry proteins is currently through their expression in transgenic crops. In the last few decades, many crops have been engineered to make them resistant to insects by expressing Cry protein-encoding genes. The most immediate advantage of insect-resistant Bt crops is that fewer insecticide applications are required (24, 34). Bt crops are considered a more environmentally friendly alternative to chemical insecticides, which have little selectivity and whose negative impact on nontarget organisms is well known. Hence, these crops have a direct benefit to farmers, consumers, and the environment at large (25, 29, 38). The most abundant of such insect-resistant crops are Bt maize and Bt cotton. Varieties of Bt maize express cry1Ab or cry1Fa, whereas varieties of Bt cotton express either cry1Ac (Bollgard I), both cry1Ac and cry2Ab (Bollgard II), or cry1F together with cry1Ac (WideStrike) (http://agbios.com/dbase.php).
Notwithstanding the high selectivity of B. thuringiensis toxins, some environmental concerns about the impact of Bt crops on nontarget organisms have been raised (4, 5, 19, 20, 21, 22). Among the beneficial insects, the green lacewing, Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae), has received special attention since its larvae are important natural enemies of insect pests in maize (10) and cotton (30). Increased mortality in immature green lacewing larvae was reported after they fed daily on Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae) or Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) larvae which had been reared on a transgenic variety of corn expressing cry1Ab (20). In the same study, lacewing larvae that reached the third instar exhibited reduced fitness compared to larvae fed non-Bt corn-reared O. nubilalis larvae, but this effect was not reported for larvae fed Bt corn-reared S. littoralis. The last result might have been due to the lower susceptibility of S. littoralis to this toxin (12), which did not affect larval health and thus prey quality so severely. Increased mortality of green lacewing larvae was also found when they were directly fed Cry1Ab (toxin)-contaminated artificial diet (22) or S. littoralis larvae reared on Cry1Ab-contaminated artificial diet (23). However, no effect of Cry1Ab and Cry2Aa protoxins was observed in the same experiments. Dutton et al. (10) performed tritrophic experiments with S. littoralis and the spider mite Tetranychus urticae (Koch) (Acari: Tetranychidae), which is not susceptible to Bt toxins. Although adverse effects were found in lacewing larvae fed S. littoralis, there was no effect on larvae fed the tolerant prey, suggesting that the detrimental effects observed when lacewing larvae were fed B. thuringiensis-treated S. littoralis larvae were due to the quality of the prey and not to a direct toxic effect of the Bt toxin. In a more recent experiment performed to avoid the possible indirect effect of prey quality, no detrimental effects were found in lacewing larvae after they ingested a large amount of Cry1Ab dissolved in a sucrose solution (36).
Taken together, the reports described above seem to indicate that decreased quality of the prey, rather than any direct effect, is responsible for the negative effects of Cry1Ab observed in some studies. Although Cry1Ab and Cry1Ac are very similar in terms of their modes of action and toxicity spectra, to our knowledge no previous laboratory study has tested the effects of Cry1Ac on lacewings. In the present work we tested the possible effects of this toxin on lacewing larvae by using direct feeding with Cry1Ac, and we performed tritrophic experiments with Cry1Ab-, Cry1Ac-, and Cry2Ab-reared Helicoverpa armigera. Moreover, below we describe a novel approach for determining the potential impact of Cry proteins on a nontarget organism. An indispensable step for the toxicity of Cry proteins is the binding to the epithelial cells of the midgut. We hypothesized that specific binding of the Cry1A proteins should be found if the proteins are toxic to the green lacewing. In vivo and in vitro binding experiments were performed to determine whether specific binding of Cry1Ab or Cry1Ac to the brush border membrane of the epithelial midgut cells of green lacewing larvae could be detected.
MATERIALS AND METHODS
Insect material.
For establishment of laboratory insect colonies, eggs and larvae of H. armigera (Hübner) (Lepidoptera: Noctuidae) and adults of C. carnea were collected in cotton fields in the province of Seville (Spain). Insects were maintained in the laboratory under controlled conditions with a photoperiod consisting of 16 h of light and 8 h of darkness and a relative humidity of 75 to 80% at 26 ± 2°C. H. armigera larvae were reared with a corn flour-based artificial diet (32), while adults were maintained with a 10% honey solution. The diet of green lacewings consisted of Ephestia kuehniella eggs (Koppert Biological Systems, The Netherlands) for larvae and a specific artificial diet for adults (39).
For the biochemical analyses, green lacewing L3 larvae were raised on the egg diet and were provided by STB-Control (Aarbergen, Germany) in shipments of 1,000 to 4,000 larvae. The larvae were harvested from the accompanying buckwheat shells used for shipping and collected in microcentrifuge tubes on ice. About 1 g of larvae was collected per tube and frozen in liquid nitrogen. The frozen larvae were stored at −80°C until further use. Spodoptera exigua (Boisduval) (Lepidoptera: Noctuidae) larvae were obtained from a laboratory colony maintained at the Virology Department of Wageningen University and were reared on an artificial diet under standard laboratory conditions until they reached the last instar.
Preparation of Cry proteins.
Cry1Ac and Cry1Ab toxins were obtained from recombinant B. thuringiensis strains EG11070 and EG7077, respectively (Ecogen, Inc., Langhorne, Pa.). Cry2Ab was prepared from recombinant B. thuringiensis strain EG7699 (Monsanto Co., Chesterfield, Mo.). Toxin preparation, solubilization, activation, and purification were performed as described previously (16), except that Cry2Ab was solubilized in carbonate buffer (50 mM Na2CO3, 0.1 M NaCl, 10 mM dithiothreitol; pH 12).
Cry1Ab and Cry1Ac used for green lacewing in vivo and in vitro binding experiments were activated by trypsin treatment, dialyzed overnight, and purified by ion-exchange chromatography to obtain highly purified activated toxin. H. armigera was fed an artificial diet containing Cry1Ac, Cry1Ab, or Cry2Ab that was trypsin activated but was not purified further. The purity of toxin samples was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the protein concentration was determined by densitometric analysis using bovine serum albumin as a standard. Before the protein preparations were used, the toxic activity was checked with larvae of the susceptible insect Plutella xylostella (L.) (Lepidoptera: Plutellidae).
C. carnea bioassays using treated prey.
All experiments were carried out in a controlled-environment chamber as described above for insect rearing. Neonate larvae of H. armigera were reared for 2 days on artificial diet containing no toxin or 1 or 10 μg/ml of toxin Cry1Ab, Cry1Ac, or Cry2Ab. On the other hand, lacewing L2 larvae were separated just after molting and kept individually in plastic cages (diameter, 2.8 cm; height, 1.5 cm). These larvae were fed five treated H. armigera larvae daily, and every other day this diet was supplemented with E. kuehniella eggs. This resulted in a total of nine treatments with three replicates per treatment. Ten lacewing larvae were used for each replicate (i.e., 30 larvae per treatment). When the adults emerged, pairs of one male and one female were put together in plastic cages, and the number of eggs per female was recorded for 3 days. Fifty eggs per treatment were put aside to evaluate larval hatching.
Green lacewing larvae were checked daily to evaluate the following parameters: larval survival, adult emergence, development time, number of H. armigera larvae ingested, fecundity, and fertility. The prey consumption data did not follow a normal distribution, so the data were analyzed with the Kruskal-Wallis test. The rest of the parameters fit a normal distribution, and therefore analysis of variance was used to determine if there were significant differences among the three treatments. Data were transformed using arcsin of the square root for the variables defined as percentages. All analyses were performed using the Statgraphics package (version 5.1; Statistical Graphics Corporation, Rockville, MD).
C. carnea bioassays using water-supplied Cry1Ac.
The first day after molting, L2 lacewing larvae were given a drop (4 μl) of fluorella blue-stained water containing either 0 or 4 μg of Cry1Ac purified toxin. After complete ingestion of the drop, larvae were fed E. kuehniella eggs for 24 h. Then the larvae were starved for 24 h and again given a new drop of water (with or without Cry1Ac). This cycle was repeated two or three times until pupation was reached. Larvae which did not drink the water drop were excluded from further analysis. The experiment was repeated three times with at least 10 larvae per replicate (i.e., 30 larvae per treatment). The parameters evaluated were the same as those described above. Data for development and fecundity were compared using Student's t test, whereas larval survival, adult emergence, and fertility were compared between treatments using contingency table analysis (χ2 test) with the Yates correction for continuity.
Tissue preparation and sectioning of Cry1Ac-treated larvae of C. carnea and H. armigera.
After 4 h of starvation, 50 L3 green lacewing larvae were given a drop (4 μl) of fluorella blue-stained water containing either 0 or 4 μg of Cry1Ac purified toxin. Similarly, 40 H. armigera L4 larvae were starved for 6 h and then given a drop (7 μl) containing either 0 or 7 μg of Cry1Ac. H. armigera was included as a control since its susceptibility to Cry1Ac is well documented (1, 26). Midguts were dissected at 4°C 15 min after larvae had completely consumed the drop. Whole midguts were fixed overnight at 4°C in the Bouin-PFA (70% picric acid, 25% paraformaldehyde, 5% acetic acid) fixative solution, washed for 1 h in distilled water, and then transferred to 70% ethanol and stored at 4°C until they were used. Dehydration of the tissue was performed with increasing concentrations of ethanol (90% and 100%; 1 h each), followed by 7 min of incubation in toluene. Paraffin embedding consisted of preincubation of the midguts in paraffin for 16 h at 56°C and then incubation for 3 h in fresh paraffin. Longitudinal sections (5 μm) were prepared with a microtome and placed on mounting glasses coated with poly-l-lysine (Menzel GmbH, Braunschweig, Germany). Tissue sections were dried by incubating them for 2 h at 40°C.
Rehydration of fixed sections was performed by incubation in decreasing concentrations of alcohol (100%, 70%, and 50%) and water. Following incubation for 5 min in lugol and for 2 min in 5% thiosulfate, tissue sections were transferred to TS-t buffer (10 mM Tris, 150 mM NaCl, 100 μM thimerosal, 0.1% Triton X-100; pH 7.6).
Bright-field microscopy.
Azokarmin staining of green lacewing sections was performed with rehydrated sections as follows. Sections were incubated for 20 min in 0.1% azokarmin in 1.5% acetic acid at 56°C. After rinsing in distilled water, sections were incubated for 1 min each in aniline (1% aniline in 70% ethanol), acid-ethanol (1% acetic acid in 96% ethanol), and distilled water. A final incubation in 5% phosphotungstic acid and water rinsing were used to prepare the sections for further dehydration in increasing concentrations of alcohol (50%, 75%, and 100%) and xylol. The sections were covered with DPX mounting medium (Fluka, Switzerland) and observed with a Nikon E800 microscope. Images were captured with a high-performance charge-coupled device camera and were processed with Adobe Photoshop 7.0 (Adobe Systems Inc., San Jose, CA).
Fluorescence microscopy.
Rehydrated sections of H. armigera and C. carnea were incubated with anti-Cry1Ac rabbit polyclonal antibody (5 μg/ml) for 16 h at room temperature. After the sections were rinsed with TS-t buffer, a fluorescent anti-rabbit antibody diluted 1:500 (Alexa Fluor 488; Molecular Probes, Madrid, Spain) was added and incubated for 2 h. Then the sections were transferred for 2 min to TS-t buffer. To counterstain the structure of the midgut, two fluorescent dyes were used. After 2 h of incubation at room temperature with Phalloidin (Sigma, St. Louis, MO) diluted 1:500, the sections were rinsed with TS-t buffer and stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, MO). Fluorescent mounting medium (Dako Cytomation, California) was added, and slides were mounted for observation. The slides were observed with a Nikon E800 microscope equipped with epifluorescence and the appropriate filters. Image capturing and processing were performed as described above. The specificity of the immunocytochemical staining was determined by omission of the primary and/or secondary antibodies and by using tissue sections from larvae that did not ingest the toxin. As expected, no staining was found in any of these controls.
BBMV preparation.
Frozen L3 green lacewing larvae were thawed in batches. The rear end of the abdomen (approximately two segments) was cut off, and the protruding midgut was pulled out, rinsed with cold MET buffer (250 mM mannitol, 17 mM Tris-HCl, 5 mM EGTA; pH 7.5), refrozen in liquid nitrogen, and stored at −80°C until use. The method used to prepare brush border membrane vesicles (BBMV) was based on the protocol described by Wolfersberger (40). Sets of pooled dissected guts were thawed and pooled in a centrifuge tube and then cooled on ice. Ten milliliters of MET buffer was added, and the midguts were homogenized by two 1-min treatments (with a 5-min interval for cooling between them) with an Ultra-Turrax homogenizer (IKA, TPE Holland, Amstelveen, The Netherlands). The homogenate was centrifuged for 5 min at 500 × g at 4°C, and the supernatant was transferred to a clean tube for further BBMV isolation. The supernatant was further homogenized by 20 strokes with a Dounce homogenizer, after which an equal volume of 24 mM MgCl2 was added and the mixture was incubated for 20 min on ice. Next, the mixture was centrifuged for 15 min at 2,500 × g at 4°C, and the supernatant was transferred to a clean tube and centrifuged for 30 min at 40,000 × g. The second pellet was resuspended in 5 ml MET buffer and homogenized in the Dounce homogenizer, and the sequential steps of precipitation with Mg2+, low-speed centrifugation, high-speed centrifugation, and homogenization were repeated three times in order to improve the enrichment for BBMV stepwise. The final membrane pellet was resuspended in 50% MET buffer and stored at −80°C until it was used in protein, enzyme, and binding assays.
BBMV from S. exigua were prepared in a similar way by using an established protocol (40).
BBMV protein and enzyme activity assays.
All assays were performed in 96-well microtiter plates. In all samples the protein concentration was measured by the BCA protein assay (Perbio Science Nederland B.V., Etten-Leur, The Netherlands). For this, 20 μl of sample was mixed with 30 μl water, 50 μl 2% sodium dodecyl sulfate, and 100 μl BCA substrate. After incubation for 2 h at 37°C, the optical density at 595 nm was determined and compared with a calibration series based on 0 to 5 μg bovine serum albumin per well in duplicate. The relative specific enzyme activities per mg and per min were calculated by dividing the change in optical density by the assay time (min) and by the amount of protein (mg) in the sample.
Aminopeptidase N (APN) activity was measured with a 20-μl protein sample in 200 μl (total volume) of buffer containing 100 mM Tris (pH 6.8) and 125 mM NaCl with the specific substrate l-leucine-p-nitroanilide at a concentration of 1 mM. After addition of the substrate, the absorbance at 405 nm was determined at 10-min intervals until the optical density reached 1 or more.
γ-Glutamyltransferase activity was measured with a 20-μl protein sample in 200 μl (total volume) of buffer containing 100 mM Tris (pH 8.0), 60 mM glycyl-glycine, and the specific substrate l-γ-glutamyl-p-nitroanilide at a concentration of 4.5 mM at 37°C. After addition of the sample, the absorbance at 405 nm was determined at 10-min intervals until the optical density reached 1 or more.
Binding studies with BBMV.
Biotinylation of trypsin-activated Cry1Ab and Cry1Ac was performed using a biotin labeling kit (Roche Diagnostics Nederlands BV, The Netherlands) according to the manufacturer's instructions. Binding studies were performed basically as described previously (2). Briefly, 10 ng of labeled toxin was incubated with 5, 10, or 20 μg of BBMV protein for 1 h at room temperature. Next, membranes with bound toxin were separated from unbound toxin by centrifugation. The sedimented membranes were washed briefly, and membrane components, along with bound toxin, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane by electroblotting. Bound biotin-labeled toxin on the membrane was then visualized by incubation with 1/1,000-diluted streptavidin-peroxidase (Roche) and a light-emitting Lumi-light Western blotting substrate (Roche), and the results were subsequently recorded with a Lumi-imager (Roche). The same experiments were performed with the labeled toxin in the presence of a 100-fold excess of unlabeled toxin. S. exigua BBMV (10 μg) were always included as a positive control.
RESULTS
Effect of Cry-treated prey on C. carnea.
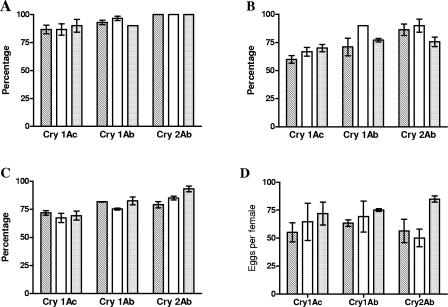
Analysis of the number of H. armigera larvae ingested in each Cry protein treatment revealed that lacewing larvae consumed most of the larvae offered (Table 1), and no differences were found with any of the B. thuringiensis Cry proteins (for Cry1Ac, Kruskal-Wallis H = 3.01 and P = 0.22; for Cry1Ab, Kruskal-Wallis H = 1.69 and P = 0.43; for Cry2Ab, Kruskal-Wallis H = 0.62 and P = 0.73). The same conclusion was drawn for the other parameters studied, including larval survival (Fig. 1A) (for Cry1Ac, F2,6 = 0.14 and P = 0.87; for Cry1Ab, F2,6 = 1.46 and P = 0.31; for Cry2Ab, all data equal to 100%), adult emergence (Fig. 1B) (for Cry1Ac, F2,6 = 0.23 and P = 0.80; for Cry1Ab, F2,6 = 1.78 and P = 0.25; for Cry2Ab, F2,6 = 1.01 and P = 0.41), fertility (Fig. 1C) (for Cry1Ac, F2,6 < 0.001 and P = 0.99; for Cry1Ab, F2,6 = 1.02 and P = 0.41; for Cry2Ab, F2,6 = 2.92 and P = 0.13), fecundity (Fig. 1D) (for Cry1Ac, F2,6 = 0.48 and P = 0.64; for Cry1Ab, F2,6 = 0.57 and P = 0.59; for Cry2Ab, F2,6 = 2.98 and P = 0.13), and development time (Table 2) (with 2 and 6 degrees of freedom and P always greater than 0.05).
TABLE 1.
Effect of Cry proteins on C. carnea prey consumption
| Treatment (μg toxin/ml diet) |
H. armigera larva consumption per daya
|
||
|---|---|---|---|
| Cry1Ac | Cry1Ab | Cry2Ab | |
| 0 | 4.0 ± 0.1 | 3.8 ± 0.1 | 4.0 ± 0.1 |
| 1 | 4.2 ± 0.1 | 4.0 ± 0.1 | 4.0 ± 0.1 |
| 10 | 4.5 ± 0.2 | 3.9 ± 0.4 | 3.4 ± 0.5 |
The values are means ± standard errors for consumption of H. armigera larvae for each toxin based on three replicates, using 10 larvae per replicate. The differences between treatments within a column are not statistically significant (P> 0.05, as determined by the Kruskal-Wallis test).
FIG. 1.
Effect of Bt corn-treated prey on C. carnea fitness. (A) Larval survival. (B) Adult emergence. (C) Fertility. (D) Fecundity. Fecundity was defined as the number of eggs per female per day. Toxins were mixed with the diet at a concentration of either 1 μg/ml (open bars) or 10 μg/ml (grey bars). The results for controls without toxin (striped bars) are also shown. The values are means ± standard errors from three replicates. Ten larvae were used for each replicate.
TABLE 2.
Effect of Bt toxin-treated prey on C. carnea development time
| Treatment
|
Development time (days)a
|
||||
|---|---|---|---|---|---|
| Toxin | Concn (μg/ml diet) | L2 | L3 | Pupa | L2-adult |
| Cry1Ac | 0 | 2.0 ± 0.4 | 5.5 ± 0.4 | 13.5 ± 0.7 | 21.0 ± 1.4 |
| 1 | 2.3 ± 0.2 | 5.8 ± 0.5 | 13.7 ± 0.6 | 21.8 ± 1.2 | |
| 10 | 2.1 ± 0.2 | 5.2 ± 0.2 | 12.8 ± 0.1 | 20.2 ± 0.4 | |
| Cry1Ab | 0 | 2.3 ± 0.3 | 5.4 ± 0.6 | 12.8 ± 0.6 | 20.5 ± 1.4 |
| 1 | 1.8 ± 0.3 | 4.9 ± 0.4 | 12.3 ± 0.2 | 19.1 ± 0.9 | |
| 10 | 1.9 ± 0.3 | 5.0 ± 0.2 | 12.6 ± 0.2 | 19.5 ± 0.4 | |
| Cry2Ab | 0 | 1.9 ± 0.1 | 5.6 ± 0.3 | 14.0 ± 0.3 | 21.6 ± 0.4 |
| 1 | 2.0 ± 0.1 | 5.7 ± 0.1 | 14.3 ± 0.3 | 22.0 ± 0.4 | |
| 10 | 1.8 ± 0.2 | 5.5 ± 0.4 | 13.8 ± 0.3 | 21.1 ± 0.8 | |
The values are means ± standard errors for the number of development days and were calculated for each instar/stage from three replicates, using 10 larvae per replicate. The differences between treatments within a column and Cry protein are not statistically significant (P > 0.05, as determined by a one-way analysis of variance with 2 and 6 degrees of freedom).
Effect of directly supplied Cry1Ac on C. carnea.
To avoid any type of indirect effects resulting from passage of the Cry toxin through the prey digestive system, the effect of Cry1Ac on the green lacewing was also determined in a direct way, by supplying the toxin in an aqueous solution which was eventually consumed by the green lacewing larvae.
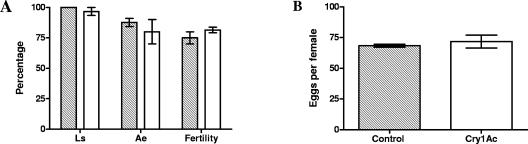
The same parameters that are described above were evaluated. The development time (Table 3) was similar for the treatments, with P values of >0.05 for all the parameters studied (for the L2 instar, t4 = 0.5 and P = 0.64; for the L3 instar, t4 = −1.34 and P = 0.25; for the pupa stage, t4 = 0.28 and P = 0.80; and from L2 to the adult stage, t4 = 0.09 and P = 0.93). For the rest of the parameters studied, there were no differences between treatments; this was true for larval survival (Fig. 2A) (χ12 = 0.07 and P = 0.80), adult emergence (Fig. 2A) (χ12 = 0.39 and P = 0.53), fertility (Fig. 2A) (χ12 = 0.47 and P = 0.49), and fecundity (Fig. 2B) (t4 = 0.64 and P = 0.56).
TABLE 3.
Effect of directly supplied Cry1Ac (4 μg) on C. carnea development time
| Treatment | Development time (days)a
|
|||
|---|---|---|---|---|
| L2 | L3 | Pupa | L2-adult | |
| Control | 2.1 ± 0.2 | 5.8 ± 0.2 | 12.3 ± 0.6 | 19.9 ± 0.9 |
| Treated | 2.2 ± 0.2 | 5.5 ± 0.1 | 12.0 ± 0.9 | 20.0 ± 0.6 |
The values are means ± standard errors for the number of development days and were calculated for each instar/stage from three replicates, using 10 larvae per replicate. The differences between treatments within a column are not statistically significant (P > 0.05, as determined by Student's t test).
FIG. 2.
Effect of directly supplied Cry1Ac on C. carnea fitness. Controls without toxin (striped bars) and larvae fed 4 μg of Cry1Ac (open bars) were used. The values are means ± standard errors from three replicates. At least 10 larvae were used for each replicate. (A) Larval survival (Ls), adult emergence (Ae), and fertility. (B) Fecundity, defined as the number of eggs per female per day.
Histopathological analysis of C. carnea larvae after ingestion of Cry1Ac.
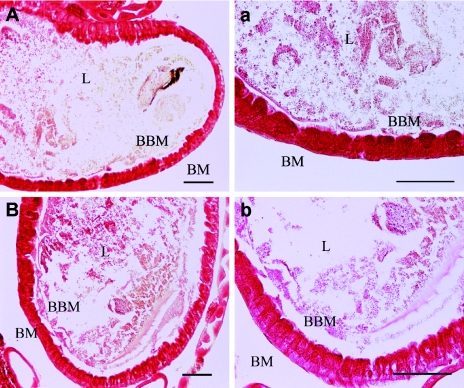
Midgut sections from green lacewing larvae that had ingested Cry1Ac, as well as midguts from control larvae that had not been exposed to the toxin, were observed under the microscope to determine any histopathological effects that could be associated with the ingested toxin. The midguts appeared as a monolayer structure of epithelial cells (Fig. 3 and 4); the membranes were red and the nuclei were blue when fluorescence microscopy was used. There were no apparent differences between midgut sections from control larvae and midgut sections from treated larvae as determined by bright-field microscopy (Fig. 3) or by fluorescence microscopy (Fig. 4).
FIG. 3.
Lack of histopathological effects induced by Cry1Ac in the midgut of C. carnea. (A and a) Larvae not exposed to Cry1Ac. (B and b) Larvae exposed to Cry1Ac for 20 min. BM, basal membrane; L, lumen; BBM, brush border membrane. Bars = 50 μm. No evidence of cell damage was observed.
FIG. 4.
Immunodetection of Cry1Ac in the midguts of H. armigera and C. carnea larvae. (A and C) Larvae exposed to Cry1Ac for 20 min. (B and D) Control larvae not exposed to the toxin. BM, basal membrane; L, lumen; BBM, brush border membrane; PM, peritrophic membrane. Bars = 50 μm. Binding of Cry1Ac to the brush border membrane and epithelial cell damage in H. armigera were observed. No binding was detected in Cry1Ac-treated C. carnea.
In vivo binding assays with Cry1Ac.
Immunodetection of ingested Cry1Ac toxin was performed with midgut sections of larvae exposed to toxin or control larvae using a green fluorescent anti-Cry1Ac antibody. In midguts of H. armigera fed Cry1Ac, the antibody was detected bound to patches of the brush border membrane in cells that were still intact and also with high intensity to the peritrophic membrane (Fig. 4A). However, no binding was detected either in nontreated H. armigera larvae (Fig. 4B) or in treated and nontreated lacewing larvae (Fig. 4C and D). In H. armigera sections, the well-organized global structure observed in controls (Fig. 4B) was not found in insects fed Cry1Ac (Fig. 4A), in which typical cell damage signs were found and migration of nuclei to the apical membrane was evident.
BBMV preparation from green lacewing larvae.
Two BBMV preparations of dissected guts were obtained by starting with 8.3 and 7.2 g (fresh weight) of larvae. This corresponded to dissection of approximately 5,000 insects per experiment. The BBMV preparation protocol was identical for both experiments. Three rounds of differential centrifugation yielded 360 and 290 μg of protein in the final BBMV pellets. The enrichment of membrane enzymes was followed by measuring the activities of APN and γ-glutamyltransferase. The mean enrichment ratios in the BBMV fraction, compared to the original homogenate, were 3.9 for APN and 7.4 for γ-glutamyltransferase in the first experiment. In the second experiment, the BBMV fraction was enriched 4.0- and 5.0-fold for APN and γ-glutamyltransferase, respectively. Therefore, the enrichment of these two enzymes suggests that the protocol for preparing BBMV from lepidopterans also works with green lacewing larvae and that, therefore, we purified BBMV from this insect.
In vitro binding assays with BBMV preparations from dissected midguts.
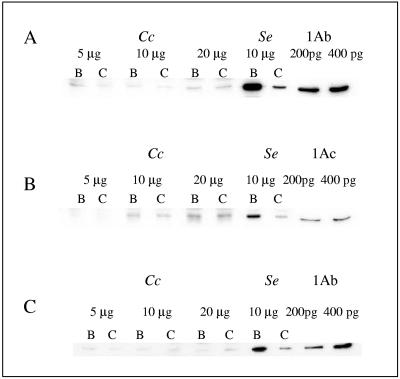
Different concentrations of lacewing BBMV were tested for binding of biotinylated Cry1Ab and Cry1Ac (Fig. 5A and B), and a replicate experiment with biotinylated Cry1Ab was performed with an independent batch of BBMV (Fig. 5C). Binding experiments with labeled toxin were always performed in the presence or absence of an excess of unlabeled toxin. When toxin binding is specific and saturable, the excess unlabeled toxin (which binds to the same binding sites) should strongly decrease the intensity of the toxin band on the blot. BBMV from S. exigua were always included as a positive control, since it is well known that these BBMV readily and specifically bind Cry1Ab (8, 14). In the three experiments we observed that there was clear binding to S. exigua BBMV (Fig. 5, lane B), which was specific because it was competed off by the unlabeled competitor (lane C). In all cases lacewing BBMV bound minute amounts of labeled toxin (compared to S. exigua BBMV), which in the case of Cry1Ac increased with the amount of BBMV protein (Fig. 5B). However, the addition of an excess of unlabeled competitor did not reduce this binding, which was therefore considered nonspecific.
FIG. 5.
Binding of biotin-labeled toxins to C. carnea and S. exigua BBMV. (A) Binding of Cry1Ab to C. carnea BBMV (batch I) and S. exigua BBMV. (B) Binding of Cry1Ac to C. carnea BBMV (batch I) and S. exigua BBMV. (C) Binding of Cry1Ab to C. carnea BBMV (batch II) and S. exigua BBMV. Cc, lanes containing C. carnea BBMV; Se, lanes containing S. exigua BBMV; 1Ab and 1Ac, lanes containing 200 and 400 pg of labeled toxin directly applied to the gel for comparison. All membrane fractions were incubated with 10 ng labeled toxin in the absence (lanes B) or in the presence (lanes C) of 1,000 ng unlabeled toxin.
DISCUSSION
Assessment of the risk of transgenic Bt crops for nontarget organisms is necessary to determine the toxicity of Cry proteins and to analyze the likelihood of protein ingestion in the field. To assess the environmental benefits and risks, appropriate baseline scenarios and representative species should be selected, and when no effects are reported in a worst-case first-tier analysis, further analyses may not be needed (11). A standardized procedure for assessing the risks of transgenic crops for entomophagous arthropods has been proposed previously (11, 33).
With regard to C. carnea, a worst-case tiered approach is an approach in which the toxicity of the Cry protein is determined after the predator is either directly given the toxin or fed a natural or artificial diet containing the toxin. Different methodologies have led to contradictory results for the possible effect of Cry1Ab toxin on C. carnea larvae. One study showed that there were detrimental effects when 100 μg/ml of Cry1Ab was delivered in microspheres containing an artificial diet (22), whereas another study showed that there was no effect after ingestion of 31 μg of Cry1Ab per g (fresh weight) of larvae, which is about 10,000-fold higher than the concentration which can be ingested through the prey (36). Negative effects have also been reported in some tritrophic studies (9, 20, 22, 23), but it was suggested that the observed effects might be mediated by poor prey quality (as a result of toxin action on the prey) rather than by direct toxin action of the protein (10). Other studies in which Cry toxins were administrated to green lacewing larvae performed to fulfill the registration requirements before the release of commercial Bt crops did not detect (13) detrimental effects of Cry toxins on C. carnea.
In the present work we tried to shed light on this controversy by using a holistic approach to study the possible effects of Cry proteins on the green lacewing. This holistic approach consisted of five independent, complementary experimental approaches: (i) ingestion of intoxicated prey and then measurement of fitness parameters, (ii) direct feeding and then measurement of fitness parameters, (iii) histopathological observation of the midgut after direct feeding, (iv) immunological detection of the toxin after direct feeding (in vivo binding experiments), and (v) in vitro binding experiments with BBMV preparations. The results obtained in our study show that none of these approaches resulted in detection of any detrimental effect of the Cry proteins tested on lacewing larvae.
The effects of the three most common toxins found in commercial Bt crops were tested by using a tritrophic scenario, in which the activated toxins were given to H. armigera larvae, which in turn were used to feed lacewing larvae. No significant differences in several fitness parameters between control and treated larvae were found. The discrepancies with previous published studies could be due, among other methodological differences, to the fact that we supplied the treated larvae with E. kuehniella eggs. The reason for the diet supplementation was that lepidopteran larvae are not major natural prey for this predator and in the field green lacewing larvae feed mainly on aphids, mites, and lepidopteran eggs (11). The quality of the prey seems to be crucial in this type of laboratory experiment in which fitness parameters are measured in the lacewing outside its natural environment. Trypsin-activated toxins were used in our experiments to simulate the form in which these toxins are found in transgenic plants; all of them are in the form of truncated proteins.
We also tested the worst-case scenario, in which a measured amount of purified Cry1Ac was directly given to the larvae. In this way, unintended effects, such as poor prey quality and degradation or sequestration of the ingested toxin within the prey, could be eliminated. As in the tritrophic experiments, toxin ingestion was also supplemented with E. kuehniella eggs, and again, no significant differences in fitness parameters were found between control and treated larvae. Our results agree with those of a recent study in which direct ingestion of Cry1Ab resulted in no deleterious effects on lacewing larvae (36).
It has been reported that sublethal doses of Cry toxins can be overcome by tolerant insects, and although originally there is tissue damage, the final outcome cannot be distinguished from the outcome for the nontreated controls (28). Therefore, although unlikely, if Cry proteins had temporary subtle deleterious effects on lacewing larvae after exposure to the toxin for a limited time, these effects could be overcome and thus not be detected by an observer. For this reason we used the third approach, microscopic examination of the midgut, to try to detect any signs of cell damage that could be associated with direct ingestion of Cry1Ac. Microscopic observation of midgut tissue from Cry1Ac-treated and nontreated larvae revealed similar morphologies of the epithelial cells and no signs of cell damage in either case.
Techniques other than bioassays, such as in vitro or in vivo binding experiments, could provide insight into the fate of the toxin if it was ingested by the nontarget organism. Therefore, an independent way to look for possible negative effects of Cry toxins on the lacewing would be to show specific binding of these toxins to the midgut brush border membrane. The mode of action of Cry toxins in all susceptible insect species tested, as well as in nematodes, requires binding to the midgut epithelium (6, 37). Normally, specific binding of Cry proteins is shown using BBMV preparations, although immunological detection of ingested toxin has also been used as an alternative method (3, 28, 35). For small insect species, for which preparation of BBMV could be cumbersome or for which in vitro binding conditions have not been determined, the immunological detection of in vivo bound toxin can be very convenient. This approach was used to try to detect in vivo binding of Cry1Ac in lacewing larvae and H. armigera larvae (as a control) which were directly fed this toxin. We used a fluorescent secondary antibody instead of colorimetric detection of the bound secondary antibody, as was done in the previous studies. Although Cry1Ac was detected bound to brush border membrane patches of intact epithelium of intoxicated H. armigera larvae, no fluorescent signal was found in tissue sections of lacewing larvae. Since binding to the epithelium is an obligatory step in the toxic action of Cry1Ac, these results corroborate the conclusions reached from the approaches described above and strongly suggest that the lacewing larval midgut lacks specific receptors for Cry1Ac.
Because the most conventional way to show the occurrence of specific receptors for Cry toxins is testing the in vitro binding to BBMV (17, 18), we also included this approach in our study and tested binding of biotin-labeled Cry1Ab or Cry1Ac to BBMV prepared from lacewing larvae. Since there were no protocols for preparing BBMV from the lacewing, we used a protocol which is widely used for other insect larvae. We first attempted to purify BBMV from intact green lacewing larvae by using a method described previously for whole small lepidopteran larvae (15, 27), with a number of variations. To assess the supposedly increasing enrichment for BBMV in the course of the purification protocol, we measured the specific enzyme activities of various fractions taken during the purification. Enzymes like aminopeptidase N and γ-glutamyltransferase are rather specific for brush border membranes, and therefore as fractions become enriched for BBMV, the specific activities of these enzymes should increase compared to the activities in the original homogenate. Unfortunately, with intact green lacewing larvae the results invariably indicated that there was not enrichment of specific enzyme marker activities throughout the protocol but there was a loss (data not shown). Therefore, we undertook the task of preparing BBMV from dissected midguts. The enrichment of the enzyme marker activities (3.9- to 7.4-fold) suggests that the protocol for preparing BBMV yielded a suitable BBMV preparation from lacewing larvae. Although slight binding of biotin-labeled Cry1Ab and Cry1Ac was detected, this binding was not specific because it could not be competed off by an excess of unlabeled toxin. The fact that no specific in vitro binding was found agrees with the lack of Cry1Ac in vivo binding to the lacewing epithelium.
The amounts of Cry toxins tested in our experiments are much higher than the amounts ingested by the green lacewing through its prey. A recent study showed that the amounts of Cry1Ab ingested by the green lacewing through prey reared on Bt corn were around 0.3 μg/g (fresh weight) when the insects were fed S. littoralis and around 2.2 μg/g (fresh weight) when they were fed the mite T. urticae (31). The levels of Cry1Ab found in Bt corn-fed prey insects were around 0.7 μg/g for S. littoralis and between 2.5 and 4.7 μg/g for T. urticae (9, 31). Bt crops express Cry proteins at levels of micrograms per gram (fresh weight) (13). Thus, for the lacewing to ingest microgram amounts of toxins, its prey would need to feed on amounts of plant tissues that far exceed their consumption capacity.
In conclusion, using our holistic approach, we were unable to show any negative effect of Cry1Ab, Cry1Ac, or Cry2Ab on lacewing larvae or the occurrence of Cry1A binding sites in the midgut epithelium. These results are in agreement with those of several laboratories (9, 36) in which no direct effect of Cry1A proteins on lacewing larvae was detected. Considering that the green lacewing is a generalist predator which, in addition to feeding on lepidopteran larvae and mites, also feeds on aphids and insect eggs in the field, it is highly unlikely that Bt crops pose any risk to this beneficial predator.
Acknowledgments
We are indebted to Carmen Ramírez-Castillejo (University of Valencia) for her invaluable help and advice with the microscopic techniques and to Jörg Romeis for his thorough comments on the manuscript.
This research was funded by grants to J.F. and A.R.-S. from the Spanish Ministry of Science and Technology (project AGL2033-09282-C03) and Generalitat Valenciana (GRUPOS2004-21). R.A.D.M., P.L.B., and J.M. were supported by the European Union 5th Framework project “Bt-BioNoTa” (contract QLK3-CT-2000-00547) and by the Dutch Ministry of Agriculture, Fisheries and Nature Management as part of DWK Research Program 347 on Biological Safety of Transgenic Plants. C.A. and J.E.G.-Z. were funded by the Spanish Ministry of Science and Technology (project AGL2033-09282-C03).
REFERENCES
- 1.Avilla, C., E. Vargas-Osuna, J. González-Cabrera, J. Ferré, and J. E. González-Zamora. 2005. Toxicity of several δ-endotoxins of Bacillus thuringiensis against Helicoverpa armigera (Lepidoptera: Noctuidae) from Spain. J. Invertebr. Pathol. 90:51-54. [DOI] [PubMed] [Google Scholar]
- 2.Bosch, D., B. Schipper, H. van der Kleij, R. A. de Maagd, and W. J. Stiekema. 1994. Recombinant Bacillus thuringiensis crystal proteins with new properties: possibilities for resistance management. Bio/Technology 12:915-918. [DOI] [PubMed] [Google Scholar]
- 3.Bravo, A., S. Jansens, and M. Peferoen. 1992. Immunocytochemical localization of Bacillus thuringiensis insecticidal crystal proteins in intoxicated insects. J. Invertebr. Pathol. 60:237-246. [Google Scholar]
- 4.de Maagd, R. A. 2002. Environmental impact of insect-resistant crop plants expressing a Bt-toxin. II. Non-target effects (updated) and Bt-resistance in target insects. Note 218. Plant Research International. [Online.] http://library.wur.nl/wasp/bestanden/LUWPUBRD_00322140_A502_001.pdf.
- 5.de Maagd, R. A. 2004. Biotechnology meets ecology, p. 117-131. In J. P. Nap, A. Atanassov, and W. J. Stiekema (ed.), Genomics for biosafety in plant biotechnology. IOS Press, Amsterdam, The Netherlands.
- 6.de Maagd, R. A., A. Bravo, C. Berry, N. Crickmore, and H. E. Schnepf. 2003. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet. 37:409-433. [DOI] [PubMed] [Google Scholar]
- 7.de Maagd, R. A., A. Bravo, and N. Crickmore. 2001. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 17:193-199. [DOI] [PubMed] [Google Scholar]
- 8.de Maagd, R. A., M. S. Kwa, H. van der Kleij, T. Yamamoto, B. Schipper, J. M. Vlak, W. J. Stiekema, and D. Bosch. 1996. Domain III substitution in Bacillus thuringiensis delta-endotoxin CryIA(b) results in superior toxicity for Spodoptera exigua and altered membrane protein recognition. Appl. Environ. Microbiol. 62:1537-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutton, A., H. Klein, J. Romeis, and F. Bigler. 2002. Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperla carnea. Ecol. Entomol. 27:441-447. [Google Scholar]
- 10.Dutton, A., H. Klein, J. Romeis, and F. Bigler. 2003. Prey-mediated effects of Bacillus thuringiensis spray on the predator Chrysoperla carnea in maize. Biol. Control 26:209-215. [Google Scholar]
- 11.Dutton, A., J. Romeis, and F. Bigler. 2003. Assessing the risks of insect resistant transgenic plants on entomophagous arthropods: Bt-maize expressing Cry1Ab as a case study. BioControl 48:611-636. [Google Scholar]
- 12.Dutton, A., J. Romeis, and F. Bigler. 2005. Effects of Bt maize expressing Cry1Ab and Bt spray on Spodoptera littoralis. Entomol. Exp. Appl. 114:161-169. [Google Scholar]
- 13.Environmental Protection Agency. 2001. Bt plant-incorporated protectants. Biopesticides action document, p. IIC1-IIC109. http://www.epa.gov/pesticides/biopesticides/index.htm.
- 14.Escriche, B., J. Ferré, and F. J. Silva. 1997. Occurrence of a common binding site in Mamestra brassicae, Phthorimaea operculella, and Spodoptera exigua for the insecticidal crystal proteins CryIA from Bacillus thuringiensis. Insect Biochem. Mol. Biol. 27:651-656. [DOI] [PubMed] [Google Scholar]
- 15.Escriche, B., F. J. Silva, and J. Ferré. 1995. Testing suitability of brush border membrane vesicles prepared from whole larvae from small insects for binding studies with Bacillus thurigiensis CryIA(b) crystal protein. J. Invertebr. Pathol. 65:318-320. [Google Scholar]
- 16.Estela, A., B. Escriche, and J. Ferré. 2004. Interaction of Bacillus thuringiensis toxins with larval midgut binding sites of Helicoverpa armigera (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 70:1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández, C. S., and J. Ferré. 2005. A common receptor for Bacillus thuringiensis toxins Cry1Ac, Cry1Fa, and CryJa in Helicoverpa armigera, Helicoverpa zea, and Spodoptera exigua. Appl. Environ. Microbiol. 71:5627-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrero, S., J. González-Cabrera, B. E. Tabashnik, and J. Ferré. 2001. Shared binding sites in Lepidoptera for Bacillus thuringiensis Cry1Ja and Cry1A toxins. Appl. Environ. Microbiol. 67:5729-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilbeck, A. 2001. Implications of transgenic, insecticidal plants for insect and plant biodiversity. Persp. Plant Ecol. Evol. Syst. 4:43-61. [Google Scholar]
- 20.Hilbeck, A., M. Baumgartner, P. M. Friend, and F. Bigler. 1998. Effects of transgenic Bacillus thuringiensis corn-fed prey on mortality and development time of immature Chrysoperla carnea (Neuroptera: Chrysopidae). Environ. Entomol. 27:480-487. [Google Scholar]
- 21.Hilbeck, A., M. Meier, and A. Raps. 2000. Review on non-target organisms and Bt-plants. EcoStrat GmbH. [Online.] http://www.greenpeace.ca/e/campaign/gmo/documents/hillbeckreport.pdf.
- 22.Hilbeck, A., W. J. Moar, M. Pusztai-Carey, A. Filippini, and F. Bigler. 1998. Toxicity of Bacillus thuringiensis Cry1Ab toxin to the predator Chrysoperla carnea (Neuroptera: Chrysopidae). Environ. Entomol. 27:1255-1263. [Google Scholar]
- 23.Hilbeck, A., W. J. Moar, M. Pusztai-Carey, A. Filippini, and F. Bigler. 1999. Prey-mediated effects of Cry1Ab toxin and protoxin and Cry2A protoxin on the predator Chrysoperla carnea. Entomol. Exp. Appl. 91:305-316. [Google Scholar]
- 24.Huang, J., R. Hu, S. Rozelle, and C. Pray. 2005. Insect-resistant GM rice in farmers' fields: assessing productivity and health effects in China. Science 308:688-690. [DOI] [PubMed] [Google Scholar]
- 25.James, C. 2004. Global status of commercialized biotech/GM crops: 2004. Preview. International Service for the Acquisition of Agri-Biotech Applications, Ithaca, N.Y.
- 26.Liao, C., D. G. Heckel, and R. Akhurst. 2002. Toxicity of Bacillus thuringiensis insecticidal proteins for Helicoverpa armigera and Helicoverpa punctigera (Lepidoptera: Noctuidae), major pests of cotton. J. Invertebr. Pathol. 80:55-63. [DOI] [PubMed] [Google Scholar]
- 27.MacIntosh, S. C., B. D. Lidster, and C. L. Kirkham. 1994. Isolation of brush border membrane vesicles from whole diamondback moth (Lepidoptera: Plutellidae) larvae. J. Invertebr. Pathol. 63:97-98. [Google Scholar]
- 28.Martínez-Ramírez, A., F. Gould, and J. Ferré. 1999. Histopathological effects and growth reduction in a susceptible and resistant strain of Heliothis virescens (Lepidoptera: Noctuidae) caused by sublethal doses of pure Cry1A crystal proteins from Bacillus thuringiensis. Biocontrol Sci. Technol. 9:239-246. [Google Scholar]
- 29.Nester, E. W., L. S. Thomashow, N. Metz, and M. Gordon. 2002. 100 Years of Bacillus thuringiensis: a critical scientific assessment. American Academy of Microbiology, Washington, D.C. [PubMed]
- 30.Novillo, C., J. Soto, and J. Costa. 1999. Resultados en España con variedades de algodón, protegidas genéticamente contra orugas de las cápsulas. Bol. San. Veg. Plagas 25:383-393. [Google Scholar]
- 31.Obrist, L. B., A. Dutton, J. Romeis, and F. Bigler. Biological activity of Cry1Ab toxin expressed by Bt maize following ingestion by herbivorous arthropods and exposure of the predator Chrysoperla carnea. BioControl, in press.
- 32.Poitout, S., and R. Bues. 1974. Élevage des chenilles de vint huit espèces de lépidoptères Noctuidae et deux espèces d'Arctiidae sur milieu artificiel simple. Particularités de l'élevage selon les espèces. Ann. Zool. Ecol. Anim. 6:431-441. [Google Scholar]
- 33.Poppy, G. M., and J. P. Sutherland. 2004. Can biological control benefit from genetically-modified crops? Tritrophic interactions on insect-resistant transgenic plants. Physiol. Entomol. 29:257-268. [Google Scholar]
- 34.Pray, C. E., J. Huang, R. Hu, and S. Rozelle. 2002. Five years of Bt cotton in China—the benefits continue. Plant J. 31:423-430. [DOI] [PubMed] [Google Scholar]
- 35.Ravoahangimalala, O., J. F. Charles, and J. Schoeller-Raccaud. 1993. Immunological localization of Bacillus thuringiensis serovar israelensis toxins in midgut cells of intoxicated Anopheles gambiae larvae (Diptera: Culicidae). Res. Microbiol. 144:271-278. [DOI] [PubMed] [Google Scholar]
- 36.Romeis, J., A. Dutton, and F. Bigler. 2004. Bacillus thuringiensis toxin (Cry1Ab) has no direct effect on larvae of the green lacewing Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). J. Insect Physiol. 50:175-183. [DOI] [PubMed] [Google Scholar]
- 37.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shelton, A. M., J. Z. Zhao, and R. T. Roush. 2002. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu. Rev. Entomol. 47:845-881. [DOI] [PubMed] [Google Scholar]
- 39.Vogt, H., P. Degrande, J. Just, S. Klepa, C. Kühner, A. Nickless, A. Ufer, M. Waldburger, A. Waltersdorfer, and F. Bigler. 1998. Side-effects of pesticides on larvae of Chrysoperla carnea: actual state of laboratory method, p. 123-138. In P. T. Haskell (ed.), Ecotoxicology: pesticides and beneficial organisms. Chapman, Andover, United Kingdom.
- 40.Wolfersberger, M. G. 1993. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the gypsy moth (Lymantria dispar). Arch. Insect Biochem. Physiol. 24:139-147. [DOI] [PubMed] [Google Scholar]