Abstract
Recurrence of osteomyelitis by the same bacterial strain is well known. We report three patients with a second episode of osteomyelitis at the same site caused by different strains of bacteria from the original. Formerly infected and altered bone surface might present a region of diminished resistance for a new infection.
CASE REPORTS
Case 1.
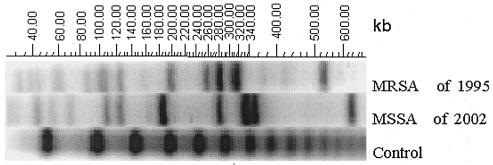
In November 2002, an otherwise healthy 45-year-old immunocompetent man was hospitalized following a week of temperatures up to 37.9°C, with mild swelling and pain over the right tibia. Pertinent medical history revealed that in 1980 the patient underwent osteosynthesis of a fracture of the tibia sustained in a motor vehicle accident. In 1995 he developed an infection due to methicillin-resistant Staphylococcus aureus, which was successfully treated by orthopedic and antibiotic management with removal of the implant material. The antibiogram showed a susceptibility to fluoroquinolones, fosfomycin, rifampin, clindamycin, and glycopeptide. The methicillin-resistant S. aureus isolate was resistant to trimethoprim-sulfamethoxazole, aminoglycosides, and erythromycin. At that time, he had presented with pain, swelling, and local tenderness, without drainage or erythema, and the radiographs revealed a healed fracture. The patient remained asymptomatic from 1995 to the present episode without a history of new trauma or bacteremia. There was no history of a draining lesion, and the bone was radiologically healed. With the most recent episode, there was again no drainage or inflammation of the overlying skin. While the radiographs were difficult to interpret due to previous fracture and osteomyelitis, there were no lytic areas, signs of acute periosteal reaction, or obvious sequestra. Magnetic resonance imaging (MRI) and computerized tomography (CT) revealed an intramedullary abscess. Surgical treatment consisted of incision and drainage, excision of inflamed material, and open bone biopsy culture, which revealed S. aureus. Except for sensitivity to methicillin, the antibiogram was the same as that for the first strain of staphylococcus isolated in 1995. It should be noted that at this institution bacterial strains from severe infections or bacteremia are often maintained at −80°C for many years. It was initially thought that the same S. aureus strain had lost its mecA gene and had therefore become methicillin sensitive. Epidemiological typing by pulsed-field gel electrophoresis (PFGE) (Pulsaphor System; Pharmacia-Biotech) of chromosomal DNA digested by restriction endonuclease SmaI (Boehringer) (9) showed a different result: the two strains of S. aureus were clearly different from each other (Fig. 1).
FIG. 1.
Comparison of Staphylococcus aureus typing (PFGE) results for the isolates obtained from the tibial osteomyelitis of patient 1 in 1995 and 2002. MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus.
Case 2.
In October 1997, a 38-year-old farmer was hospitalized with the predominant clinical symptom of nonresolving pain in the right femur. Other than a history of excessive alcohol intake, he reported no comorbidities. However, one thing that was significant in his history was a previous infection at the same site in 1979, which was caused by methicillin-sensitive S. aureus. This followed an osteosynthesis of a fracture secondary to a traffic accident. At the time of the first episode there was no draining fistula and the radiographs revealed a healed fracture. The infection had been successfully treated by orthopedic and antibiotic management, and the patient remained symptom-free until the new episode. The implant material had been removed several years previously, and the bone was considered as healed. At the time of the present episode, there was no local cellulitis or draining fistula. The radiographs did not reveal any acute changes, but imaging studies (MRI and CT) were consistent with intramedullary infection. The patient underwent irrigation and debridement, and bone biopsy culture revealed Pseudomonas aeruginosa. There had been no history of previous bacteremia, new trauma, draining lesions, or inflammation elsewhere. The origin of this gram-negative recurrence could not be determined.
Case 3.
In May 2002, a 57-year-old healthy, immunocompetent man was hospitalized with a 1-week history of pain in the leg without trauma. His history was remarkable for a first infection at the same site caused by methicillin-sensitive S. aureus after an osteosynthesis of a fracture secondary to a traffic accident in 1971. At the time of that first infection, radiographs revealed a healed fracture. There were no sites of drainage. After successful treatment and presumed bone recovery, he remained symptom free until the present episode. At the time of presentation, there was swelling and local tenderness but no inflammation of the overlying skin or draining fistula. There was no history of disease suggesting a transient bacteremia, and the patient had no signs of inflammation at any other site. The implant material had been removed many years before. Radiographs revealed a healed fracture with no clear signs of acute infection. Imaging studies (MRI and CT) did reveal intramedullary infection. Treatment was by incision, drainage, and debridement of inflamed tissue. Open bone culture grew Enterobacter cloacae.
Each bacterial isolate was routinely monitored for its overall antimicrobial susceptibility by disk diffusion (Sirscan) according to the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards). Epidemiological typing of sequential S. aureus isolates was performed by PFGE of chromosomal DNA digested with SmaI (Bio-Rad) using a CHEF MAPPER system (Bio-Rad, Hercules, CA) according to established protocols (2). The banding pattern of the different gels was analyzed with the software Gel-Compar (V 4.1; Applied Math, Belgium). Interpretation of the fragment patterns was based on published criteria (1). Patterns that differed by one to six DNA fragments were considered as subtypes, and those distinguished by seven or more DNA fragments were considered as distinct types (3).
The characteristics of the three patients are summarized in Table 1.
TABLE 1.
Characteristics of the three patients and therapy
| Characteristic | Result fora:
|
||
|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | |
| Age (yr) at first episode/sex | 33/male | 20/male | 26/male |
| 1st episode (yr) | 1995 | 1979 | 1971 |
| Patient's occupation | House painter | Farmer | Company director |
| 1st bacterial isolate | MRSA | MSSA | MSSA |
| Surgery (1st episode) | Incision, drainage, debridement | Incision, drainage, debridement | Incision, drainage, debridement |
| Antibiotics (1st episode) | Rifampin, fusidic acid | Cloxacillin | Cloxacillin |
| Asymptomatic delay (yr) | 7 | 18 | 31 |
| 2nd episode (yr) | 2002 | 1997 | 2002 |
| 2nd bacterial isolate | MSSA | P. aeruginosa | E. cloacae |
| Surgery (2nd episode) | Incision, drainage, debridement | Incision, drainage, debridement | Incision, drainage, debridement |
| Antibiotics (2nd episode) | Ciprofloxacin, and rifampin for 6 wk | Ceftazidime and ciprofloxacin for 12 wk | Garamycinb beads for 3 wk; cefepime for 6 wk |
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus.
Gentamicin C complex sulfate (Schering).
Reactivation of osteomyelitis, even after a 50-year disease-free interval, has been reported in the literature (6). In daily clinical practice, these recurrences are not rare and usually occur at the prior anatomical site of infection without any history of concomitant disease, bacteremia, or new trauma. Their real incidence is not known and has been estimated at 25 to 30% (7). The responsible bacteria are almost always the same as those from the prior infection, as determined by microbiological identification and their antibiogram (4, 10).
However, the assumption that all recurrences are due to the same strain of bacteria is probably not the reality. A different bacterium responsible for a second infection at the same anatomical site would be proof positive for a true reinfection. This is demonstrated by the patients in cases 2 and 3 who had a “repetition” of their osteomyelitis, but which was caused by a reinfection with a gram-negative bacterium following an initial episode of methicillin-sensitive S. aureus infection. This was also shown by the patient in case 1, who had a second infection with a different strain of S. aureus. We initially thought that the same S. aureus strain had lost its mecA gene and had become methicillin sensitive, as has been described in the literature (3, 5, 8). Yet PFGE showed a completely different strain, which can only mean a new second infection. For even if a deletion of the mecA gene was possible for this patient, a mutation of an underlying existing colony of S. aureus passing the strain barrier to become a gram-negative rod as in the other cases is impossible.
In all three patients, there was no history of trauma, open wounds, chronic fistulas, current or past evidence of bacteremia, concomitant disease, or episode of recent illness. All patients were young, otherwise healthy, fully immunocompetent, and without any implant material in place. All previous fractures were well healed. Despite a careful history and examination, we were unable to find any explanation for the origin and incubation time of the new infection. In addition, it is not known why the reinfection occurred at exactly the same anatomic site so successfully cured many years previously. Transitory bacteremia, silent and asymptomatic, can occur for almost any reason. We suggest that bacteria such as S. aureus, with its extended adhesion capacities, could adhere to a previously altered bone area with the resultant development of a symptomatic infectious disease. Since normal bone is highly resistant to infection (6), reinfection would occur primarily at formerly compromised sites. Previously infected bone must be considered a lifetime focus of diminished resistance, and thus osteomyelitis should be considered a risk factor for a second episode at the same site due to pathologically altered bone surfaces. This theory strongly resembles the established facts about altered valvular surfaces and infectious endocarditis. One cannot exclude, however, that during surgery another bacterium was introduced that remained dormant for many years.
From a clinician's point of view, it is only important to have the antibiogram in order to plan for appropriate treatment. However, from a scientific point of view, it would be interesting to determine the approximate ratio of new infection versus a true reactivation of osteomyelitis, particularly in S. aureus infections. Such a question could be easily answered by conducting a prospective study using PFGE in a large number of patients.
Acknowledgments
None of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article. No funds were received in support of this study. No potential conflict of interest exists.
REFERENCES
- 1.Blanc, D. S., D. Pittet, C. Ruef, A. F. Widmer, K. Muhlemann, C. Petignat, S. Harbarth, R. Auckenthaler, J. Bille, R. Frei, R. Zbinden, P. Moreillon, P. Sudre, and P. Francioli. 2002. Molecular epidemiology of predominant clones and sporadic strains of methicillin resistant Staphylococcus aureus in Switzerland and comparison with European epidemic clones. Clin. Microbiol. Infect. 8:419-426. [DOI] [PubMed] [Google Scholar]
- 2.Blanc, D. S., M. J. Struelens, A. Deplano, R. De Ryck, P. M. Hauser, C. Petignat, and P. Francioli. 2001. Epidemiological validation of pulsed-field gel electrophoresis patterns for methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 39:3442-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deplano, A., P. T. Tassios, Y. Glupczynski, E. Godfroid, and M. J. Struelens. 2000. In vivo deletion of the methicillin resistance mec region from the chromosome of Staphylococcus aureus strains. J. Antimicrob. Chemother. 46:617-620. [DOI] [PubMed] [Google Scholar]
- 4.Donati, L., P. Quadri, and M. Reiner. 1999. Reactivation of osteomyelitis caused by Staphylococcus aureus after 50 years. J. Am. Geriatr. Soc. 47:1035-1037. [DOI] [PubMed] [Google Scholar]
- 5.Donnio, P.-Y., L. Louvet, L. Preney, D. Nicolas, J.-L. Avril, and L. Desbordes. 2002. Nine-year surveillance of methicillin-resistant Staphylococcus aureus in a hospital suggests instability of mecA DNA region in an epidemic strain. J. Clin. Microbiol. 40:1048-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lew, D. P., and F. A. Waldvogel. 1997. Osteomyelitis. N. Engl. J. Med. 336:999-1007. [DOI] [PubMed] [Google Scholar]
- 7.Mader, J., J. Calhoun, and L. Lazzarini. 2003. Adult long bone osteomyelitis, p. 173. In J. Calhoun and J. Mader (ed.), Musculoskeletal infections. Marcel Dekker, New York, N.Y.
- 8.Wagenvoort, J. H., H. M. Toenbreker, M. E. Heck, W. J. van Leeuwen, and W. J. Wannet. 2000. Hospital outbreak of methicillin-resistant Staphylococcus aureus followed by an in vivo change to a mecA-negative mutant with loss of epidemicity. Eur. J. Clin. Microbiol. Infect. Dis. 19:976-977. [DOI] [PubMed] [Google Scholar]
- 9.Wei, M. Q., F. U. Wang, and W. B. Grubb. 1992. Use of contour-clamped homogeneous electric field (CHEF) electrophoresis to type methicillin-resistant Staphylococcus aureus. J. Med. Microbiol. 36:172-176. [DOI] [PubMed] [Google Scholar]
- 10.Widmer, A., G. E. Barraud, and W. Zimmerli. 1988. Reactivation of Staphylococcus aureus osteomyelitis after 49 years. Schweiz. Med. Wochenschr. 118:23-26. [PubMed] [Google Scholar]