Abstract
Environmental and economic factors predicate the need for efficient processing of renewable sources of fuels and chemicals. To fulfill this need, microbial biocatalysts must be developed to efficiently process the hemicellulose fraction of lignocellulosic biomass for fermentation of pentoses. The predominance of methylglucuronoxylan (MeGAXn), a β-1,4 xylan in which 10% to 20% of the xylose residues are substituted with α-1,2-4-O-methylglucuronate residues, in hemicellulose fractions of hardwood and crop residues has made this a target for processing and fermentation. A Paenibacillus sp. (strain JDR-2) has been isolated and characterized for its ability to efficiently utilize MeGAXn. A modular xylanase (XynA1) of glycosyl hydrolase family 10 (GH 10) was identified through DNA sequence analysis that consists of a triplicate family 22 carbohydrate binding module followed by a GH 10 catalytic domain followed by a single family 9 carbohydrate binding module and concluding with C-terminal triplicate surface layer homology (SLH) domains. Immunodetection of the catalytic domain of XynA1 (XynA1 CD) indicates that the enzyme is associated with the cell wall fraction, supporting an anchoring role for the SLH modules. With MeGAXn as substrate, XynA1 CD generated xylobiose and aldotetrauronate (MeGAX3) as predominant products. The inability to detect depolymerization products in medium during exponential growth of Paenibacillus sp. strain JDR-2 on MeGAXn, as well as decreased growth rate and yield with XynA1 CD-generated xylooligosaccharides and aldouronates as substrates, indicates that XynA1 catalyzes a depolymerization process coupled to product assimilation. This depolymerization/assimilation system may be utilized for development of biocatalysts to efficiently convert MeGAXn to alternative fuels and biobased products.
The increasing cost and demand of fossil fuels highlight the need to develop efficient methods to utilize renewable resources for conversion to alternative energy sources such as fuel ethanol (1, 63). Supplementing the energy infrastructure with ethanol may help to shift economic dependence from petroleum-based energy. Microbial biocatalysts, both yeast and bacteria, have been developed for the conversion of glucose derived from cellulose and pentoses derived from hemicellulose to ethanol (22, 35, 37, 38), and similar approaches with bacteria have been successfully applied to the formation of value-added products such as optically pure lactic acid (23, 66, 67). Current research efforts are directed at improving the pretreatment processes to maximize the release of fermentable pentoses as well as glucose and to further develop bacterial biocatalysts for specific fermentations. The adoption of microbial strategies for the efficient depolymerization and assimilation of hemicellulose-derived carbohydrates offers promise for maximizing the conversion of the hemicellulose fraction of lignocellulosic biomass to alternative fuels and biobased products (56).
Hemicellulose represents 20% to 30% of lignocellulosic biomass, and methylglucuronoxylan (MeGAXn) is the predominant form of hemicellulose found in hardwood and crop residues (56, 61). This polymer consists of β-1,4-linked xylan in which 10% to 20% of the xylose residues are periodically substituted with α-1,2-linked 4-O-methylglucuronic acid (MeGA) moieties. Complete enzymatic hydrolysis of MeGAXn requires the combined action of several families of glycosyl hydrolases, including β-1,4-endoxylanase, α-1,2-4-O-methylglucuronidase, and β-1,4-xylosidase (56). Secreted microbial xylanases that catalyze the depolymerization of MeGAXn are primarily represented by two families of glycosyl hydrolase, glycosyl hydrolase family 10 (GH 10) and GH 11, based on sequence similarity and hydrophobic cluster analysis (Carbohydrate-Active Enzymes website, http://afmb.cnrs-mrs.fr/CAZY) (29, 31, 32).
Both GH 10 and GH 11 endoxylanases generate xylobiose (X2), xylotriose (X3), and aldouronates as prominent products during the depolymerization of glucuronoxylan. A feature of the GH 11 xylanases that distinguishes them from the GH 10 xylanases is found in the aldouronates generated upon enzyme-catalyzed hydrolysis of MeGAXn. The GH 11 xylanases generate the predominant limit product aldopentauronate (MeGAX4), in which the xylose immediately following the nonreducing residue of β-1,4-xylotetraose is substituted with MeGA, while GH 10 endoxylanases generate aldotetrauronate (MeGAX3) as the predominate limit product, in which the nonreducing xylose residue of β-1,4-xylotriose is directly substituted with MeGA (8). Based upon this observation, the GH 11 xylanase is presumed to cleave a xylosidic bond penultimate to a xylose residue that is substituted with MeGA, while the GH 10 xylanase is presumed to cleave at a xylose linked directly with the residue substituted with MeGA. Structural studies of GH 10 xylanases provide a basis for this cleavage relative to the site of MeGA substitution (28, 54).
In bacteria capable of utilizing MeGAXn, the metabolism of the aldouronates generated by enzyme-catalyzed depolymerization is dependent on their assimilation and cleavage of the MeGA substitution. Most substrate and structural studies of α-glucuronidases, the enzymes required to initiate complete degradation of MeGA-substituted xylooligosaccharides, have clearly established that only aldouronates in which MeGA is linked to the nonreducing terminal xylose are suitable substrates (49, 52). This distinguishes the role of GH 10 xylanases from GH 11 xylanases in generating products for direct assimilation and metabolism. This argument is further supported by evidence that aldotetrauronate acts as a catabolic signaling molecule for its further metabolism (60). Studies of the glucuronic acid utilization gene cluster of Geobacillus stearothermophilus have identified a putative MeGAX3 transporter in an operon composed of genes involved with the degradative and catabolic processing of glucuronoxylan. The uxuR gene product, a DNA binding protein, was found to be a self-regulating element of this operon that acts to repress transcription. Binding of MeGAX3 by UxuR alleviates repression. From this, it appears that GH 10 xylanases play a prominent role, both directly and indirectly, in the processing of MeGAXn for its complete catabolism. There is no evidence to support a similar role for the GH 11 xylanases. It is possible that GH 11 xylanases act to hydrolyze polymeric xylan primarily into shorter fragments that can then be further acted upon by GH 10 xylanases and β-xylosidases (53).
Here we describe the properties of an extracellular multidomain endoxylanase from an aggressively xylanolytic Paenibacillus sp. (strain JDR-2). The association of XynA1 with cell wall preparations indicates an anchoring role for the surface layer homology (SLH) domains near the C terminus of the 155-kDa enzyme. The marked preference of this organism for polymeric MeGAXn as a growth substrate compared to xylose or the aldouronates generated by the action of the GH 10 endoxylanase supports a role for this enzyme in the vectoral processing of MeGAXn for subsequent metabolism.
MATERIALS AND METHODS
Isolation and identification of Paenibacillus sp. strain JDR-2.
Paenibacillus sp. strain JDR-2 was isolated from fresh-cut disks (diameter, 5 cm; thickness, 2 to 4 mm) of sweet gum stem wood (Liquidambar styraciflua) incubated about 1 in. below the soil surface in a sweet gum stand for approximately 3 weeks. Disks were suspended in 50 ml of sterile deionized water and sonicated in a 125-W Branson Ultrasonic Cleaner water bath for 10 min. The sonicate was inoculated into 0.2% sweet gum (SG) MeGAXn containing the mineral salts of Zucker and Hankin (Z-H) (68) at pH 7.4 and incubated at 30°C. The SG MeGAXn was prepared and characterized by 13C nuclear magnetic resonance as described previously (34, 39, 40). Isolated colonies were passed several times in MeGAX1 broths and agars until pure. A culture growing on 0.2% MeGAXn Z-H medium was cryostored by mixing 0.5 ml of exponentially growing culture with 0.5 ml of 50% sterile glycerol and freezing at −70°C. The purified isolate was submitted to MIDI Labs (http://www.midilabs.com) for partial 16S rRNA sequencing. The organism was identified as Paenibacillus sp. with 96% identity to Paenibacillus granivorans by BLASTN submission of 530 nucleotides of sequenced 16S rRNA.
Growth studies.
A common protocol was applied in the maintenance and analysis of Paenibacillus sp. strain JDR-2 cultures. Each time a culture was prepared for study, a sample from the cryostored stock culture was transferred into 4 ml of 0.5% SG MeGAXn Z-H medium in 16- by 100-mm test tubes. After 36 to 48 h of growth, the culture was plated on agar medium containing 1% yeast extract (YE) and 0.5% oat spelt xylan in Z-H medium and grown for 36 to 48 h until appropriately sized colonies were observed. In a slight deviation, inoculum from the cryostored culture was plated directly onto the agar medium and grown for 48 to 72 h before an isolated colony was picked. All colonies regularly displayed the expected phenotype, i.e., a clearing zone on the opaque oat spelt xylan background with the expected colony morphology. For the various growth studies described below, a single colony was inoculated into medium specified for the particular experiment. All growth was performed at 30°C.
Growth optimization studies were performed aerobically in 16- by 100-mm test tubes containing 4-ml volumes of medium, and the optical densities of cultures were measured at 600 nm (OD600) with a Beckman DU500 series spectrophotometer with a 16- by 100-mm test tube holder. Individual 4-ml cultures for study were inoculated with 200 μl (5% volume) of an exponentially growing culture (4 ml of medium containing 1% YE in Z-H medium). For these test tube cultures, agitation was achieved by setting a test tube rack in a large flask holder on a New Brunswick G-2 gyratory shaker at an angle of approximately 45°. Under these conditions, rotation at 200 rpm yielded the best agitation in comparison to simple rotation.
Studies comparing Paenibacillus sp. strain JDR-2 utilization of MeGAXn, with or without xylose or glucose as cosubstrates, were performed in 125-ml baffle flasks with shaking at 150 rpm on a G-2 gyratory shaker. Cultures were initiated by the addition of 4 ml (8% volume) of Z-H mineral salts-washed cells from an overnight culture (25 ml) of 1% YE Z-H medium. Growth was monitored using an HP diode array spectrophotometer at 600 nm in a 1.00-cm cuvette. For these cultures, sample dilutions were performed to obtain OD600 readings between 0.2 and 0.8 absorbance units, and the resulting values were corrected by the dilution factor. Culture aliquots were centrifuged, supernatants were filtered, and carbohydrate utilization was measured by high-pressure liquid chromatography (HPLC) using a complete modular Waters chromatography system comprised of a 600 controller, 610 solvent delivery unit, 2410 RI detector, and a 710B WISP automated injector. Carbohydrate separation was achieved with a Bio-Rad HPX-87H column running in 0.01 N H2SO4 with a flow rate of 0.8 ml/min at 65°C. Data analysis was performed using Waters Millennium Software.
The differential utilization of MeGAXn and catalytic domain of XynA1 (XynA1 CD)-generated products from MeGAXn as growth substrates by the organism were evaluated by the initiation of 50-ml cultures with 4 ml (8% volume) of Z-H washed cells from 25-ml overnight cultures in 0.5% SG MeGAXn Z-H medium. Growth was monitored as described above and aliquots were examined by thin-layer chromatography (TLC; see procedure below).
DNA cloning, sequencing, and analysis.
A genomic library of Paenibacillus sp. strain JDR-2 DNA, prepared in pUC18 with gel-purified 6- to 9-kb fragments obtained from a partial Sau3AI digest, was kindly provided by Loraine Yomano from the laboratory of Lonnie Ingram. All cloning and general DNA manipulation methods derive from Molecular Cloning: A Laboratory Manual (58). In addition, DNA purification and gel extraction were performed using kits purchased from QIAGEN (Valencia, Calif.). Cloning analysis and planning and image preparation were performed with Clone Manager 6 and Enhance (Scientific and Educational Software, Cary, NC). Analysis of sequences for regulatory elements was conducted using the online tools available through Softberry (http://www.softberry.com/berry.phtml). The pUC18-based 6- to 9-kb library was transformed into Escherichia coli DH5α and screened for xylanase-positive clones by plating transformed cells on Remazol Brilliant Blue xylan plates and observing agar clearing after 24 h (13). Sequencing of cloned DNA was done in house by subcloning the insert into smaller sizes and using pUC18 M13 priming sites for sequencing of both strands. Primer walking at the ICBR Genome Sequencing Services Laboratory at the University of Florida filled in gaps and completed 2× coverage. All sequencing employed the Sanger dideoxy chain termination method. The final sequence was assembled using the CAP3 sequence assembly program (33) located on the Pôle Bio-Informatique Lyonnais server (http://pbil.univ-lyon1.fr/). Sequence analysis was performed with online resources available through the NCBI (http://www.ncbi.nlm.nih.gov) and BCM (http://searchlauncher.bcm.tmc.edu) websites. The main tools employed were BLAST and CD-Search of the CDD (Conserved Domain Database) (46) on the NCBI site and the 6 Frame Translation and Readseq utility at the BCM site.
Phylogenetic analysis of Paenibacillus sp. strain JDR-2 XynA1.
All presented phylogenetic analyses resulted from sequences that had been trimmed to contain only the highly conserved catalytic domain from the proton donor (WDVVNE) to the catalytic nucleophile (ITELDI). These sequences were aligned using ClustalX and phylogenetic trees constructed using MEGA 2.1 (Molecular Evolutionary Genetics Analysis, version 2.1) (43). The domain arrangement of the whole xylanase was determined with CDD (46) at NCBI (http://www.ncbi.nih.gov/Structure/cdd/wrpsb.cgi). Signal sequences were analyzed by the on-line program Signal-P (7) (http://www.cbs.dtu.dk/services/SignalP). Eighty-four bacterial GH 10 xylanases were downloaded from the CAZy(ModO) database (http://afmb.cnrs-mrs.fr/CAZY/) and processed as described above. This processing ensured the strictest comparison between all the bacterial GH 10 xylanases. Four xylanases showed high similarity to Paenibacillus sp. strain JDR-2 XynA1. These and 11 randomly chosen sequences from the set of 84 are presented in figures for this publication.
Carbohydrate and protein assays.
Total carbohydrate concentrations related to substrate preparations and enzymatic kinetic analysis were determined by a phenol-sulfuric acid assay (26). In conjunction with the total carbohydrate assay, measurements to define the degree of polymerization of substrate and increased reducing terminus levels due to xylanolytic activities were determined by the method of Nelson (50). Xylose was used as the reference for both assays. Protein levels were determined using Bradford assay reagents (Bio-Rad) with bovine serum albumin (fraction V) as the standard (12).
XynA1 CD cloning, overexpression, and purification.
The expression vector pET15b+ (Novagen, San Diego, CA) was used to overexpress the catalytic domain of XynA1 independent of other modules. Primers were designed to delimit the CD based on the modular endpoints identified by Pfam (5). The forward primer (5′-AAGCATATGGCTCCACTCAAA) included an NdeI site (underlined) for in-frame fusion with the His-tagged sequence, and the reverse primer (5′-TGTGCTCAGCCGGATCAAT) contained a BlpI (Bpu1102I) site (underlined) for directional cloning into pET15b+. This primer selection method added a Gly-Ser-His-Met sequence to the N terminus just prior to the beginning of the Pfam-designated sequence. This additional sequence was derived from the pET15 expression open reading frame coding sequence. There was additional sequence corresponding to Ala-Glu-Gln at the C-terminal end resulting from vector-derived sequence just upstream from the vector-encoded stop codon. The PCR product was generated using Proof Start high-fidelity PCR (QIAGEN, Valencia, CA). The construct was verified by sequencing. The pET15XynA1 CD N-terminal His construct was expressed in E. coli Rosetta (DE3). Protocols for expression and purification were derived from those in the pET System manual (10th edition) and Amersham Biosciences HiTrap Chelating HP instruction booklet (Piscataway, NJ). Expression was performed at 37°C, and expression culture was induced with 1 mM isopropyl-β-d-thiogalactopyranoside and grown for 3 h after induction. Affinity purification yielded a single expected band detected with Coomassie blue (CB) following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Removal of the N-terminal His tag was accomplished using a thrombin cleavage capture kit available through Novagen. XynA1 CD and XynA1 CD N-terminal His proteins were stored for short periods of time at 4°C in 50 mM potassium phosphate buffer, pH 6.5. For longer storage, these stocks were split with equal volumes of glycerol and stored at −20°C. Enzyme analysis by activity measurement and protein profiles following SDS-PAGE staining with CB were the same after 6 months of storage at −20°C.
Xylanase activity measurements for enzyme optimization and kinetic analysis.
The temperature optimum for XynA1 CD xylanase activity was determined by incubation in 0.25-ml reaction mixtures containing 1.0% SG MeGAXn in 0.1 M potassium phosphate, pH 7.0, for 10 to 30 min over a 40°C to 60°C range. Reactions were halted by the addition of 0.25 ml of Nelson's A:B reagent (25:1, vol/vol), and the increase in reducing termini was determined (50). The resulting temperature optimum (45°C) was subsequently used to determine the optimal activity for the enzyme over a pH range from 5.5 to 7.0 in reaction mixtures containing 1% SG MeGAXn in 0.1 M potassium phosphate. The optimal conditions from these determinations (pH 6.5 at 45°C) were used in experiments to examine the reaction kinetics of the enzyme with SG MeGAXn as a substrate. Activity units are described as the amount of enzyme producing 1 μmol of reducing termini per minute at 45°C. Production rates were linear through 30 min, and data obtained are averages of three separate experiments performed in triplicate.
Chromatographic resolution and detection of aldouronates and xylooligosaccharides.
Standards were obtained by acid and enzymatic hydrolysis of SG MeGAXn. Aldouronate oligomers, MeGAX1 through MeGAX5, were prepared by acid hydrolysis of MeGAXn in 0.1 N H2SO4 at 121°C for 60 min. The acid hydrolysate was neutralized with BaCO3, and the aldouronates were adsorbed onto Bio-Rad AG2-X8 anion exchange resin in the acetate form. Xylose and xylooligosaccharides were eluted with water, and the aldouronates were then eluted with 20% acetic acid. After concentration by flash evaporation, aldouronates were fractionated with 50 mM formic acid eluent on a 2.5-cm by 160-cm BioGel P-2 column (Bio-Rad, Hercules, CA) equilibrated in the same buffer. Identities of MeGAX1 and MeGAX2 were confirmed by 13C and 1H nuclear magnetic resonance spectrometry (K. Zuobi-Hasona, F. J. St. John, J. D. Rice, and J. F. Preston, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. O-20, 2001). Identities of MeGAX3, MeGAX4, and MeGAX5 are based upon the elution profile from the BioGel P-2 column and TLC analysis of aldouronates resulting from GH 10- and GH 11-catalyzed MeGAXn hydrolysis. Xylobiose and xylotriose were generated by hydrolysis with a GH 11 xylanase, XynII of Trichoderma longibrachiatum (Hampton Research, Aliso Viejo, CA), and fractionated using water-based BioGel P-2 column chromatography. These methods allowed the isolation of X2, X3, and the aldouronates MeGAX1 through MeGAX5.
To follow the depolymerization of MeGAXn catalyzed by XynA1 CD, a 250-μl reaction containing 0.5 U of enzyme and 5 mg of SG MeGAXn in potassium phosphate buffer, pH 6.5, was incubated at 30°C. Samples (5 μl) were removed every 10 min up to 120 min and spotted on 20- by 20-cm precoated 0.25-mm Silica Gel 60 TLC plates (EM Reagents, Darmstadt, Germany). An additional 0.5 U of XynA1 CD was added after the initial 120 min, and incubation was continued for an additional 16 h. A 5-μl sample representing the reaction limit products was also spotted on the plate. Oligomers were separated by ascension with a solvent system containing chloroform:glacial acetic acid:water (6:7:1, vol/vol/vol) two times for 4 h each with at least 1 h of drying time between each solvent presentation. After the second development the plate was allowed to dry for at least 30 min and then sprayed with 6.5 mM N-(1-naphthyl)ethylenediamine dihydrochloride in methanol containing 3% sulfuric acid with subsequent heating to detect the carbohydrates (10).
To compare the ability of Paenibacillus sp. strain JDR-2 to utilize MeGAXn, aldouronates, and xylooligosaccharides, MeGAXn was digested with XynA1 CD to generate primarily X2, X3, and MeGAX3. For digestions with XynA1 CD, 50 ml of substrate containing 30 mg/ml SG MeGAXn was prepared with 10 mM sodium phosphate buffer, pH 6.5. Digestions were initiated by the addition of 3.5 U of XynA1 CD and incubated with rocking at 30°C for 24 h. An additional 1 U was added after 24 h and incubation was continued for 40 h.
Digests were processed by stir-cell filtration under nitrogen pressure through YM-3 ultrafiltration membranes (3-kDa molecular mass cutoff) (Millipore, Billerica, MA). The filtrates containing oligomers with molecular sizes less than 3,000 Da were concentrated by flash evaporation and analyzed by the phenol-sulfuric acid assay (26). These concentrated fractions then served as substrates comparing the growth rates and yield of Paenibacillus sp. strain JDR-2 on MeGAXn. Cultures were incubated at 30°C in baffle flasks containing 50 ml of medium supplemented with 10 mg/ml of anhydroxylose equivalents (determined by the total carbohydrate assay). Growth was followed by measuring the OD600. Samples of 250 μl were removed at selected times and centrifuged at maximum speed in a microcentrifuge, and the supernatant and pellet were separated and saved. The supernatant was incubated at 70°C for 10 min prior to storage. A volume of 6 μl was used for each sample on the TLC plate. TLC plates were developed as described above.
Immunolocalization of XynA1.
SDS-PAGE was performed according to Laemmli (44) using a Mini-Protean 3 electrophoresis cell, a 12% Ready Gel, and Precision Plus Dual Color prestained molecular weight standards (Bio-Rad Laboratories, Hercules, CA), following the instructions in the Mini-Protean 3 manual (Bio-Rad Laboratories, Hercules, Calif.). Immunodetection was performed as previously described (59). XynA1 CD was purified to homogeneity as judged by SDS-PAGE after staining with CB and used as an antigen in the preparation of polyclonal chicken immunoglobulin Y (IgY) (19, 55).
Immunolocalization studies were performed with cell fractions following growth on SG MeGAXn. Bacillus subtilis 168 was cultured as a negative control and compared with Paenibacillus sp. strain JDR-2. Colonies of B. subtilis and strain JDR-2 were suspended in 2 ml of 1× Z-H medium and vortexed until cells were fully suspended. The complete 2-ml volume was used to inoculate 50 ml of medium in 250-ml baffle flasks containing 0.2% YE with 0.36% SG MeGAXn in Z-H medium. Cultures were grown overnight (16 h) at 30°C with shaking at 150 rpm on a New Brunswick G-2 gyratory shaker. Cells were harvested at an OD600 of 1.0 (Paenibacillus sp.) and 0.7 (B. subtilis). Cultures were centrifuged at 5,000 × g for 15 min at room temperature, and the supernatant was recovered. Cell pellets were resuspended in 50 mM sodium phosphate buffer, pH 6.5, and centrifuged as above. The procedure was repeated with 50 mM sodium phosphate, pH 6.5, containing 0.5 M NaCl. The final cell pellet was resuspended in 5 ml of 50 mM sodium phosphate, pH 6.5. Some cell lysis of Paenibacillus sp. strain JDR-2 was apparent, observed as increased viscosity, probably due to osmotic shock. A volume of 50 μl of Promega DNase RQ1 at 1 U/μl was added with 1/10 the volume of 10× DNase RQ1 buffer (0.40 M Tris-HCl, 0.10 M MgCl2, 0.01 M CaCl2, pH 8.0), and the suspension was incubated for 30 min at room temperature. Cells were then lysed by two passes at 16,000 lb/in2 through a French pressure cell. Lysates were centrifuged at 30,600 × g for 20 min at 4°C. The supernatant was collected as the cell extract, and the pellet was resuspended in 1 ml of 50 mM sodium phosphate, pH 6.5, and designated the cell wall suspension. All supernatants were concentrated using YM-10 Centriprep concentrators (Millipore, Billerica, MA) to volumes of less than 4 ml. Samples of the medium supernatant concentrate (MSC), NaCl wash (NaCl), cell extract, and cell wall suspension (CWS) were analyzed by SDS-PAGE. Reactive antigens were detected on immunoblots using rabbit anti-chicken alkaline phosphatase conjugate (Sigma, St. Louis, Mo.) as previously described, and proteins were detected in gels with CB (59).
Nucleotide sequence accession numbers.
The partial 16S ribosomal RNA sequence used to identify Paenibacillus sp. strain JDR-2 has been deposited with EMBL (http://www.ebi.ac.uk/embl/) under accession number AM180751. A culture of Paenibacillus sp. strain JDR-2 has been deposited with the Bacillus Genetic Stock Center (http://www.bgsc.org) under accession number 35A1. The DNA sequence coding for XynA1 has been deposited in the EMBL database under accession number AJ938162.
RESULTS
Growth analysis of Paenibacillus sp. strain JDR-2.
Based on OD600 measurements, the initial growth analysis of Paenibacillus sp. strain JDR-2 indicated that the organism utilized MeGAXn more efficiently than glucose or xylose as substrates (Fig. 1A). More detailed studies (Fig. 1B to D) using HPLC to follow substrate concentration showed that MeGAXn is nearly entirely utilized. Additionally, Paenibacillus sp. strain JDR-2 preferentially utilized the MeGAXn in the presence of glucose or xylose. Under these conditions, the concentrations of glucose and xylose in the medium decreased more slowly and at a nearly linear rate. Figure 1B also shows that xylose accumulates in the medium to a small extent during growth on MeGAXn, indicating that what is produced during the extracellular depolymerization may not be directly assimilated.
FIG. 1.
Growth analysis of Paenibacillus sp. strain JDR-2. (A) Paenibacillus sp. strain JDR-2 growth characterization on individual sugar substrates. OD600 measurements were determined for 4-ml cultures. For these cultures 4 ml of 1% carbohydrate in Z-H mineral salts was inoculated with 200 μl from an overnight culture of 1% YE in Z-H mineral salts. This was started from a single colony of Paenibacillus sp. strain JDR-2 from a 2-day culture in 1% YE with 0.5% oat spelt xylan on Z-H agar plates. (B) Paenibacillus sp. strain JDR-2 growth on 0.5% SG MeGAXn. (C) Paenibacillus sp. strain JDR-2 growth on 0.5% SG MeGAXn and 0.5% glucose. (D) Paenibacillus sp. strain JDR-2 growth on 0.5% SG MeGAXn and 0.5% xylose. (B to D) OD600 measurements of 50-ml cultures in 125-ml baffle flasks. For these 50-ml baffle flask cultures, 4 ml of inoculum was used from an overnight culture in 1% YE in Z-H mineral salts. HPLC was used to quantify carbohydrate concentrations. (A) Square, growth on SG MeGAXn; triangle, growth on xylose; circle, growth on glucose. (B to D) Diamond, OD600; square, SG MeGAXn; triangle, xylose; circle, glucose.
Identification and sequencing of xynA1 encoding a secreted modular GH 10 endoxylanase.
Analysis of the Paenibacillus sp. strain JDR-2 chromosomal DNA library for xylanases led to the isolation of four clones. Restriction analysis of these clones showed that the inserts were from the same genomic DNA location. Plasmid pFSJ4 was selected for sequencing, which revealed an insert (Fig. 2) including a large modular xylanase (xynA1) of 4,401 nucleotides (1,467 amino acids). Sequencing of the complete genomic DNA insert identified genes flanking xynA1. In the 5′ direction on the same chain there is an mdep gene encoding a putative multidrug efflux permease with 43% amino acid identity to the same in Bacillus halodurans (gene BH3482) determined by BLASTP. In the 3′ direction on the opposite strand there is a putative α-1,6-mannanase gene (amanA) that codes for a protein with 67% identity to Aman6 protein (aman6 gene) from Bacillus circulans. Domain analysis revealed that AmanA has the exact modular structure of Aman6, with a GH 76 catalytic module followed by triplicate family 6 carbohydrate binding modules (CBM). In silico sequence analysis identified a probable promoter region and rho-independent terminator for xynA1 but only a terminator for the mdep gene and a promoter for the gene encoding AmanA.
FIG. 2.
Genetic map of xynA1 and surrounding sequence resulting from sequencing of the Paenibacillus sp. strain JDR-2 genomic DNA insert of pFSJ4. Graphic textures refer to indicated modules as identified by Pfam. Putative promoters and rho-independent terminators are identified by an arrow and the symbol Ω, respectively.
Much like many other glycosyl hydrolases, XynA1 is a modular protein composed of eight separate modules (Fig. 2). The domains include a triplicate N-terminal set of CBM 22 modules that have previously been shown to bind soluble xylan and β-1,3-1,4-glucan (21, 65). These modules are followed in sequence by a GH 10 CD and a CBM 9, which has been shown to bind to the reducing end of carbohydrate chains (9, 51). Following CBM 9 is an undefined sequence with high similarity to the same region in Xyn5, a GH 10B xylanase of Paenibacillus sp. strain W-61. This region, as previously reported, has high identity to the lysine-rich region of the SdbA protein of C. thermocellum. Xyn5 and XynA1 have 36% and 35% amino acid identity, respectively, to this region of SdbA, and this region in XynA1 has 49% identity to the same region in Xyn5. Although these identities to the lysine rich-region of SdbA are relatively high, XynA1 and Xyn5 contain only about 5% and 6.5% lysine, respectively, to the same region of SdbA, which has 13% lysine (data not shown) (36, 45). The C-terminal region includes a triplicate set of SLH modules which are predicted to function in surface anchoring (14, 42, 48).
Phylogenetic analysis of XynA1.
Initial phylogenetic analysis revealed that XynA1 and XynA1 CD amino acid sequences, when subjected to BLASTP, had high bit scores to the same set of four modular GH 10 xylanases (Fig. 3). Comparison of the top nine BLASTP hits to XynA1 of Paenibacillus sp. strain JDR-2 shows the comparative modular structures. Additionally, the bit scores are represented for the whole sequence BLASTP and the CD sequence BLASTP. XynA1 and the top four hits were classified as GH 10B and the lower set was classified as GH 10A based on the number of amino acids separating the glutamate residue functioning as the catalytic proton donor from the glutamate functioning as the catalytic nucleophile. Although there are some exceptions, most catalytic domains of GH 10 xylanases have about 105 amino acids separating the two catalytic residues. In the case of sequence group GH 10B, the distance separating the catalytic residues is about 123 amino acids (Table 1). For further analysis we reasoned that the catalytic residue bridge sequence was probably the most highly conserved portion of GH 10 xylanases and compared this sequence from many xylanases. Figure 4 represents a phylogenetic comparison of the GH 10B subset to 11 randomly selected GH 10A xylanases. Table 1 characterizes the modular structures for the xylanases, indicating significant diversity among those represented in subset 10A. In Fig. 4A the Clustal alignment of the CD region used to prepare the phylogenetic tree revealed three areas in which the GH 10B subset differs from GH 10A. The additional sequences accounted for the extra length between the catalytic proton donor and nucleophilic glutamate residues. A phylogenetic tree developed with the neighbor-joining method for the alignment in Fig. 4A shows sequences within a clade distinct from the others (Fig. 4B). It should be noted that this comparison set is biased to the extent that it contains five very similar GH 10B sequences with 11 other random GH 10A sequences. However, the presentation of data identifies a relationship that indicates that GH 10B sequences have a common lineage. Large-scale analysis of 84 bacterial GH 10 xylanases obtained from CAZy identified GH 10B as a subset and allowed few other subsets to be created with a >95% bootstrap value. Many of these sequences did not place with confidence in any subset potentially allowing for only a few well-defined subgroups of GH 10 xylanases.
FIG. 3.
Domain alignment of GH 10 subset B and subset A sequences. The CDD was used to predict the domains from the nine most similar sequences identified through a BLAST search. Similarities relative to Paenibacillus sp. strain JDR-2 are arranged in descending order. Next to each organism name is the BLAST bits score (similarity to Paenibacillus sp. strain JDR-2) as (X/Y). X, whole XynA1 sequence BLASTP bits score; Y, Pfam-designated CD module BLASTP bits score.
TABLE 1.
Source and characteristics of sequences used for phylogenetic comparison
| Sequence no. | Organism | Swiss-Prot/TrEMBL/GenPept accession no. | Acid-to-nucleophile distancea | Domain arrangementb | Predicted signal sequencec |
|---|---|---|---|---|---|
| 1 | Clostridium stercorarium | Q9XDV5 | 123 | CBM4-9/CD/CBM9 | Yes |
| 2 | Clostridium stercorarium | Q8GJ37 | 123 | CBM4-9/CD/CBM9 | Yes |
| 3 | Paenibacillus sp. JDR-2 | Q53I45 | 123 | 3CBM4-9/CD/CBM9/3SLH | Yes |
| 4 | Clostridium josui | Q9F1V3 | 123 | CBM4-9/CD/CBM9/tSLH | Yes |
| 5 | Paenibacillus sp. W-61 | BAC45001.1 | 122 | 2CBM4-9/CD/CBM9/2SLH | Yes |
| 6 | Bacillus stearothermophilus | P45703 | 106 | CD | No |
| 7 | Thermoanaerobacter thermosulfurogenes | Q60046 | 104 | 2CBM4-9/CD/2CBM9/3SLH | Yes |
| 8 | Cellvibrio mixtus | O68541 | 104 | CD | Yes |
| 9 | Bacteroides ovatus | P49942 | 104 | CD | Yes |
| 10 | Cellvibrio japonicus | P14768 | 118 | CBM2/CBM6/CD | Yes |
| 11 | Streptomyces lividans | P26514 | 107 | CD/RBT | Yes |
| 12 | Cellulomonas fimi | P07986 | 105 | CD/CBM2 | Yes |
| 13 | Clostridium stercorarium | P40942 | 107 | CD | Yes |
| 14 | Caldicellulosiruptor saccharolyticus | O30427 | 107 | 2CBM4-9/CD | Yes |
| 15 | Clostridium thermocellum | O32374 | 116 | CBM4-9/CD/DCK | Yes |
| 16 | Clostridium acetobutylicum | Q97TP5 | 106 | CD | Yes |
Number of amino acids.
Domain identification performed through NCBI Conserved Domain Database.
Signal sequence determined using Signal-P online tool.
FIG. 4.
Phylogenetic analysis of a randomly selected set of GH 10 xylanases with respect to the XynA1 CD GH 10B subset (Table 1). Sequence for comparison consists of the highly conserved bridge between the catalytic nucleophile and proton donor glutamate residues. Sequences 1 to 5 represent the top four BLASTP hits to Paenibacillus sp. strain JDR-2 XynA1 (Table 1). All other sequences were randomly selected from a list of bacterial xylanases. (A) Clustal alignment of the analyzed sequences. (B) Neighbor-joining/bootstrap phylogenetic tree analysis.
XynA1 localization.
Chicken polyclonal IgY generated against XynA1 CD (anti-CD) was used to examine the localization of XynA1 in Paenibacillus sp. strain JDR-2 cell fractions. CB-stained SDS-PAGE bands of both Paenibacillus sp. strain JDR-2 and B. subtilis 168 proteins were primarily greater than 100 kDa for all fractions (Fig. 5A). However, anti-CD showed reactivity with Paenibacillus sp. strain JDR-2 CWS protein (approximately 150 kDa), which was not apparent with the CB-stained gel (Fig. 5B). The antibody reacted well with XynA1 CD with essentially no cross-reactivity toward B. subtilis fractions. Size estimation of the reactive Paenibacillus sp. strain JDR-2 CWS protein at approximately 150 kDa compares favorably with the molecular mass (154 kDa) obtained from the translated amino acid sequence of the XynA1 modular enzyme. The band identified as XynA1 in the immunoblot is not visible in the CB-stained gel (Fig. 5A), indicating that XynA1 represents a minor component of the surface protein complement. What is obvious from the CB-stained gel is a band size of approximately 80 kDa. This undoubtedly is the most prominent protein overshadowing all others. Observing that XynA1 is anchored to the surface supports the possibility that Paenibacillus sp. strain JDR-2 produces a crystalline surface layer. The size of the prominent band at 80 kDa is roughly the same size as the Sap and 80K surface layer proteins from Bacillus anthracis and Bacillus sphaericus, respectively (11, 27).
FIG. 5.
Localization of modular XynA1 in subcellular fractions. (A) SDS-PAGE analysis of protein content stained with Coomassie blue. (B) Immunodetection of XynA1 in companion gel blot using anti-XynA1 CD IgY polyclonal preparation. Cells were grown in medium containing 1% YE with 0.5% SG xylan in Z-H mineral salts. Recombinant XynA1 CD was used as a positive control. B. subtilis 168 was grown in the same manner for use as a gram-positive negative control. Lane designations: MSC, media supernatant concentrate; NaCl, concentrate from 0.5 M NaCl wash of cells; CFE, supernatant of French press lysate; CWS, cell wall suspension. Protein amounts (μg) loaded for SDS-PAGE are as follows for Paenibacillus sp. lanes in panel A: MSC, 3.0; NaCl, 3.0; CFE, 4.0; CWS, 4.0. For B. subtilis the lanes in panel A are as follows: MSC, 2.5; NaCl, 3.0; CFE, 3.0; and CWS, 3.0. For the immunoblots in panel B, the Paenibacillus sp. lanes are as follows (μg of protein): MSC, 1.0; NaCl, 1.0; CFE, 1.0; and CWS, 1.0. For B. subtilis the lanes are as follows: MSC, 1.0; NaCl, 1.0; CFE, 1.0; and CWS, 1.0. The XynA1 CD positive control, 3.0 μg, was loaded for SDS-PAGE and detected with CB; 1.0 μg was loaded for the Western blotting.
Kinetic and product analysis of XynA1 CD.
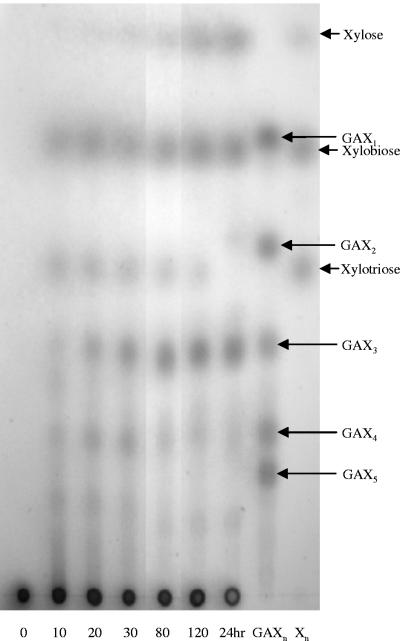
XynA1 CD was overexpressed in pET15b+ and affinity purified using an N terminus His tag. Removal of the affinity tag by thrombin protease treatment was judged as complete by SDS-PAGE and resulted in increased activity against SG MeGAXn of approximately 50%. Initial characterization showed XynA1 CD to have an optimal pH and temperature of 6.5 and 45°C, respectively (data not shown). Kinetic analysis with SG MeGAXn as a substrate (Fig. 6) showed XynA1 CD to have Vmax and Km values of 8 U/mg and 1.96 mg/ml, respectively, and a kcat of 306.8/min. Analysis of products by TLC (Fig. 7) showed that XynA1 CD is a typical GH 10 xylanase hydrolyzing MeGAXn primarily to X2 and MeGAX3. Small amounts of X3 and MeGAX4 were also produced. True limit products of the reaction included xylose, which built up from the seemingly slow conversion of X3 and MeGAX4 to X2 and MeGAX3. Figure 7 shows that by 30 min after reaction initiation, X2 and MeGAX3 are the predominant products. The small amounts of X3 and MeGAX4 disappeared by 24 h.
FIG. 6.
Lineweaver-Burk kinetic analysis of XynA1 CD. The inset represents a graph of XynA1 CD velocity versus SG MeGAXn concentration. These data were used to prepare the double reciprocal plot. XynA1 CD was analyzed using SG MeGAXn as a substrate and measuring the increase in reducing terminus by Nelson's test. All samples were analyzed in triplicate, and the final data represent the average of three separate assays.
FIG. 7.
Kinetic analysis of product formation catalyzed by XynA1 CD hydrolysis of SG MeGAXn. A 250-μl reaction mixture containing 20 mg/ml SG MeGAXn in 10 mM KH2PO4, pH 6.5, was digested with 0.5 U of XynA1 CD. The reaction mixture was incubated at 30°C with sampling every 10 min. Another 0.5 U of XynA1 CD was added after 2 h and then incubated overnight. Samples (5 μl) were subjected to TLC at indicated times, and resolved products were detected as described in Materials and Methods.
Paenibacillus sp. strain JDR-2 utilization of aldouronates and xylooligosaccharides in comparison to MeGAXn.
Xylooligosaccharides and aldouronates were generated by hydrolysis of SG MeGAXn with XynA1 CD. A 3-kDa molecular mass cutoff ultrafiltration filtrate product was used to evaluate growth of Paenibacillus sp. strain JDR-2 on xylanase-generated aldouronates and xylooligosaccharides. Growth was compared for SG MeGAXn and XynA1 CD filtrate. Figure 8 shows the aldouronates and xylooligosaccharides resolved by TLC during the growth of Paenibacillus sp. strain JDR-2. Through the time course for growth on SG MeGAXn, neither aldouronates nor xylooligosaccharides were detected in the medium during exponential growth. In contrast to growth observed on the XynA1 CD-generated products, higher growth rates and yields were observed with MeGAXn as the substrate, indicating preferred utilization of polymeric glucuronoxylan compared to aldouronates and xylooligosaccharides generated by the in vitro XynA1 CD-catalyzed depolymerization of MeGAXn.
FIG. 8.
Differential carbohydrate utilization by Paenibacillus sp. strain JDR-2. Growth of Paenibacillus sp. strain JDR-2 was compared using SG MeGAXn and the concentrated, filter-sterilized YM-3 filtrate from an overnight XynA1 SG MeGAXn digest as 1% substrates in Z-H mineral salts. (A) Growth of 50-ml cultures after inoculation with 4 ml from an overnight culture of Paenibacillus sp. strain JDR-2 in 0.5% SG MeGAXn in Z-H mineral salts. Cultures were incubated at 30°C at 150 rpm in 125-ml baffle flasks; 0.25-ml samples (S1 through S6) were removed at the times indicated. (B) TLC analysis of 6-μl aliquots of S1 through S6 supernatants after microcentrifugation and heat inactivation of xylanase activity at 70°C for 10 min.
DISCUSSION
Based upon growth and substrate utilization analysis, Paenibacillus sp. strain JDR-2 has been shown to more efficiently utilize the biomass polymer MeGAXn compared to simple sugars such as glucose and xylose. In addition, growth on MeGAXn with competing simple sugars does not seem to affect its utilization of MeGAXn (Fig. 1). This observation stands in contrast to a similar xylanolytic system from Paenibacillus sp. strain W-61, in which the investigators found that glucose strongly repressed xylanase activity (64). Although there appear to be metabolic differences, Paenibacillus sp. strain W-61 produces Xyn5, a GH 10B xylanase that is the top BLASTP hit of XynA1. Sharing 51% identity, the two full sequences are very similar, with Xyn5 differing by having only two CMB 22 modules rather than three. The kinetic properties of the two xylanases are similar, but the generation of aldouronates by Paenibacillus sp. strain W-61 was not determined, precluding a comparison to XynA1 secreted by Paenibacillus sp. strain JDR-2 (36).
Even though Paenibacillus sp. strain JDR-2 utilizes MeGAXn very efficiently, it is probable that XynA1 is the only extracellular xylanase responsible for this ability. Genomic library screening led to the isolation of four xylanolytic clones with identical restriction profiles, each containing the same xynA1 coding sequence. Only one other xylanase gene has been identified from this organism during intensive cosmid library screening, and this encodes a 40-kDa GH 10 catalytic domain designated XynA2. The primary amino acid sequence for XynA2 does not have a detectable secretion signal sequence and is expected to be localized to the cytosol. The xynA2 gene sequence is located within an operon including aguA, encoding a GH 67 α-glucuronidase, and encodes a GH 10 xylanase that may be involved in the intracellular processing of aldouronates and xylooligosaccharides generated by the action of XynA1 on the cell surface (G. Nong, V. Chow, J. D. Rice, F. St. John, and J. F. Preston, Abstr. 105th ASM Gen. Meet., abstr. O-055, 2005). MeGAX3, the primary aldouronate limit product of GH 10 xylanases (8), is presumably efficiently assimilated as it has been identified as an inducer for genes involved in hydrolysis and catabolism of glucuronoxylan in Geobacillus stearothermophilus (8, 60).
Phylogenetic characterization of XynA1 placed the sequence with a highly similar set of GH 10 xylanases referred to in this paper as the GH 10B subset (Fig. 3 and 4). This classification is supported by differences observed in the CD coding sequence. Specifically, the area bridging the two catalytic glutamate residues contains three areas of additional sequence that are not observed in other xylanases. Although GH 10B xylanases are modular, there are many similarly modular xylanases that may not be classified as GH 10B. This suggests a unique mode of action for GH 10B xylanases. It is interesting that this subset includes GH 10 xylanases in anaerobic Clostridium spp. and aerobic Paenibacillus spp., all of which are found in soil environments. The common modular architecture that, in this case, includes anchoring motifs, suggests a positive role in niche development of these bacteria. The variability in the number of CBM and SLH modules suggests these may be mobile elements that may be combined from different genes during evolution.
XynA1 CD analysis identified XynA1 as a typical GH 10 endoxylanase, producing primarily X2, X3, and MeGAX3 in the early stages of the reaction (Fig. 7) (8, 56). Hydrolysis seemed to proceed in two stages. The first stage resulted in formation of X2 and MeGAX3 with small amounts of xylose, X3, and MeGAX4. The second stage included the slow conversion of the minor products to X2 and MeGAX3 with increased formation of xylose. The XynA1 CD Km for SG MeGAXn was within a comparable range found for other GH 10 xylanases; however, the rate of catalysis was significantly lower than that found in some other GH10 enzymes (17, 18, 53). This may be due in part to the special attention given to xylanases showing high activity. Purification of Xyn5 from Paenibacillus sp. strain W-61 yielded an enzyme with a specific activity similar to that of the XynA1 purified from Paenibacillus sp. strain JDR-2 (57). It may be that the members of the GH 10B subset have lower kcat values compared to those classified herein as GH 10A.
Extracellular GH 10 xylanases may also occur as large multimodular surface-anchored enzymes separate from other glycosyl hydrolases. Representative GH 10 xylanases from Clostridium, Thermoanaerobacterium, Caldicellulosirupter, Thermotoga, Promicromonospora, Paenibacillus, and several other genera have been shown to have similar modular architectures. Typically, the N-terminal region is comprised of two to three family 22 carbohydrate binding modules (CBM 22) that are followed by a GH 10 CD. This may be followed by one or two CBM 9 modules, and the C-terminal region is sometimes comprised of up to three SLH domains. Biochemical and structural studies have characterized the roles of representative CBMs associated with xylanases showing similarities to XynA1 (2-4, 9, 15, 16, 20, 21, 47, 51, 65). The SLH modules have not been structurally characterized but have been found to play a primary role in anchoring the associated protein to the cell surface (14, 48).
It seems likely that there is a competitive advantage in colonizing a niche for an organism that utilizes surface-anchored enzymes to hydrolyze biomass polymers. The proximity of the resulting hydrolysis products would decrease diffusion-dependent assimilation rates. This strategy has been attributed to Clostridium spp. that produce the cell surface-localized cellulosome (6, 24, 25). Inclusion of xylanolytic enzymes in the cellulosome does not establish that these organisms can utilize the products resulting from hydrolysis of MeGAXn. It is probable that hemicellulolytic activities are associated with the cellulosome to increase cellulase access to cellulose by removing associated noncellulose polymers (6). It has been reported that the mesophilic Clostridium cellulovorans can ferment xylan, but there is no clear analysis of hydrolysis products and the extent to which the xylan is utilized (41, 62). Additionally, although there is increasing evidence of GH 10 xylanases in the Clostridium, there is no evidence of accessory enzymes such as an α-glucuronidase, which is thought to be required for complete utilization of MeGAXn (30). All of these characteristics pertaining to the cellulosomal systems seem to stand in contrast to the MeGAXn hydrolytic system of Paenibacillus sp. strain JDR-2. This system does not utilize the hydrolytic products of XynA1 CD efficiently and seems to require the activity of XynA1 anchored to the cell surface for efficient utilization of MeGAXn. This would suggest that the XynA1 anchoring/vectoral transport mechanism has evolved to yield almost complete recovery of hydrolytic products as an advantage against potential competitors.
Considering the efficient utilization of MeGAXn by Paenibacillus sp. strain JDR-2, this organism may provide a platform for future biocatalyst development. Under conditions of low oxygen, Paenibacillus sp. strain JDR-2 produces succinate and acetate as fermentation products (unpublished data). Alternatively, the genes encoding the cell surface-anchored XynA1, as well as those involved in the assimilation and metabolism of XynA1-generated products, may be used to engineer other bacterial platforms to efficiently convert MeGAXn to the desired fermentation products. The aggressive utilization of MeGAXn by Paenibacillus sp. strain JDR-2 supports its further development and genetic exploitation for the conversion of lignocellulosic biomass to alternative fuels and bio-based products.
Acknowledgments
We appreciate the assistance provided by K. T. Shanmugam and L. O. Ingram for general guidance and facility as well as review of this work. We also thank Loraine Yomano of L. O. Ingram's laboratory for preparing the genomic DNA library of Paenibacillus sp. strain JDR-2 and L. M. Schmidt for supplying eggs from chickens immunized with XynA CD from Paenibacillus sp. strain JDR-2.
This work was supported by U.S. Department of Energy grants DE FC36-99GO10476 and DE FC36-00GO10594, The Consortium for Plant Biotechnology Research Project GO12026-198 (DE-FG36-02GO12026), and the Institute of Food and Agricultural Sciences, University of Florida Experiment Station, as CRIS Project MCS 3763.
REFERENCES
- 1.Aldhous, P. 2005. Energy: China's burning ambition. Nature 435:1152-1154. [DOI] [PubMed] [Google Scholar]
- 2.Ali, E., G. Zhao, M. Sakka, T. Kimura, K. Ohmiya, and K. Sakka. 2005. Functions of family-22 carbohydrate-binding module in Clostridium thermocellum Xyn10C. Biosci. Biotechnol. Biochem. 69:160-165. [DOI] [PubMed] [Google Scholar]
- 3.Ali, M. K., H. Hayashi, S. Karita, M. Goto, T. Kimura, K. Sakka, and K. Ohmiya. 2001. Importance of the carbohydrate-binding module of Clostridium stercorarium Xyn10B to xylan hydrolysis. Biosci. Biotechnol. Biochem. 65:41-47. [DOI] [PubMed] [Google Scholar]
- 4.Araki, R., M. K. Ali, M. Sakka, T. Kimura, K. Sakka, and K. Ohmiya. 2004. Essential role of the family-22 carbohydrate-binding modules for beta-1,3-1,4-glucanase activity of Clostridium stercorarium Xyn10B. FEBS Lett. 561:155-158. [DOI] [PubMed] [Google Scholar]
- 5.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:138-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer, E. A., J. P. Belaich, Y. Shoham, and R. Lamed. 2004. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 58:521-554. [DOI] [PubMed] [Google Scholar]
- 7.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 8.Biely, P., M. Vrsanska, M. Tenkanen, and D. Kluepfel. 1997. Endo-beta-1,4-xylanase families: differences in catalytic properties. J. Biotechnol. 57:151-166. [DOI] [PubMed] [Google Scholar]
- 9.Boraston, A. B., A. L. Creagh, M. M. Alam, J. M. Kormos, P. Tomme, C. A. Haynes, R. A. Warren, and D. G. Kilburn. 2001. Binding specificity and thermodynamics of a family 9 carbohydrate-binding module from Thermotoga maritima xylanase 10A. Biochemistry 40:6240-6247. [DOI] [PubMed] [Google Scholar]
- 10.Bounias, M. 1980. N-(1-Naphthyl)ethylenediamine dihydrochloride as a new reagent for nanomole quantification of sugars on thin-layer plates by a mathematical calibration process. Anal. Biochem. 106:291-295. [DOI] [PubMed] [Google Scholar]
- 11.Bowditch, R. D., P. Baumann, and A. A. Yousten. 1989. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J. Bacteriol. 171:4178-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 13.Braun, E. J., and C. A. Rodrigues. 1993. Purification and properties of an endoxylanase from a corn stalk rot strain of Erwinia chrysanthemi. Phytopathology 83:332-337. [Google Scholar]
- 14.Cava, F., M. A. de Pedro, H. Schwarz, A. Henne, and J. Berenguer. 2004. Binding to pyruvylated compounds as an ancestral mechanism to anchor the outer envelope in primitive bacteria. Mol. Microbiol. 52:677-690. [DOI] [PubMed] [Google Scholar]
- 15.Cazemier, A. E., J. C. Verdoes, A. J. van Ooyen, and H. J. Op den Camp. 1999. Molecular and biochemical characterization of two xylanase-encoding genes from Cellulomonas pachnodae. Appl. Environ. Microbiol. 65:4099-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charnock, S. J., D. N. Bolam, J. P. Turkenburg, H. J. Gilbert, L. M. Ferreira, G. J. Davies, and C. M. Fontes. 2000. The X6 “thermostabilizing” domains of xylanases are carbohydrate-binding modules: structure and biochemistry of the Clostridium thermocellum X6b domain. Biochemistry 39:5013-5021. [DOI] [PubMed] [Google Scholar]
- 17.Charnock, S. J., J. H. Lakey, R. Virden, N. Hughes, M. L. Sinnott, G. P. Hazlewood, R. Pickersgill, and H. J. Gilbert. 1997. Key residues in subsite F play a critical role in the activity of Pseudomonas fluorescens subspecies cellulosa xylanase A against xylooligosaccharides but not against highly polymeric substrates such as xylan. J. Biol. Chem. 272:2942. [DOI] [PubMed] [Google Scholar]
- 18.Charnock, S. J., T. D. Spurway, H. Xie, M. H. Beylot, R. Virden, R. A. Warren, G. P. Hazlewood, and H. J. Gilbert. 1998. The topology of the substrate binding clefts of glycosyl hydrolase family 10 xylanases are not conserved. J. Biol. Chem. 273:32187-32199. [DOI] [PubMed] [Google Scholar]
- 19.Chen, S. Y. C., J. Preston, J. F. Dickson, D. W. Rice, and J. D. 1997. Antibodies from chicken eggs as probes for antigens from Pasteria penetrans endospores. J. Nemat. 29:268-275. [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke, J. H., K. Davidson, H. J. Gilbert, C. M. Fontes, and G. P. Hazlewood. 1996. A modular xylanase from mesophilic Cellulomonas fimi contains the same cellulose-binding and thermostabilizing domains as xylanases from thermophilic bacteria. FEMS Microbiol. Lett. 139:27-35. [DOI] [PubMed] [Google Scholar]
- 21.Dias, F. M., A. Goyal, H. J. Gilbert, A. M. P. Jose, L. M. Ferreira, and C. M. Fontes. 2004. The N-terminal family 22 carbohydrate-binding module of xylanase 10B of Clostridium themocellum is not a thermostabilizing domain. FEMS Microbiol. Lett. 238:71-78. [DOI] [PubMed] [Google Scholar]
- 22.Dien, B. S., M. A. Cotta, and T. W. Jeffries. 2003. Bacteria engineered for fuel ethanol production: current status. Appl. Microbiol. Biotechnol. 63:258-266. [DOI] [PubMed] [Google Scholar]
- 23.Dien, B. S., N. N. Nichols, and R. J. Bothast. 2002. Fermentation of sugar mixtures using Escherichia coli catabolite repression mutants engineered for production of L-lactic acid. J. Ind. Microbiol. Biotechnol. 29:221-227. [DOI] [PubMed] [Google Scholar]
- 24.Doi, R. H., and A. Kosugi. 2004. Cellulosomes: plant-cell-wall-degrading enzyme complexes. Nat. Rev. Microbiol. 2:541-551. [DOI] [PubMed] [Google Scholar]
- 25.Doi, R. H., A. Kosugi, K. Murashima, Y. Tamaru, and S. O. Han. 2003. Cellulosomes from mesophilic bacteria. J. Bacteriol. 185:5907-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for the determination of sugars and related substances. Anal. Chem. 28:350-356. [DOI] [PubMed] [Google Scholar]
- 27.Etienne-Toumelin, I., J. C. Sirard, E. Duflot, M. Mock, and A. Fouet. 1995. Characterization of the Bacillus anthracis S-layer: cloning and sequencing of the structural gene. J. Bacteriol. 177:614-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimoto, Z., S. Kaneko, A. Kuno, H. Kobayashi, I. Kusakabe, and H. Mizuno. 2004. Crystal structures of decorated xylooligosaccharides bound to a family 10 xylanase from Streptomyces olivaceoviridis E-86. J. Biol. Chem. 279:9606-9614. [DOI] [PubMed] [Google Scholar]
- 29.Gilkes, N. R., B. Henrissat, D. G. Kilburn, R. C. Miller, Jr., and R. A. Warren. 1991. Domains in microbial beta-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol. Rev. 55:303-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han, S. O., H. Yukawa, M. Inui, and R. H. Doi. 2004. Isolation and expression of the xynB gene and its product, XynB, a consistent component of the Clostridium cellulovorans cellulosome. J. Bacteriol. 186:8347-8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henrissat, B., and A. Bairoch. 1993. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henrissat, B., and G. Davies. 1997. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 7:637-644. [DOI] [PubMed] [Google Scholar]
- 33.Huang, X., and A. Madan. 1999. CAP3: A DNA sequence assembly program. Genom. Res. 9:868-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurlbert, J. C., and J. F. Preston III. 2001. Functional characterization of a novel xylanase from a corn strain of Erwinia chrysanthemi. J. Bacteriol. 1 83:2093-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram, L. O., H. C. Aldrich, A. C. Borges, T. B. Causey, A. Martinez, F. Morales, A. Saleh, S. A. Underwood, L. P. Yomano, S. W. York, J. Zaldivar, and S. Zhou. 1999. Enteric bacterial catalysts for fuel ethanol production. Biotechnol. Prog. 15:855-866. [DOI] [PubMed] [Google Scholar]
- 36.Ito, Y., T. Tomita, N. Roy, A. Nakano, N. Sugawara-Tomita, S. Watanabe, N. Okai, N. Abe, and Y. Kamio. 2003. Cloning, expression, and cell surface localization of Paenibacillus sp. strain W-61 xylanase 5, a multidomain xylanase. Appl. Environ. Microbiol. 69:6969-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffries, T. W., and Y. S. Jin. 2004. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl. Microbiol. Biotechnol. 63:495-509. [DOI] [PubMed] [Google Scholar]
- 38.Jin, Y. S., J. M. Laplaza, and T. W. Jeffries. 2004. Saccharomyces cerevisiae engineered for xylose metabolism exhibits a respiratory response. Appl. Environ. Microbiol. 70:6816-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones, J. K. N., C. B. Purves, and T. E. Timell. 1961. Constitution of 4-O-methylglucuronoxylan from the wood of trembling aspen (Populus tremuloides Michx.). Can. J. Chem. 39:1059-1066. [Google Scholar]
- 40.Kardosova, A., M. Matulova, and A. Malovikova. 1998. (4-O-Methyl-alpha-d-glucurono)-d-xylan from Rudbeckia fulgida, var. sullivantii (Boynton et Beadle). Carbohydr. Res. 308:99-105. [DOI] [PubMed] [Google Scholar]
- 41.Kosugi, A., K. Murashima, and R. H. Doi. 2001. Characterization of xylanolytic enzymes in Clostridium cellulovorans: expression of xylanase activity dependent on growth substrates. J. Bacteriol. 183:7037-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kosugi, A., K. Murashima, Y. Tamaru, and R. H. Doi. 2002. Cell-surface-anchoring role of N-terminal surface layer homology domains of Clostridium cellulovorans EngE. J. Bacteriol. 184:884-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 44.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 45.Leibovitz, E., H. Ohayon, P. Gounon, and P. Beguin. 1997. Characterization and subcellular localization of the Clostridium thermocellum scaffoldin dockerin binding protein SdbA. J. Bacteriol. 179:2519-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchler-Bauer, A., J. B. Anderson, C. Weese-Scott, N. D. Fedorova, L. Y. Geer, S. He, D. I. Hurwitz, J. D. Jackson, A. R. Jacobs, C. J. Lanczycki, C. A. Liebert, C. Liu, T. Madej, G. H. Marchler, R. Mazumder, A. N. Nikolskaya, A. R. Panchenko, B. S. Rao, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, S. Vasudevan, Y. Wang, R. A. Yamashita, J. J. Yin, and S. H. Bryant. 2003. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 31:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meissner, K., D. Wassenberg, and W. Liebl. 2000. The thermostabilizing domain of the modular xylanase XynA of Thermotoga maritima represents a novel type of binding domain with affinity for soluble xylan and mixed-linkage beta-1,3/beta-1,4-glucan. Mol. Microbiol. 36:898-912. [DOI] [PubMed] [Google Scholar]
- 48.Mesnage, S., T. Fontaine, T. Mignot, M. Delepierre, M. Mock, and A. Fouet. 2000. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 19:4473-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagy, T., K. Emami, C. M. Fontes, L. M. Ferreira, D. R. Humphry, and H. J. Gilbert. 2002. The membrane-bound α-glucuronidase from Pseudomonas cellulosa hydrolyzes 4-O-methyl-d-glucuronoxylooligosaccharides but not 4-O-methyl-d-glucuronoxylan. J. Bacteriol. 184:4925-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson, N. 1944. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 153:375-380. [Google Scholar]
- 51.Notenboom, V., A. B. Boraston, D. G. Kilburn, and D. R. Rose. 2001. Crystal structures of the family 9 carbohydrate-binding module from Thermotoga maritima xylanase 10A in native and ligand-bound forms. Biochemistry 40:6248-6256. [DOI] [PubMed] [Google Scholar]
- 52.Nurizzo, D., T. Nagy, H. J. Gilbert, and G. J. Davies. 2002. The structural basis for catalysis and specificity of the Pseudomonas cellulosa alpha-glucuronidase, GlcA67A. Structure 10:547-556. [DOI] [PubMed] [Google Scholar]
- 53.Pell, G., L. Szabo, S. J. Charnock, H. Xie, T. M. Gloster, G. J. Davies, and H. J. Gilbert. 2004. Structural and biochemical analysis of Cellvibrio japonicus xylanase 10C: how variation in substrate-binding cleft influences the catalytic profile of family GH-10 xylanases. J. Biol. Chem. 279:11777-11788. [DOI] [PubMed] [Google Scholar]
- 54.Pell, G., E. J. Taylor, T. M. Gloster, J. P. Turkenburg, C. M. Fontes, L. M. Ferreira, T. Nagy, S. J. Clark, G. J. Davies, and H. J. Gilbert. 2004. The mechanisms by which family 10 glycoside hydrolases bind decorated substrates. J. Biol. Chem. 279:9597-9605. [DOI] [PubMed] [Google Scholar]
- 55.Polson, A., T. Coetzer, J. Kruger, E. von Maltzahn, and K. J. van der Merwe. 1985. Improvements in the isolation of IgY from the yolks of eggs laid by immunized hens. Immunol. Investig. 14:323-327. [DOI] [PubMed] [Google Scholar]
- 56.Preston, J. F., J. C. Hurlbert, J. D. Rice, A. Ragunathan, and F. J. St. John. 2003. Microbial strategies for the depolymerization of glucuronoxylan: leads to biotechnological applications of endoxylanases, p. 191-210. In S. D. Mansfield and J. N. Sadler (ed.), Applications of enzymes to lignocellulosics. American Chemical Society, Washington D.C.
- 57.Roy, N., N. Okai, T. Tomita, K. Muramoto, and Y. Kamio. 2000. Purification and some properties of high-molecular-weight xylanases, the xylanases 4 and 5 of Aeromonas caviae W-61. Biosci. Biotechnol. Biochem. 64:408-413. [DOI] [PubMed] [Google Scholar]
- 58.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 59.Schmidt, L., J. Preston, D. Dickson, J. Rice, and T. Hewlett. 2003. Environmental quantification of Pasteuria penetrans endospores using in situ antigen extraction and immunodetection with a monoclonal antibody. FEMS Microbiol. Ecol. 44:17-26. [DOI] [PubMed] [Google Scholar]
- 60.Shulami, S., O. Gat, A. L. Sonenshein, and Y. Shoham. 1999. The glucuronic acid utilization gene cluster from Bacillus stearothermophilus T-6. J. Bacteriol. 181:3695-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh, S., A. M. Madlala, and B. A. Prior. 2003. Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol. Rev. 27:3-16. [DOI] [PubMed] [Google Scholar]
- 62.Sleat, R., R. A. Mah, and R. Robinson. 1984. Isolation and characterization of an anaerobic, cellulytic bacterium, Clostridium cellulovorans sp. nov. Appl. Environ. Microbiol. 48:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun, Y., and J. Cheng. 2002. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour. Technol. 83:1-11. [DOI] [PubMed] [Google Scholar]
- 64.Viet, D., Y. Kamio, N. Abe, J. Kaneko, and K. Izaki. 1991. Purification and properties of beta-1,4-xylanase from Aeromonas caviae W-61. Appl. Environ. Microbiol. 57:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie, H., H. J. Gilbert, S. J. Charnock, G. J. Davies, M. P. Williamson, P. J. Simpson, S. Raghothama, C. M. Fontes, F. M. Dias, L. M. Ferreira, and D. N. Bolam. 2001. Clostridium thermocellum Xyn10B carbohydrate-binding module 22-2: the role of conserved amino acids in ligand binding. Biochemistry 40:9167-9176. [DOI] [PubMed] [Google Scholar]
- 66.Zhou, S., T. B. Causey, A. Hasona, K. T. Shanmugam, and L. O. Ingram. 2003. Production of optically pure d-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl. Environ. Microbiol. 69:399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou, S., K. T. Shanmugam, and L. O. Ingram. 2003. Functional replacement of the Escherichia coli d-(-)-lactate dehydrogenase gene (ldhA) with the l-(+)-lactate dehydrogenase gene (ldhL) from Pediococcus acidilactici. Appl. Environ. Microbiol. 69:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zucker, M., and L. Hankin. 1970. Regulation of pectate lyase synthesis in Pseudomonas fluorescens and Erwinia carotovora. J. Bacteriol. 104:13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]