Abstract
African swine fever (ASF) is an infectious and economically important disease of domestic pigs. The absence of vaccine renders the diagnostic test the only tool that can be used for the control of new outbreaks of the disease. At present, the enzyme-linked immunosorbent assay (ELISA) test is the most useful method for large-scale ASF serological studies, although false positives have been detected, mainly on poorly preserved sera. In order to improve the current diagnostic test available for ASF, we have studied the antigenic properties of the ASF virus polyprotein pp62 and its suitability for use in a novel ELISA. Two well-known antigenic proteins of ASF virus, p32 and p54, were also included in this study. These proteins were expressed in the baculovirus expression system and used as antigens in ASF serological tests. Our results indicate that the use of these recombinant proteins as antigens in the ELISAs improves the sensitivity and specificity obtained with the conventional diagnosis test used to detect antibodies against ASF virus. Furthermore, the use of polyprotein pp62 in an ELISA for testing poorly preserved sera allows performance of the diagnosis of ASF without the need to confirm the results by the immunoblot test. These features make pp62 one of the most interesting viral proteins to be used for serological ASF diagnosis.
African swine fever (ASF) was first reported in 1921 in Kenya as a highly contagious swine disease that caused extensive mortality (17). The disease was epidemic in many European and African countries in the 1950s and 1990s and caused heavy losses in the swine industry. Currently ASF is prevalent in Italy (Sardinia) and many sub-Saharan African countries, and it remains one of the most serious viral diseases threatening the swine industry.
The causative agent of ASF is an icosahedral cytoplasmic deoxyvirus that has been assigned to a new family, Asfarviridae, as a member in the genus Asfivirus (10). The genome of ASF virus (ASFV) is a linear double-stranded DNA molecule ranging in size from 170 to 190 kbp; about 150 open reading frames (ORFs) in ASFV have been identified (29). The extracellular virions contain more than 50 proteins, with molecular masses ranging from 9.5 to 150 kDa, including the enzymatic machinery required for synthesis and processing of early mRNA (1, 3, 8, 12, 23, 25, 26).
An essential feature of ASFV infection is the lack of fully neutralizing antibodies, which has hampered the development of an effective vaccine to control the disease, although in vitro virus inhibition without complete neutralization has been reported (13, 14). Therefore, the lack of a vaccine makes diagnostic procedures the only methodology that can help to perform a complete eradication of the disease in affected countries. The PCR is an important diagnostic tool for ASFV, particularly when the viral isolates causing the disease are highly virulent and kill pigs before an antibody response is mounted. However, due to the presence of strains of reduced virulence that result in a low mortality (7, 15), the ASF disease is diagnosed mainly by detection of specific antibodies. Thus, it is important to study those viral components potentially able to induce humoral immune responses and therefore suitable for use as diagnosis reagents.
In this context, the present study was undertaken to investigate the antigenic properties of the polyprotein pp62 encoded by the ORF CP530R. This polyprotein was identified as a late protein which, after proteolytic processing, produces two major structural proteins, p35 and p15 (27).
We describe the expression of the polyprotein pp62 in the baculovirus expression system and its use for ASFV diagnosis, in the enzyme-linked immunosorbent assay (ELISA) and immunoblotting (IB) serological tests. The results obtained by the analysis of sera from infected pigs were compared with the results obtained with the ELISA prescribed for international trade (18) and with the results of ELISAs using recombinant proteins (rP-ELISAs) p32 (p32-ELISA) and p54 (p54-ELISA) (p32 and p54 are two of the most antigenic ASFV proteins [2]).
MATERIALS AND METHODS
Recombinant transfer vector.
The complete sequence of the ASFV polyprotein pp62 encoded by the ORF CP530R was obtained from the pKS-CP530R plasmid (27). This recombinant plasmid was digested with NdeI, end filled with the Klenow fragment to create blunt termini, and digested with SpeI. The 1,880-bp fragment containing the complete pp62 coding sequence was isolated and inserted into the StuI/SpeI-cut plasmid pHta (FastBac system; Gibco-BRL) to generate plasmid pHTa.CP530R.
The pL29-E183L plasmid containing the complete p54-encoding gene has been previously described (24). To construct the vector pHTa.E183L, a fragment containing the complete p54 coding sequence was excised from pL29-E183L using NcoI and PstI restriction enzymes and inserted into NcoI/PstI-digested plasmid pHTa (FastBac system; Gibco-BRL).
Recombinant baculoviruses expressing proteins p54 (Bacp54) and pp62 (Bacpp62) were constructed using the corresponding pHTa vectors and following the manufacturer's instructions (Bac-to-Bac system; Gibco-BRL). The recombinant baculovirus expressing the p32 ASFV protein was previously described by Carrascosa (9).
Cells and viruses.
A Spanish strain of ASF virus isolated in 1970 (E70) and adapted to grow in a monkey stable cell line (MS) was used for antigen production, following the method of Escribano et al. (11).
Three different recombinant baculoviruses (Bacpp62, Bacp54, and Bacp32) were used to express the ASFV proteins pp62, p54 and p32, respectively. Wild-type and recombinant baculoviruses were propagated in Spodoptera frugiperda (Sf-9) or High Five (H5) insect cell lines (Invitrogen Corp.).
Immunoreagents.
Porcine field sera were collected during a series of ASF outbreaks in Spain between 1989 and 1992. A total of 678 porcine sera were analyzed. Out of 678 field sera, 253 were used to assess the suitability of ELISAs using recombinant protein p32, p54, and pp62 (pp62-ELISA) as the antigen to carry out serodiagnosis of poor quality samples. In order to do that, the 253 field sera were tested by Office International des Epizooties (OIE)-prescribed tests (conventional ELISA in which whole ASFV produced in MS cells was used as the coated antigen [MS-ELISA] and IB), kept at 37°C for a month to generate “poorly preserved” sera, and then analyzed again using OIE-prescribed tests and rP-ELISAs.
The anti-ASFV serum used as a positive reference serum was produced by oronasal inoculation of a pig with 105 50% tissue culture infective doses of the attenuated ASFV isolate E75. Negative-control serum was obtained from a blood donor pig reared at our institute (INIA).
Protein A conjugated to horseradish peroxidase (HRPO) was purchased from SIGMA.
Optimization of recombinant protein expression.
To determine the optimal conditions for the expression of ASFV recombinant proteins by baculovirus-infected cells, H5 cells were infected with recombinant baculoviruses at a multiplicity of infection of 4 and harvested at 0, 24, 48, 72, and 96 h postinfection. Harvested cells were rinsed with phosphate-buffered saline (PBS), suspended in PBS (p32, pp62, and p54 extracts) or 7 M urea (p54 extract) at 106 cells/ml, and then sonicated on ice for 30 seconds. The protein extracts obtained were quantified following the instructions of a commercial kit (protein assay; Bio-Rad) and were used as antigens in ELISA and immunoblotting assays.
MS-ELISA.
The conventional ELISA makes use of ASFV (E70 viral isolate) produced in MS cells as the coated antigen and protein A labeled to HRPO as the indicator, and it was carried out following the protocol described in the OIE Manual of Standards for Diagnostic Tests and Vaccines for Terrestrial Animals (18).
rP-ELISA.
Briefly, microtiter plates (Polysorp immunoplates; Nunc) were incubated at 4°C overnight with 50 μl/well of extracts from cultures of H5 cells infected with recombinant baculoviruses or with extracts from cultures of H5 cells infected with wild-type baculovirus, at a previously determined optimal concentration, in coating buffer (0.1 M carbonate buffer, pH 9.6). The coated plates were washed with PBS-T (PBS, pH 7.5, containing 0.05% [vol/vol] Tween 20) and used immediately or stored at −20°C until use. The plates were subsequently blocked with PBS-TM (PBS, pH 7.5, 0.05% [vol/vol] Tween 20, 3% [wt/vol] skim milk), and duplicate samples of porcine sera were tested at a 1:20 dilution in PBS-TM by incubation for 1 h at 37°C. Positive and negative reference sera were included on each plate. HRPO-labeled protein A was added diluted 1:1,500 in PBS-TM, and the plates were incubated for 1 h at 37°C. After the plates were washed, 0.2 ml of 7,12-dimethyl-1,2-benz[a]anthracene (DMBA)-3-methyl-2-benzothiazolinone hydrazone (MBTH) substrate (Sigma) was added per well. The reaction was stopped by the addition of 50 μl of 3 N H2SO4, and the optical density at 620 nm (OD620) was measured after incubation for 10 min at room temperature.
In order to standardize the assay, respective ΔODs (OD in recombinant antigen well minus OD in mock antigen well) for each serum sample tested was expressed as percent reactivity, calculated by the following formula: percent reactivity of sample = (ΔOD of the sample − ΔOD of the negative control)/(ΔOD of the positive control − ΔOD of the negative control).
IB test.
The expression of recombinant proteins p32, p54, and pp62 was analyzed on 12.5% sodium dodecyl sulfate (SDS)-polyacrylamide gels stained with Coomassie blue. After electrophoresis, proteins were electrotransferred to a nitrocellulose filter and the IB tests were performed as described previously (21), with minor modifications. The nitrocellulose filter was incubated with PBS-TM as the blocking solution for 1 h at 37°C and allowed to react for 1 h with test sera at a 1:30 dilution. The immunocomplexes were detected by using HRPO-labeled protein A and 4-chloronaphthol as the substrate.
RESULTS
Production, expression, and characterization of recombinant proteins pp62, p32, and p54.
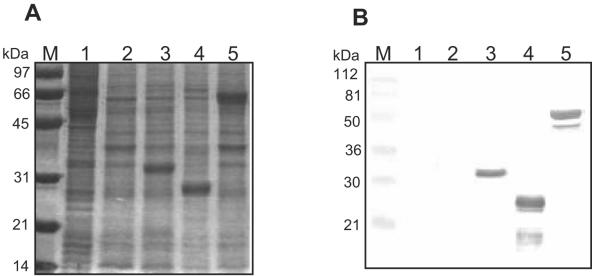
Transfer vectors pHta.CP530R and pHta.E183L were constructed as described in Materials and Methods and BacPAK-p32 was constructed as described by Carrascosa (9). The transfer vectors were used to engineer recombinant baculoviruses expressing ASFV protein pp62 (Bacpp62), p54 (Bacp54), and p32 (Bacp32), respectively. In order to confirm expression of these proteins, H5 cells infected with the recombinant viruses were harvested at 72 h postinfection and subjected to 12.5% SDS-polyacrylamide gel electrophoresis and IB test (Fig. 1). Extracts from wild-type baculovirus-infected cells and mock-infected cells served as negative controls.
FIG. 1.
Expression of ASFV recombinant proteins p32, p54, and pp62 in insect H5 cells. (A) Extracts corresponding to recombinant proteins and controls were separated by 12.5% SDS-polyacrylamide gel electrophoresis followed by Coomassie blue staining. The leftmost lane contains molecular mass markers. Lane 1, mock-infected cell extracts; lane 2, wild-type baculovirus-infected cells; lane 3, Bacp32 extracts; lane 4, Bacp54 extracts; lane 5, Bacpp62 extracts. (B) Western blot analysis of extracts shown in panel A using an ASFV-positive reference serum diluted 1:30.
As shown in Fig. 1A, the three recombinant proteins were expressed with the predicted molecular masses: 32 kDa (Bacp32), 28 kDa (Bacp54), and 62 kDa (Bacpp62a). These proteins were not detected in extracts from wild-type baculovirus-infected cells or in uninfected cells. The authenticity of each protein band was confirmed by IB analysis using an ASF reference serum sample from one experimentally infected pig. The IB shown in Fig. 1B demonstrated that the three recombinant proteins were specifically recognized by antibodies present in the positive reference serum and that no immunoreactive proteins were detected in protein extracts from wild-type baculovirus-infected or uninfected cells.
The recombinant protein p32 was recognized as a single band of 32 kDa with an electrophoretic mobility similar to the mobility of the protein expressed in ASFV-infected cells. The recombinant proteins p54 and pp62 were recognized as major bands of 25 kDa and 62 kDa, respectively, besides two minor bands of 17 kDa (p54) and 52 kDa (pp62).
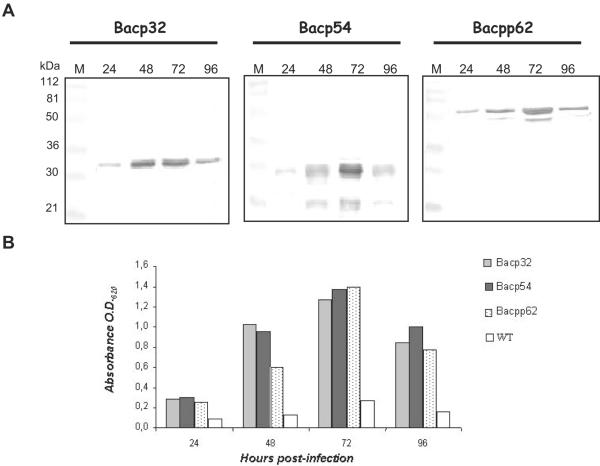
Optimization of the production of recombinant proteins pp62, p32, and p54 in baculovirus-infected insect cells was carried out by detecting the proteins by IB analysis at 24, 48, 72, and 96 h postinfection. This time course expression experiment showed that the three recombinant proteins were first detected in H5 cells harvested 24 h postinfection, while the maximum protein expression level was reached at 72 h postinfection (Fig. 2A).
FIG. 2.
Time course of p32, p54, and pp62 recombinant protein expression in insect cells. Extracts from H5 cells infected with Bacp32, Bacp54, and Bacpp62 and collected at 24, 48, 72, or 96 h postinfection, were analyzed by Western blotting (A) or ELISA (B). Wild-type (WT) baculovirus-infected cells were used as negative controls in ELISA tests. ASF-positive reference serum diluted 1:30 (Western blot) or 1:20 (ELISA) was used to reveal the ASFV proteins. Lane M contains molecular mass markers.
In ELISA tests where the plates were coated with recombinant proteins collected at 24, 48, 72, and 96 h postinfection, the absorbance values were higher with extracts from 72 h postinfection, confirming the highest level of protein expression at this time (Fig. 2B). At 96 h postinfection, the cell cultures showed severe cytopathic effect and the recombinant protein accumulation was reduced. A maximum OD620 value of 0.199 at 72 h postinfection was obtained by coating the wells with wild-type baculovirus-infected cells, therefore confirming the specificity of this analysis (Fig. 2B).
Standardization of rP-ELISA.
Once the conditions for optimal recombinant protein production in infected insect cells had been established, we determined the optimal antigen concentration to coat ELISA microtiter plates for serodiagnosis of ASF using a positive and a negative reference serum.
The absorbance values of wells tested by ELISAs with the recombinant antigens were found to be optimal when the microtiter plates were coated with 0.6 μg of antigen (p32 and p54) or 0.3 μg (pp62) per well. No reactivity (less than 0.2 OD620 unit) was detected when the positive serum was incubated with wild-type baculovirus-infected H5 cell extracts or when the negative serum was incubated with the recombinant proteins (data not shown).
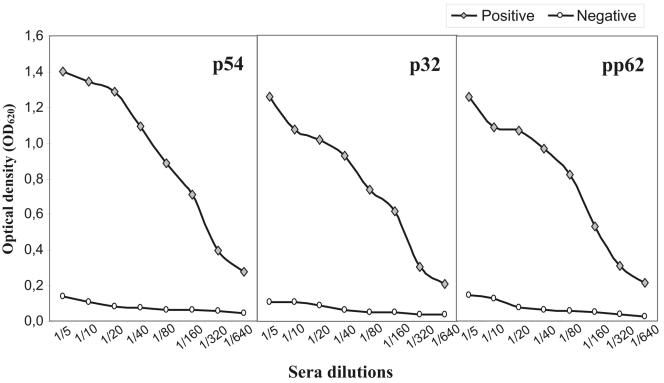
In addition, the optimal dilution of sera to be tested by the rP-ELISA test was determined. A 1:20 dilution of serum offered the best results. At this dilution, the absorbance corresponding to positive reference sera was 10 times greater than that corresponding to the negative reference serum (Fig. 3).
FIG. 3.
Titration curves of reference sera by rP-ELISAs using p54, p32, and pp62. Duplicate serum samples were tested in twofold dilutions from 1:5 to 1:640. The results are expressed as OD620 and correspond to the average value obtained in at least three different analyses.
Evaluation of the rP-ELISAs analyzing field pig sera.
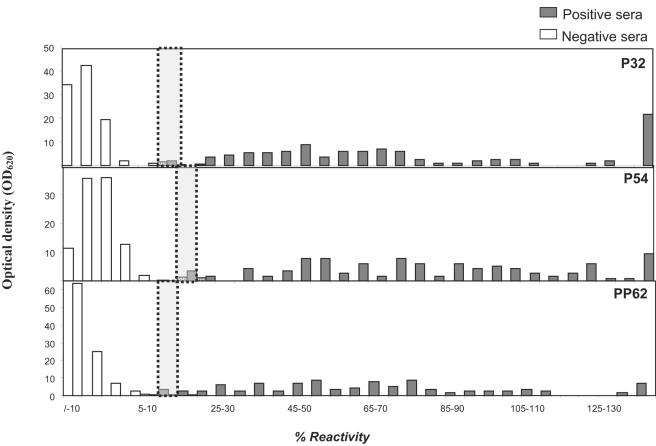
A total of 425 ASF field sera, 309 negative and 116 positive as measured by MS-ELISA and conventional IB test (using whole virus), were tested by each rP-ELISA. The results were expressed as a percentage of reactivity calculated by the formula described in Materials and Methods and represented as a frequency distribution chart (Fig. 4). Cutoff values for each recombinant ELISA were established comparing the reactivity from 309 ASFV-negative and 116 ASFV-positive field sera. The cutoffs were set at 10 to 15% for the recombinant pp62-ELISA and p32-ELISA and at 15 to 20% for the recombinant p54-ELISA. Sera scoring within the cutoff were considered doubtful. From the 424 sera analyzed, the percentages of doubtful sera were 2% by p32 and p54 ELISAs and 1.5% by pp62 ELISA. No false negatives were detected by p54-ELISA, and only one false-negative serum sample was detected by p32 and pp62 ELISAs. The numbers of false-positive sera were three, two, and one with the recombinant protein p54, p32, and pp62, respectively.
FIG. 4.
Distribution of the percentage of reactivity in 5% intervals, of field ASFV-positive and -negative swine sera. Duplicate serum samples were diluted 1:20 and analyzed by rP-ELISAs (p30, p54, and pp62). Each bar corresponds to the percentage of sera scoring within a 5% reactivity interval. The dotted lines indicate the cutoffs of the assays, while white and gray bars represent negative and positive sera, respectively, as classified by MS-ELISA and IB (OIE reference tests).
In summary, on the basis of test results on 424 sera obtained from ASF outbreaks in Spain, the sensitivity and specificity of the recombinant ELISA ranged between 96 and 99% for the three recombinant proteins.
Assessment of suitability of the new assay to test poorly preserved sera.
In order to assess the influence of the preservation state of samples on the new recombinant ELISA tests described above, 253 sera, 112 of them negative and 141 positive, were kept at 37°C for 1 month, and tested by conventional and recombinant ELISAs (Table 1). By using the conventional ELISA, 9% of the sera were doubtful. However, in ELISAs using the recombinant proteins, this percentage was reduced to 3% and 2% using protein p54 and p32, respectively. In the case of pp62, this percentage was 0%. Therefore, the sensitivity and specificity of this assay with the recombinant protein pp62 to test poorly preserved sera were 100%. Likewise, these results revealed that the p32- and p54-ELISAs also provide more sensitivity and specificity than the conventional ELISA (Table 1).
TABLE 1.
Sensitivity and specificity of rP-ELISAs (p32, p54, and pp62) compared to conventional ELISA (MS-ELISA) for analysis of poorly preserved seraa
| Test | Sensitivity (%) | Specificity (%) |
|---|---|---|
| p32-ELISA | 98.5 | 96.4 |
| p54-ELISA | 97.1 | 93.7 |
| pp62-ELISA | 100.0 | 100.0 |
| MS-ELISA | 80.8 | 92.8 |
Data were obtained by the analysis of 253 porcine field sera and using the following formulas: sensitivity = 100 × (number of positive sera in the test/total number of positive sera in the reference test [MS-ELISA plus IB]); specificity = 100 × (number of negative sera in the test/total number of negative sera in the reference test [MS-ELISA plus IB]). In order to make these calculations, all the doubtful sera (those in the cutoff interval) were considered positive.
DISCUSSION
In this study we have analyzed the antigenicity of the ASFV recombinant protein pp62 expressed in insect cells and assessed the use of this antigen as a diagnostic tool in new assays (ELISA and IB) for ASF serological diagnosis. Furthermore, the antigenicity and diagnostic potential of pp62 have been compared to viral proteins p32 and p54, also expressed in the baculovirus expression system and previously described by Alcaraz et al. (4) and Oviedo et al. (19) as antigenic proteins.
The expression of the three recombinant ASFV proteins studied (pp62, p32, and p54) in H5 insect cells started at 24 h postinfection, but the peak of maximal accumulation was reached at 72 h postinfection and the amounts accumulated were similar for all three proteins. The recombinant protein pp62 was expressed as a major band of 62 kDa, which correlates with the band observed in ASFV-infected cells, and a minor band of 52 kDa. Different authors have reported that the presence of an Asn amino acid within the amino end of the protein might provoke the processing of a sequence of 8 kDa from the precursor pp62, generating a fragment of 52 kDa (5, 27, 28). However, as the ASFV protease is not expressed in our extracts, a degradation of the recombinant protein is the most likely reason for the presence of the 52-kDa band observed in the production of recombinant pp62. The recombinant protein p54 was expressed as a major band of 28 kDa and a minor band of 17 kDa, while the recombinant protein p32 appeared as a unique band of 32 kDa, as reported previously by different authors (16, 22).
The three recombinant proteins were used as antigens in ELISA and IB tests, assaying first sera from pigs experimentally infected with ASFV. The positive sera similarly recognized the three recombinant proteins by IB, but not by ELISA in which the reactivity against proteins pp62 and p32 was higher than that observed for protein p54. Oviedo et al. (19) previously described a similar difference in the reactivity against p32 and p54 and concluded that the p54 protein was not suitable for use as an antigen in ELISA tests. However, in the study shown here, we have established that when the p54 protein was solubilized in 7 M urea, the reactivity of the positive sera against this protein was similar to that observed against pp62 and p32, and therefore in this solution the recombinant protein p54 is suitable for use in an ELISA. These results could imply that, in the course of ASFV infection, antibodies recognizing linear epitopes against protein p54 are induced and that they may not be accessible when the protein adopts its native conformation.
Analysis of different serum dilutions using the optimal concentration of recombinant proteins shows that the antibody titer against protein pp62 is greater than those against proteins p32 and p54. This could be mainly due to the presence of more epitopes exposed on the pp62 protein than on the p32 and p54 proteins or could be due to the epitopes exposed on pp62 being more antigenic than those on p32 and p54. Validation of ELISAs using the recombinant proteins was carried out by testing 425 field sera. The results obtained indicate that the use of the recombinant protein pp62 as an antigen in ELISA tests renders an specificity of 99%, while the use of proteins p32 and p54 shifts this value to 97%. The sensitivity of ELISA was around 97% with the three proteins.
As none of the sera analyzed was doubtful or false positive or negative at the same time by the three ELISAs, a possible serodiagnosis strategy could be the analysis of sera by ELISA against the three recombinant proteins and successive confirmation of doubtful sera by IB using the recombinant proteins p32 and p54.
One of the disadvantages of using the conventional ELISA (with semipurified virions as the antigen) is the lack of reliability for analysis of poorly preserved samples. The main consequence of this fact is the increase of false-positive results, making the confirmation of the diagnostic results by IB essential (2, 6, 20). In order to evaluate the ability of the new ELISAs to analyze properly samples of poor quality, 253 field sera were tested, before and after incubation for 1 month at 37°C, using the prescribed test and recombinant assays. The results obtained showed that the recombinant ELISAs have higher sensitivity and specificity than the conventional ELISA. Among the recombinant proteins, the best results were obtained using the pp62 protein, reaching a sensitivity and specificity of 100% for sera analyzed with this protein. A possible explanation for the improvement of poorly preserved serum diagnosis with the pp62-ELISA could be that the antibodies against this protein are more stable or display higher affinity than others, which could allow them to react even after the thermal treatment.
In summary, the results shown here allowed us to develop a new serodiagnosis assay for ASF in which recombinant proteins pp62, p32, and p54 were used, improving its specificity, sensitivity, and reliability mainly for the analysis of poorly preserved field sera. Furthermore, the use of the recombinant proteins has additional advantages. (i) It eliminates the use of infectious ASFV in antigen production. (ii) It makes interpretation of the results easier. (iii) It improves the reproducibility of the assay, since it is easier to standardize the antigen protein. In addition, our results demonstrate for the first time the antigenicity of the pp62 polyprotein against sera from infected pigs. Further studies are under way to determine which of the proteins from the pp62 polyprotein is responsible for the antigenicity observed and to analyze the kinetics of the antibody response against these proteins in pigs infected with ASFV. Another relevant aspect is to evaluate the rP-ELISAs with sera from pigs infected with diverse ASFV isolates. Given that the variability of ASFV isolates in Africa is higher than that detected in Europe, it would be important to use serum samples from African countries in order to adequately validate the ELISAs. Work is in progress to address this issue by using serum samples collected from areas affected by recent ASFV outbreaks in countries from East and West Africa.
Acknowledgments
We thank E. Martin for her valuable technical assistance.
This work has been supported by EU grant QLK2-CT2001-02216 and by grant AGL 2004-07857 from the Ministerio de Educación y Ciencia of Spain.
REFERENCES
- 1.Alcaraz, C., B. Pasamontes, F. Ruiz Gonzalvo, and J. M. Escribano. 1989. African swine fever virus-induced proteins on the plasma membranes of infected cells. Virology 168:406-408. [DOI] [PubMed] [Google Scholar]
- 2.Alcaraz, C., M. De Diego, M. J. Pastor, and J. Escribano. 1990. Comparison of a radioimmunoprecipitation assay to immunoblotting and ELISA for detection of antibody to African swine fever virus. J Vet. Diagn. Investig. 2:191-196. [DOI] [PubMed] [Google Scholar]
- 3.Alcaraz, C., A. Alvarez, and J. Escribano. 1992. Flow cytometric analysis of African swine fever virus-induced plasma membrane proteins and their humoral immune response in infected pigs. Virology 189:266-273. [DOI] [PubMed] [Google Scholar]
- 4.Alcaraz, C., F. Rodriguez, J. M. Oviedo, A. Eiras, M. De Diego, C. Alonso, and J. M. Escribano. 1995. Highly specific confirmatory Western blot test of African swine fever virus antibody detection using the recombinant virus protein p54. J. Virol. Methods 52:111-119. [DOI] [PubMed] [Google Scholar]
- 5.Andres, G., A. Alejo, J. Salas, and M. L. Salas. 2002. African swine fever virus polyproteins pp220 and pp62 assemble into the core shell. J. Virol. 76:12473-12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias, M., J. M. Escribano, and J. M. Sanchez-Vizcaino. 1993. Persistence of African swine fever antibody reactivity on ELISA and immunoblotting assays. Vet. Rec. 133:189-190. [DOI] [PubMed] [Google Scholar]
- 7.Bech-Nielsen, S., M. L. Arias, J. Panadero, J. M. Escribano, C. Gomez Tejedor, Q. Perez-Bonilla, and J. M. Sánchez Vizcaíno. 1993. Laboratory diagnosis and disease occurrence in the current African swine fever eradication program in Spain 1989-1991. Prevent. Vet. Med. 17:225-234. [Google Scholar]
- 8.Carrascosa, A. L., M. del Val, J. F. Santarén, and E. Viñuela. 1985. Purification and properties of African swine fever virus. J. Virol. 54:337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrascosa, A. L. 1994. Enhancement of baculovirus plaque assay in insect cell monolayers by DEAE-dextran. BioTechniques 16:1078-1081, 1083-1085. [PubMed] [Google Scholar]
- 10.Dixon, L. K., J. V. Costa, J. M. Escribano, D. L. Rock, E. Vinuela, and P. J. Wilkinson. 2000. Asfarviridae, p. 159-165. In M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 11.Escribano, J. M., M. J. Pastor, and J. M. Sánchez Vizcaíno. 1989. Antibodies to bovine serum albumin in swine sera: implication for false-positive reactions in the serodiagnosis of African swine fever. Am. J. Vet. Res. 50:1118-1122. [PubMed] [Google Scholar]
- 12.Esteves, A., M. I. Marques, and J. V. Costa. 1986. Two-dimensional analysis of African swine fever virus proteins and proteins induced in infected cells. Virology 152:192-206. [DOI] [PubMed] [Google Scholar]
- 13.Gómez-Puertas, P., F. Rodríguez, J. M. Oviedo, F. Ramiro-Ibanez, F. Ruiz Gonzalvo, C. Alonso, and J. M. Escribano. 1996. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 70:5689-5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gómez-Puertas, P., F. Rodríguez, J. M. Oviedo, A. Brun, C. Alonso, and J. M. Escribano. 1998. The African swine fever virus protein p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody mediated protective immune response. Virology 243:461-471. [DOI] [PubMed] [Google Scholar]
- 15.Hess, W. R. 1971. African swine fever virus. Virol. Monogr. 9:1-3. [DOI] [PubMed] [Google Scholar]
- 16.López-Otín, C., C. Simón-Mateo, L. Martinez, and E. Viñuela. 1989. Gly-Gly-X, a novel consensus sequence for the proteolytic processing of viral and cellular proteins. J. Biol. Chem. 264:9107-9110. [PubMed] [Google Scholar]
- 17.Montgomery, R. E. 1921. On a form of swine fever occurring in British East Africa (Kenya colony). J. Comp. Pathol. 34:159-191. [Google Scholar]
- 18.Office International des Epizooties. 2004. Manual of standards for diagnostic tests and vaccines for terrestrial animals, 5th ed. Office International des Epizooties, Paris, France.
- 19.Oviedo, J. M., F. Rodriguez, P. Gomez-Puertas, A. Brun, N. Gomez, C. Alonso, and J. M. Escribano. 1997. High level expression of the major antigenic African swine fever virus proteins p54 and p30 in baculovirus and their potential use as diagnosis reagents. J. Virol. Methods 64:27-35. [DOI] [PubMed] [Google Scholar]
- 20.Pastor, M. J., M. D. Laviada, J. M. Sanchez-Vizcaino, and J. M. Escribano. 1989. Detection of African swine fever virus antibodies by immunoblotting assay. Can. J. Vet. Res. 53:105-107. [PMC free article] [PubMed] [Google Scholar]
- 21.Pastor, M. J., M. Arias, C. Alcaraz, M. De Diego, and J. M. Escribano. 1992. A sensitive dot immunobinding assay for serodiagnosis of African swine fever virus with application in field conditions. J. Vet. Diagn. Investig. 4:254-257. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez, F., C. Alcaraz, A. Eiras, R. J. Yánez, J. M. Rodríguez, C. Alonso, J. F. Rodríguez, and J. M. Escribano. 1994. Characterization and molecular basis of heterogeneity of the African swine fever virus envelope protein p54. J. Virol. 68:7244-7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez, J. M., M. L. Salas, and J. F. Santaren. 2001. African swine fever virus-induced polypeptides in porcine alveolar macrophages and in Vero cells: two-dimensional gel analysis. Proteomics 1:1447-1456. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez, J. M., R. García-Escudero, M. L. Salas, and G. Andrés. 2004. African swine fever virus structural protein p54 is essential for the recruitment of envelope precursors to assembly sites. J. Virol. 78:4299-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salas, M. L., J. Kuznar, and E. Vinuela. 1981. Polyadenylation, methylation, and capping of the RNA synthesized in vitro by African swine fever virus. Virology 113:484-491. [DOI] [PubMed] [Google Scholar]
- 26.Santarén, J. F., and E. Viñuela. 1986. African swine fever virus-induced polypeptides in Vero cells. Virus Res. 5:391-405. [DOI] [PubMed] [Google Scholar]
- 27.Simón-Mateo, C., G. Andrés, F. Almazán, and E. Viñuela. 1997. Proteolytic processing in African swine fever virus: evidence for a new structural polyprotein, pp62. J. Virol. 71:5799-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varshavsky, A. 1992. The end rule of protein turnover. Cell 69:725-735. [DOI] [PubMed] [Google Scholar]
- 29.Yáñez, R. J., J. M. Rodríguez, M. L. Nogal, L. Yuste, C. Enríquez, J. F. Rodriguez, and E. Viñuela. 1995. Analysis of the complete nucleotide sequence of African swine fever virus. Virology 208:249-278. [DOI] [PubMed] [Google Scholar]