Abstract
We report here on the case of a child who was infected with scrub typhus, and we made the diagnosis according to the serology and by performing PCRs on the child's eschar. The patient was treated with azithromycin, and he did not experience any complications. Performing nested PCR on the eschar might be both a rapid diagnostic test for scrub typhus in the early acute stage and a differential test as to whether or not a scab is a scrub typhus eschar.
CASE REPORT
A 7-year-old boy was admitted to Seonam University Hospital because of his 7-day history of a high fever and rash. The physical examination at the time of admission revealed a papulomacular skin rash on his trunk that soon spread to his extremities. Enlarged tender lymph nodes were palpable in his right neck and back of the neck areas. The body temperature was 39°C, the heart rate was 115/min, and the respiration rate was 28. The patient's leukocyte count was 5,700/mm3 (68.5% polymorphonuclear cells and 23.9% lymphocytes), his hemoglobin level was 11.2 g/dl, and the platelet count was 91,000/mm3. Other laboratory values were as follows: aspartate aminotransferase, 170 U/liter; alanine aminotransferase, 189 U/liter; lactate dehydrogenase, 230 U/liter; and creatine phosphokinase, 255 U/liter. We suspected that the patient was suffering from viral exanthema or infectious mononucleosis with a mobiliform rash. The patient was treated symptomatically with acetaminophen. Over the next 2 days, dyspnea developed and his temperature increased to 39.8°C. Radiographs of the chest showed an interstitial pneumonia pattern. On the third hospital day, we inadvertently found an eschar-like crust lesion on the front part of the scalp (Fig. 1), but his mother insisted that the crust was caused by minor trauma. We carefully took the history once again. Seven days before admission, he had played with his brother on the grass of Citizen's Park in Gwangju City. We wanted to know whether the crust was eschar or not. An informed consent was obtained to take a sample of the eschar and blood samples, and then a piece of crust was removed from the scalp. We immersed the crust into about 1 ml of saline. We performed nested PCR for the gene that encodes the 56-kDa protein that is specific for Orientia tsutsugamushi. Based on the clinical diagnosis of scrub typhus, azithromycin therapy was initiated. Defervescence occurred within 12 h. However, because the optimal dosage of azithromycin for the treatment of scrub typhus has not been determined, especially for children, we added another 500 mg of azithromycin at 24 h after the first dose to experimentally reduce the risk of relapse or therapeutic failure. Sixty hours after the azithromycin administration was stopped, a fever of 38.0°C, malaise, and headache were again developed. The therapy with azithromycin was restarted. Azithromycin (250 mg) once a day for an additional 5 days was added. After this administration, his symptoms, such as fever and malaise, were relieved and he was discharged without complications. A second serology for O. tsutsugamushi by using indirect immunofluorescence was performed during the second week after admission, and it revealed positive conversion (the titer of immunoglobulin M was 1:1,280; the titer of immunoglobulin G was 1:4,096 or more). To exclude other infections, the patient was evaluated for laboratory evidence of murine typhus, leptospirosis, hemorrhagic fever with renal syndrome, infectious mononucleosis, and measles. But these results were all negative.
FIG. 1.
Eschar on frontal part of the scalp of our patient with scrub typhus.
Scrub typhus is an acute febrile illness that is caused by Orientia tsutsugamushi, and this is transmitted from rodents to humans by the larval-stage trombiculid mites (4). An infection is heralded by an eschar at the site of the inoculating chigger bite, and this is followed by the development of a disseminated papulomacular rash, fever, malaise, myalgia, and anorexia. The diagnosis of scrub typhus is made either by recovering the causal O. tsutsugamushi from the blood of a patient during the febrile period or by demonstrating an increase of the serum antibodies against O. tsutsugamushi during convalescence (1). However, the identification of O. tsutsugamushi in cultured cells or infected mice requires at least several weeks (9). There is also a delay of several weeks between the onset of the illness and the increase in the titer of antibody. Consequently, a simple and more rapid laboratory diagnostic method for scrub typhus is needed. PCR assay of a blood sample has proven to be very useful for the early diagnosis of scrub typhus (5). Yet, until now, there have been no previous reports on the clinical utility of eschar PCR for the diagnosis of scrub typhus. We describe here a case of scrub typhus, and the diagnosis was made by performing PCR on the eschar and by the patient's serology.
Discussion.
The initial lesion of scrub typhus is a papule that develops at the site of the inoculation and it subsequently forms an eschar. Approximately 1 to 2 weeks after the development of the initial lesion, an abrupt onset of high fever, headache, and skin rash develops. The diagnosis of scrub typhus has been based on the assessment of the titer of antibody in serum samples that are obtained during the acute and convalescent phases of the illness. However, it takes several weeks to confirm the diagnosis by serologic testing to establish a fourfold or greater titer increase.
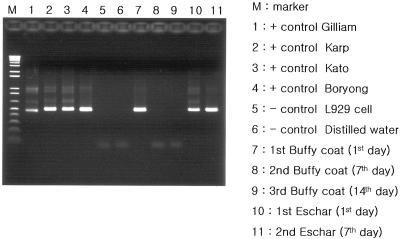
We thought that performing PCR assay for eschar could be one of the possible diagnostic methods in the early stage of the illness because the eschar is easily accessible as a diagnostic specimen, especially in children, and the eschar is the inoculation site of O. tsutsugamushi and there should be a lot of Orientia organisms present at this site. For the eschar PCR assay, we removed an eschar on the scalp and a new crust formed 7 days later at the site of the eschar extraction. The newly formed crust was removed for the second eschar PCR. The blood samples and the eschars were analyzed by a nested PCR method as described previously by Furuya et al. (5; see also reference 7) with slight modification. The DNAs were extracted from the eschar and the buffy coat by using a QIAamp DNA mini kit (QIAGEN, Hilden, Germany). The nucleotide primers were based on the nucleotide sequences of a gene encoding the 56-kDa antigen of a Gilliam strain of O. tsutsugamushi. Primers 34 (5′-TCA AGC TTA TTG CTA GTG CAA TGT CTGC-3′) and 55 (5′-AGG GAT CCC TGC TGC TGT GCT TGC TGC G-3′) were used for the first PCR, and nested PCR primers 10 (5′-GAT CAA GCT TCC TCA GCC TAC TAT AAT GCC-3′) and 11 (5′-CTA GGG ATC CCG ACA GAT GCA CTA TTA GGC-3′) were used to amplify a 483-bp fragment. The strains that were used as positive controls in this study were three prototype strains (Gilliam, ATCC VR 312; Karp, ATCC VR 150; and Kato, ATCC VR 609) and a Korea isolate (Boryong). Distilled water and L929 cells were used as the negative control. A 483-bp DNA fragment was amplified by nested PCR (Fig. 2). A 56-kDa-protein-specific band was detected in both the buffy coat and the eschar sample that was collected at the time that antibiotics were administered. However, on the follow-up PCR study at 7 days after administration of antibiotics, a 56-kDa-protein-specific band was detected in only the newly formed eschar, not in the buffy coat (Fig. 2). This means that eschar PCR can be a useful diagnostic test for patients with scrub typhus who are undergoing appropriate antibiotic therapy. We also performed sequencing of the amplicons and the restriction fragment length polymorphism. We identified the strain as being the O. tsutsugamushi Boryong strain (data not shown).
FIG. 2.
Agarose gel electrophoresis of the amplified DNA fragments that were obtained by nested PCR on the buffy coat and the eschar sample that was obtained from the patient at the time that antibiotics were administered, as well as 7 days and 14 days after administration of antibiotics. A 483-bp DNA fragment was amplified by nested PCR.
Physicians sometimes encounter eschar-like crust lesions in clinical practice in Korea, especially in the autumn season. In some cases, the patient or his family insists that the crust is caused by minor trauma or is a mark from scratching. In our case, we wanted to know whether the crust was a scrub typhus eschar or not. In such a case, especially for a patient who does not have any symptoms of suspected rickettial disease, such as skin rash or fever, we can consider the use of nested PCR on the eschar for the diagnosis. Furthermore, eschar PCR can be useful after antibiotic administration, and the test results can be obtained within 24 h after getting a part of the eschar.
This case gives us another message about using azithromycin treatment for scrub typhus. Although chloramphenicol and doxycycline have been used as the drugs of choice, they have the potential for such complications as aplastic anemia and tooth discoloration in children (2). Recently, azithromycin has been proven to be more effective than doxycycline in an in vitro assay system against the doxycycline-susceptible and the doxycycline-resistant strains of Orientia tsutsugamushi (3, 8, 10). But, to our surprise, there have been no previous reports of azithromycin administration to children for the treatment of scrub typhus in the English literature. Therefore, the efficacy and optimal dosage of azithromycin for the treatment of scrub typus have not been evaluated via a clinical study in children. A prospective, open-labeled randomized trial was recently conducted (6). The results reported that a single dose of azithromycin was as effective as a 1-week course of daily 200-mg doses of doxycycline for the treatment of mild scrub typhus in patients who were 18 years of age or older. But for moderate-to-severe scrub typhus, such as in our case, or for the cases accompanying with pneumonitis in children, a single dose of azithromycin might run the risk of clinical failure.
This case is clinically significant in two aspects. First, this is the first case report of the clinical usefulness of eschar PCR for the diagnosis of O. tsutsugamushi. The nested PCR method for eschar might be both a rapid diagnostic test for tsutsugamushi disease in the early acute stage and a differential test for whether or not a crust is a scrub typhus eschar. Second, to our knowledge, this is the first case report in the English literature of a child with scrub typhus who was treated with azithromycin.
Acknowledgments
This study was supported by research funds from Chosun University, 2005.
We thank Myoung-don Oh for his keen ideas and his review of the manuscript.
REFERENCES
- 1.Bozeman, G. W., and B. L. Elisberg. 1963. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc. Soc. Exp. Biol. Med. 112:568-573. [DOI] [PubMed] [Google Scholar]
- 2.Brown, G. W. 1988. Scrub typhus; pathogenesis and clinical syndrome, p. 93-100. In D. H. Walker (ed.), Biology of rickettsial disease, vol. 1. CRC Press, Boca Raton, Fla. [Google Scholar]
- 3.Choi, E. K., and H. Pai. 1998. Azithromycin therapy for scrub typhus during pregnancy. Clin. Infect. Dis. 27:1538-1539. [DOI] [PubMed] [Google Scholar]
- 4.Elisberg, B. L., J. M. Campbell, and F. M. Bozeman. 1968. Antigenic diversity of Rickettsia tsutsugamushi: epidemiologic and ecologic significance. J. Hyg. Epidemiol. Microbiol. Immunol. 12:18-25. [PubMed] [Google Scholar]
- 5.Furuya, Y., Y. Yoshida, T. Katayama, S. Yamamoto, and A. Kawamura, Jr. 1993. Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J. Clin. Microbiol. 31:1637-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim, Y. S., H. J. Yun, S. K. Shim, S. H. Koo, and S. Kim. 2004. A comparative trial of a single dose of azithromycin versus doxycycline for the treatment of mild scrub typhus. Clin. Infect. Dis. 39:1329-1335. [DOI] [PubMed] [Google Scholar]
- 7.Pai, H., S. Sohn, Y. Seong, S. Kee, W. H. Chang, and K. W. Choe. 1997. Central nervous system involvement in patients with scrub typhus. Clin. Infect. Dis. 24:436-440. [DOI] [PubMed] [Google Scholar]
- 8.Strickman, D., T. Sheer, K. Salata, et al. 1995. In vitro effectiveness of azithromycin against doxycycline-resistant and -susceptible strains of Rickettsia tsutsugamushi, etiologic agent of scrub typhus. Antimicrob. Agents Chemother. 39:2406-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suto, T. 1980. Rapid serologic diagnosis of tsutsugamushi disease employing the immunoperoxidase reaction with cell cultured rickettsia. Clin. Virol. 8:425-429. [Google Scholar]
- 10.Watt, G., P. Kantipong, K. Jongsakul, P. Watcharapichat, and D. Phulsuksombati. 1999. Azithromycin activities against Orientia tsutsugamushi strains isolated in cases of scrub typhus in northern Thailand. Antimicrob. Agents Chemother. 43:2817-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]