Abstract
A multiple vector system for the production and export of recombinant affinity-tagged proteins in Bacillus megaterium was developed. Up to 1 mg/liter of a His6-tagged or Strep-tagged Lactobacillus reuteri levansucrase was directed into the growth medium, using the B. megaterium esterase LipA signal peptide, and recovered by one-step affinity chromatography.
The gram-positive bacterium Bacillus megaterium has several advantages over other recombinant protein production hosts. In contrast to Bacillus subtilis, B. megaterium does not possess alkaline proteases and is known for the stable replication and maintenance of plasmids (7). The bacterium readily secretes proteins into the growth medium. For this study, the commercially interesting levansucrase Lev from Lactobacillus reuteri 121 (3, 6) was chosen as a model protein to further improve the efficiency and use of a novel B. megaterium-based secretion system for heterologous proteins.
Construction of vector pMM1525 for high-level production and secretion of recombinant proteins via the LipA signal peptide in B. megaterium.
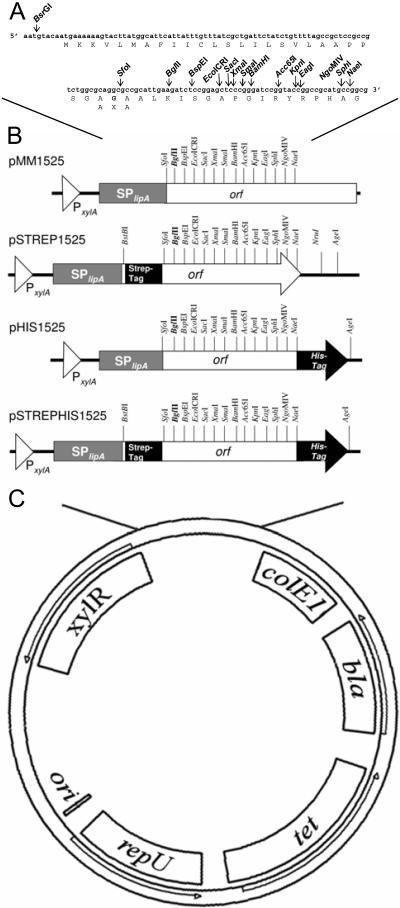
For the secretion of heterologous proteins of interest from a B. megaterium host, a vector encoding a functional signal peptide was constructed. The vector allows translational fusion of a gene of interest to a signal peptide-encoding sequence. The resulting fusion protein with the signal peptide is transported via the SEC pathway into the growth medium. The 28-amino-acid signal peptide of the recently discovered B. megaterium extracellular esterase LipA was chosen (4). Its signal peptidase cleavage site consists of an AKA motif (Fig. 1A). Because the second amino acid of the signal peptidase recognition site is variable (5), it was possible to incorporate an SfoI restriction site into its coding sequence, replacing the lysine (K) with a glycine residue (G). The introduced SfoI restriction site allows for the cloning of genes directly downstream of the signal peptide coding sequence (Fig. 1B). As a consequence, the resulting fusion protein possesses the original N-terminal amino acid sequence after processing by the signal peptidase and its secretion into the growth medium. However, this first N-terminal amino acid, which is the one next to the signal peptidase cleavage site, is not completely variable. Secreted proteins in B. subtilis have most abundantly an alanine at this position. This alanine residue is encoded in pMM1525 but can be exchanged for most other amino acids, except proline and cysteine (5).
FIG. 1.
(A) Structure of secretion vector pMM1525 encoding the signal peptide of the B. megaterium extracellular esterase LipA (SPlipA) and carrying a multiple cloning site. The amino acid sequence of the open reading frame (orf) and the cleavage site AXA of the signal peptidase type I are indicated. (B) Expression plasmids for secretion of tagged proteins of interest via the LipA signal peptide. All plasmids are based on the shuttle vector pMM1520 (1, 2). All expression plasmids shown allow parallel cloning of genes of interest into the identical multiple cloning sites, from BglII (marked in bold) to NgoMIV. (C) The elements for inducible gene expression in B. megaterium are the xylose-inducible promoter (PxylA) and the gene for the xylose repressor (xylR). The elements for plasmid replication in Bacillus sp. are the origin of plasmid replication (ori), a gene essential for plasmid replication (repU), and the tet resistance gene. The elements for plasmid replication in E. coli are the origin of replication, colE1, and the ampicillin resistance gene bla.
For insertion of the signal peptide-encoding sequence into the B. megaterium expression plasmid pMM1520 (1), an additional unique restriction site (BsrGI) had to be generated downstream of the xylose-inducible promoter (PxylA) by site-directed mutagenesis, creating pMM1522. This new restriction site provided the basis for the insertion of the signal peptide coding sequence into the BsrGI and BstBI restriction sites of pMM1522. The resulting vector, pMM1525 (Fig. 1), allows the xylose-inducible production and secretion of proteins of interest in B. megaterium. Due to the identity of this region between pMM1522 and pMM1525, parallel cloning of genes as translational fusions for intracellular production (pMM1522) and secretion into the growth medium (pMM1525) is possible.
B. megaterium gene expression plasmids for extracellular production of fusion proteins with small affinity tags.
The vectors pHIS1525 and pSTREP1525 allow the insertion of target genes for the production and secretion of a C-terminally His6-tagged and an N-terminally Strep II-tagged fusion protein, respectively (see the supplemental material). Features of these two secretion vectors were combined in pSTREPHIS1525, an expression plasmid for the extracellular production of Strep-His6-tagged proteins (Fig. 1B). Convenient parallel cloning into all described vectors is possible when genes of interest are inserted into the identical restriction endonuclease sites of the multiple cloning site (BglII to NgoMIV).
Construction of L. reuteri levansucrase gene-carrying B. megaterium expression vectors.
The extracellular levansucrase Lev from L. reuteri strain 121 was chosen as a model protein for protein export studies using B. megaterium. The soluble and stable Lev variant LevΔ773MycHis was previously generated via deletion of the DNA encoding the N-terminal Lev-specific signal peptide and the C-terminal cell wall anchor. Furthermore, a C-terminal Myc epitope and a His6 tag were added (6). For secretion of the levansucrase LevΔ773MycHis by B. megaterium, the coding sequence was inserted directly downstream of the signal peptide coding sequence of pMM1525, resulting in pMMBm7 (see the supplemental material). Furthermore, the plasmid pMGBm4, carrying solely the levΔ773 gene, was constructed after introduction of a stop codon via site-directed mutagenesis upstream of the Myc epitope and His6 tag. Finally, the levΔ773 gene was cloned into the plasmids pHIS1525, pSTREP1525, and pSTREPHIS1525 for the export of affinity-tagged levansucrase (see the supplemental material).
Evaluation of production and export of affinity-tagged levansucrase.
Using 500 ml LB broth in 1-liter shake flask cultivations, up to 4 mg/liter of untagged levansucrase was secreted as the predominant protein into the culture medium. His6- and Strep-tagged levansucrase was produced and exported at up to 2 mg per liter culture medium (Table 1). Clearly, the stepwise introduction of affinity tags decreased the amount of exported recombinant protein. Formed secondary and tertiary protein structure elements of the fused tags in the cytoplasm might be responsible for this observation. A batch incubation of the cell-free extracellular growth medium with the corresponding affinity chromatographic material allowed one-step purification of LevΔ733His (Table 1). Previously, 186.5 mg L. reuteri levansucrase per liter culture medium was produced intracellularly in Escherichia coli. However, after cell disruption, only 7.3% (4.6 mg) was recovered by chromatographic purification (6). Its specific activity of 177 U/mg compares well with the 197 U/mg reported here for purified LevΔ773His. Hence, a competitive B. megaterium-based system for the production, export, and further affinity chromatographic recovery of recombinant proteins in the milligram range per liter growth medium was established.
TABLE 1.
Recombinant production and one-step affinity purification of L. reuteri levansucrase from growth medium, using a B. megaterium expression system
| Basic secretion vector | Final secretion vector | Encoded protein | Concn of secreted LevΔ773 (mg/liter culture)a | Concn of purified LevΔ773 (mg/liter culture)b |
|---|---|---|---|---|
| pMM1525 | pMGBm4 | LevΔ773 | 4.0 | |
| pHIS1525 | pRBBm15 | LevΔ773His | 2.1 | 0.9d |
| pSTREP1525 | pRBBm13 | StrepLevΔ773 | 2.7 | 0.7c |
| pMM1525 | pMMBm7 | LevΔ773MycHis | 1.6 | |
| pSTREPHIS1525 | pRBBm16 | StrepLevΔ773His | 0.8 |
For the induction of gene expression, 0.5% (wt/vol) xylose was added at an optical density at 578 nm of 0.4 to 500-ml cultures of B. megaterium MS941 carrying the indicated final, levansucrase-encoding plasmids. Cell-free growth medium was obtained by centrifugation. Protein amounts were calculated either via measured enzyme activity and specific activity or via densitometry after sodium dodecyl sulfate polyacrylamide gel electrophoresis of proteins from the growth medium.
A Bradford assay kit (Bio-Rad, Hercules, Calif.) was used for protein measurements.
Ammonium sulfate precipitation of proteins from cell-free growth medium was followed by StrepTactin affinity purification (IBA GmbH, Göttingen, Germany).
One-step purification was achieved by batch incubation of Ni-nitrilotriacetic acid-Sepharose (Amersham-Pharmacia Biotech, Uppsala, Sweden) with cell-free growth medium prior to elution.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support granted by the Deutsche Forschungsgemeinschaft (SFB 578) and “Fonds der Chemischen Industrie.” Furthermore, we thank Mobitec GmbH and IBA GmbH, Göttingen, Germany, for commercialization of the expression system described here.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Malten, M., R. Hollmann, W.-D. Deckwer, and D. Jahn. 2005. Production and secretion of recombinant Leuconostoc mesenteroides dextransucrase DsrS in Bacillus megaterium. Biotechnol. Bioeng. 89:206-218. [DOI] [PubMed] [Google Scholar]
- 2.Malten, M., H. Nahrstedt, F. Meinhardt, and D. Jahn. 2005. Coexpression of the type I signal peptidase gene sipM increased recombinant protein production and export in Bacillus megaterium MS941. Biotechnol. Bioeng. 91:616-622. [DOI] [PubMed] [Google Scholar]
- 3.Ozimek, L. K., S. A. van Hijum, G. A. van Koningsveld, M. J. van Der Maarel, G. H. van Geel-Schutten, and L. Dijkhuizen. 2004. Site-directed mutagenesis study of the three catalytic residues of the fructosyltransferases of Lactobacillus reuteri 121. FEBS Lett. 560:131-133. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz, C., A. Blanco, F. I. Pastor, and P. Diaz. 2002. Analysis of Bacillus megaterium lipolytic system and cloning of LipA, a novel subfamily I.4 bacterial lipase. FEMS Microbiol. Lett. 217:263-267. [DOI] [PubMed] [Google Scholar]
- 5.Tjalsma, H., A. Bolhuis, J. D. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hijum, S. A., E. Szalowska, M. J. van der Maarel, and L. Dijkhuizen. 2004. Biochemical and molecular characterization of a levansucrase from Lactobacillus reuteri. Microbiology 150:621-630. [DOI] [PubMed] [Google Scholar]
- 7.Vary, P. S. 1994. Prime time for Bacillus megaterium. Microbiology 140:1001-1013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.