Abstract
Mutations in several subgenomic regions have been implicated in influencing response to interferon therapy; however, a comprehensive picture of Indian patients was lacking. Based on the viral load and clinical factors, 10 out of 15 patients were found to be complete responders, whereas 5 patients were nonresponders. The pretreatment viral RNA load of the patients was found to be between 5.20 and 6.13 log10 IU/ml, which eventually fell to 2.77 log10 IU/ml after 24 weeks of treatment, whereas in the case of nonresponders, the average was 5.38 log10 IU/ml. In order to study the influence of the hepatitis C virus genotype on the response to interferon therapy, the 5′ untranslated region sequences of the samples were analyzed, which showed that genotype 3 patients responded better than genotype 1 patients. Additionally, the mutations in the interferon sensitivity-determining region (ISDR) of the NS5A protein and the double-stranded RNA-activated protein kinase-eukaryotic initiation factor 2 alpha phosphorylation homology domain (PePHD) of the E2 envelope protein, before and after treatment, were compared with nonresponder prototype J. Although, no clear correlation was found in the case of the mutated ISDR, some significant changes in residues were observed in the PePHD region, which could be helpful in understanding the molecular basis of resistance to therapy. Interestingly, analysis of the quasispecies variations showed a change in genotype in one sample during treatment, which might have contributed to the resistance. The results suggest that the mutations in different regions of the viral genome might have a concerted effect on the response to interferon therapy.
Hepatitis C virus (HCV), a member of the family Flaviviridae, is the primary causative agent of parenterally transmitted non-A, non-B hepatitis and affects a significant part of the population worldwide (4). Together with hepatitis B virus, it is the major cause of severe liver disorders, like chronic hepatitis and hepatocellular carcinoma. The infecting genotype, the quasispecies variations, and the viral load have all been suggested to correlate with the disease severity, degree of liver damage, and response to interferon treatment (9).
HCV is an enveloped virus with a 9,500-nucleotide-long single-stranded positive RNA genome encoding a single polyprotein, which is processed by the host cell and virus-encoded proteases into three major structural proteins and several nonstructural proteins necessary for viral replication (6, 22). A comparison of genome and polyprotein sequences of HCV isolates worldwide led to the identification of six major genotypes, each with several subtypes (19). Genotypes 1, 2, and 3 are the major types observed in Japan, Western Europe, and North America; type 4 has been found in Central and Northern Africa and in the Middle East; type 5 has been described in South Africa, and type 6 in Southeast Asia (15).
Several studies have reported the presence of various HCV genotypes in India, and genotype 3 has been shown to be prevalent in most parts of the country. Earlier, HCV types 1a, 1b, 2a, 3a, 3b, and 3g were reported in the northern regions of India (16, 20), whereas genotype 1 was shown to be prevalent in southern India (24). Recent studies also suggest a similar pattern in India, with type 3 being the predominant genotype, followed by type 1 (13, 20, 5), which was further confirmed by our group (1).
Like other RNA viruses, HCV is genetically heterogeneous due to a lack of proofreading activity of RNA-dependent RNA polymerase, which results in the accumulation of nonlethal mutations during viral replication, leading to quasispecies populations that are transmitted to progeny viruses. This high genetic complexity of quasispecies populations, as well as the sequence diversity of particular regions of the viral genome, is believed to be associated with better adaptation to environmental conditions (21).
The sustained virological response (SVR) with alpha interferon (IFN-α) monotherapy in patients infected with HCV genotype 1 was found to be poor. However, the use of pegylated interferon was found to be associated with a significantly higher SVR (7). The current strategy of combination therapy using pegylated interferon plus ribavirin (a nucleoside analog) has been found to have a much higher sustained virological response (12). Response to therapy varies according to the viral genotype, as well as potential host and treatment efficacy factors. It has been shown in India that patients infected with genotypes 2 and 3 achieve a sustained virological response of up to 95% to a combination of daily IFN-α2b and ribavirin (10). Similarly, patients worldwide infected with genotype 2 or 3 achieve a sustained virological response of up to 72%, whereas those infected with genotype 1 show relatively low response rates (13%) after 24 weeks of treatment (25, 26). Though the mechanism of resistance for interferon treatment in infected patients is not thoroughly understood, it is believed that both host and viral factors, including several viral genomic regions, are essential for an effective response to interferon therapy (17).
Hepatitis C virus nonstructural protein 5A (NS5A) has been shown to bind to double-stranded RNA-dependent protein kinase (PKR) and repress its function (8). Interestingly, the double-stranded RNA-activated protein kinase-eukaryotic initiation factor 2 alpha (PKR-eIF2α) phosphorylation homology domain (PePHD) of the structural E2 gene has been shown to interact in vitro with the interferon-inducible cellular PKR (23). The 12-amino-acid domain of PePHD acts as a pseudosubstrate for PKR and thus interferes with the inhibitory effect of PKR on protein synthesis. Mutations in these regions are believed to influence the response to interferon therapy; however, the results of different studies are conflicting and not sufficiently clear. Also, a majority of these studies have been of HCV type 1, which is the prevalent genotype worldwide. In India, although genotype 3 has been shown to be the most prevalent strain, genotype 1 is frequently observed in HCV-infected patients in the south. In order to study the comparative responses to interferon therapy between the patients infected with the two genotypes, we have analyzed mutations in specific subgenomic regions and compiled the results with the respective viral load and clinical response to interferon therapy to get a detailed picture of the possible correlation (if any). For this purpose, mutations within the interferon sensitivity-determining region (ISDR) and the PePHD region were extensively analyzed in samples from 15 infected patients. The changes in the amino acids in these regions during therapy have been evaluated, along with the other reported studies, to understand their relevance as a function of response.
MATERIALS AND METHODS
Detection of HCV in patient serum.
Serum samples (10 ml) were collected, using Vacutainers, from HCV-infected patients from the Center for Liver Research and Diagnostics, Hyderabad, India, and were analyzed immediately for the presence of HCV using a third-generation enzyme immunoassay (EIA 3.0; Abbott, Chicago, IL) and reconfirmed using reverse transcription-PCR. These patients were treated by injecting pegylated IFN-α2a (1.5 μg/kg of body weight) with ribavarin (800 to 1,000 mg/day) subcutaneously for a period of 24 weeks. Serum alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) levels were measured before and after the interferon treatment.
Amplification of subgenomic-region sequences.
Isolated HCV RNA from serum was reverse transcribed by using Moloney murine leukemia virus reverse transcriptase at 42°C for 60 min, and the cDNA obtained was PCR amplified. The specific primers used to amplify the subgenomic region were as follows: 5′ untranslated region (UTR), sense (5′GCCAGCCCCCATTGGGG-3′) and antisense (5′GTTACGTTTGGTTTTTCT); PePHD, sense (5′TGCTGCATGCAACTGGA) and antisense (5′TGCACGTCCACGATGTTC); and ISDR, sense (5′AGCCAGCTGTCTGCGCC) and antisense (3′TCGAAAGAGTCCAG). PCR was carried out for 36 cycles using Taq polymerase; the annealing temperature used was 54°C, and the extension temperature used was 72°C. The PCR-amplified products were purified and cloned into the pGEM-T easy vector (Promega). Single recombinant plasmids were sequenced by Macrogen Inc., South Korea.
Measurement of HCV viral load.
The HCV viral load was assessed using a commercially available kit, a second-generation hepatitis C virus quantitative assay (COBAS AMPLICOR HCV Monitor Test, version 2.0) from Roche Diagnostic Systems. The detection limit of this kit was 600 IU/ml.
Analysis of sequences.
The 5′ UTR sequences of hepatitis C virus obtained from the samples were aligned with reported HCV genotypes using CLUSTAL W version 1.82. Briefly, the sequences in FASTA format were pasted in the submission form (available at http://www.ebi.ac.uk/clustalw/), and the output obtained was represented by a phylogenetic tree. The NCBI accession numbers of the representative HCV genotypes used for comparative analysis were as follows: 1a, M62321; 1b, D11355; 1c, D14853; 2a, D00944; 2b, D10988; 3a, D28917; 3b, D49374; 4a, Y11604; 5a, Y13184; and 6a, Y12083.
Statistical analysis.
The values representing the enzymatic activities of ALT, AST, and ALP, obtained from the patients before and after interferon treatment, were statistically analyzed using the Student t test. All tests of significance were two tailed, with P values of less than 0.05, which was considered to be statistically significant.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported here have been submitted to the GenBank nucleotide sequence database with accession numbers from DQ140282 to DQ140341 as follows: 5′ UTR before treatment (BT), samples 1 to 14, DQ140282 to DQ140295, and sample 15, DQ140301; 5′ UTR after treatment (AT), samples 16 to 20, DQ140296 to DQ140300; PePHD BT, samples 1 to 15, DQ140302 to DQ140315, and sample 10, DQ140321; PePHD AT, samples 16 to 20, DQ140316 to DQ140320; ISDR BT, samples 1 to 15, DQ140322 to DQ140336; and ISDR AT, samples 16 to 20, DQ140337 to DQ140341.
RESULTS
Levels of viremia in patients undergoing treatment.
In order to have comprehensive information about the molecular basis of the responses to interferon therapy during HCV infection in the Indian population, a random group of 15 patients were selected for our study. The group consisted of 14 males and 1 female. Different patients had different routes of infection, through blood transfusion, surgery, and unhygienic habits (Table 1).
TABLE 1.
Clinical profile of patients
| Parameter | Value |
|---|---|
| No. of patients | 15 |
| Age (yr) | 43.86 ± 15.04 |
| Sex (no. of patients/total) | |
| Female | 1/15 |
| Male | 14/15 |
| Route of infection (no. of patients) | |
| Accidental needle prick | 1 |
| Unhygienic habits | 2 |
| Blood transfusion | 3 |
| Surgery | 3 |
| Unsterilized needle use | 2 |
| Unknown | 4 |
| Mode of detection (no. of patients/total) | |
| ELISAa | 15/15 |
| PCR | 15/15 |
ELISA, enzyme-linked immunosorbent assay.
The response to interferon-based therapy was primarily monitored by measuring the viral loads at the beginning and during the course of treatment. Viral RNAs were isolated from the infected serum and were quantitated with the help of a commercially available quantitation kit, the COBAS AMPLICOR HCV Monitor Test version 2.0. The viral loads in the selected patient group were found to be between 5.20 and 6.13 log10 IU/ml at the beginning of interferon treatment (Table 2).
TABLE 2.
Clinical and virological characteristics of patients before and after therapy
| Sample | Genotype | Viral load (IU/ml)
|
ALT level (U/liter)
|
AST level (U/liter)
|
ALP level (U/liter)
|
Response | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | BT | AT | BT | AT | |||
| 1 | 3b | 1,231,000 | BDL | 52 | 28 | 59 | 19 | 288 | 220 | Fast |
| 2 | 3b | 162,000 | 293,000 | 76 | 63 | 84 | 72 | 320 | 280 | None |
| 3 | 1a | 391,000 | 171,000 | 61 | 42 | 67 | 47 | 295 | 228 | None |
| 4 | 1b | 693,000 | 231,000 | 121 | 29 | 117 | 33 | 228 | 234 | None |
| 5 | 3b | 282,000 | BDL | 44 | 21 | 51 | 18 | 292 | 198 | Fast |
| 6 | 3b | 782,000 | BDL | 94 | 32 | 105 | 37 | 388 | 370 | Fast |
| 7 | 1a | 687,000 | 24,100 | 125 | 31 | 200 | 29 | 299 | 196 | None |
| 8 | 3b | 341,000 | 9,000 | 36 | 31 | 46 | 37 | 299 | 480 | Slow |
| 9 | 1a | 471,000 | BDL | 18 | 18 | 26 | 26 | 235 | 197 | Slow |
| 10 | 1a | 341,000 | BDL | 63 | 31 | 71 | 35 | 276 | 192 | Slow |
| 11 | 1b | 706,000 | 313,000 | 63 | 21 | 58 | 26 | 310 | 298 | None |
| 12 | 3b | 451,000 | BDL | 54 | 17 | 51 | 21 | 223 | 210 | Slow |
| 13 | 1b | 373,000 | BDL | 81 | 05 | 93 | 07 | 403 | 220 | Slow |
| 14 | 1a | 1,355,000 | BDL | 121 | 32 | 108 | 35 | 191 | 286 | Slow |
| 15 | 1a | 231,000 | BDL | 31 | 32 | 28 | 35 | 281 | 295 | Fast |
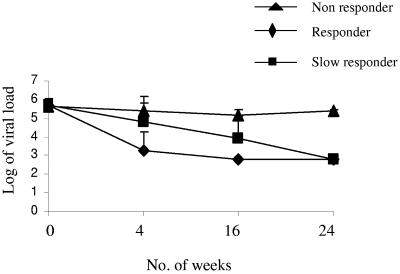
An SVR was observed in 10/15 patients after 24 weeks of treatment in which the viral load fell below a detectable level (the lower detection limit of the kit is 600 IU/ml; 2.77 log10 IU/ml) (Fig. 1). Although in some patients (4/10) the response was faster and the viral load fell below the detectable limit (BDL) within 4 weeks of treatment, in other cases (6/10) the response was relatively slow and the viral RNA was detectable up to 16 weeks of treatment (Fig. 1). However, in the case of nonresponders, HCV RNA was detectable throughout treatment, while after treatment the virus load was found to be only marginally reduced from 5.67 to 5.38 log10 IU/ml (Fig. 1).
FIG.1.
Virological status during treatment. Viral RNAs were estimated at the beginning and at different times (4, 16, and 24 weeks) during the course of treatment and plotted as log10 values against the time (in weeks) for responders, slow responders, and nonresponders.
Estimation of the ALT enzyme level before treatment showed considerably higher values compared to a healthy control (5 to 40 U/liter). The mean value in responders was estimated to be 59.4 ± 31.37 U/liter at the beginning of treatment, which eventually fell after treatment to 24.7 ± 9.16 U/liter, which is within the normal range. Similarly, in nonresponders, the mean value was found to be 89.2 ± 31.2 U/liter before treatment, which was reduced to 37.2 ± 16.25 U/liter after treatment. However, an abnormally high ALT level was observed with the genotype 1 infection (samples 3, 4, 7, 10, 11, 13, and 14) compared to samples of other genotypes.
Similarly, estimation of the AST level showed the mean value for the responders to be 63.8 ± 9.6 U/liter, and that for nonresponders was 105 ± 57.58 U/liter before treatment. However, in both cases, the enzyme levels were reduced to a normal level after treatment: responders showed 27 ± 10.4 U/liter, and for nonresponders it was 41.4 ± 18.9 U/liter. An abnormally high AST level (normal value, 5 to 40 U/liter) was found to be associated with type 1 infection before treatment.
The ALP level was also estimated both before and after treatment for responders and nonresponders, but the values did not show significant changes after treatment (Table 2). For the responders, the mean value before treatment was estimated to be 287 ± 66.6 U/liter, which became 266.8 ± 4.3 U/liter after treatment. For the nonresponders, the mean value was 290 ± 36.2 U/liter, which was reduced to 247 ± 41.29 U/liter after treatment. Considering that the normal ALP level is 60 to 306 U/liter, the values were not abnormally high or out of range. Thus, these marginal changes in level during treatment did not seem to reflect the response pattern.
Student's t test was performed for all enzyme levels (ALT, AST, and ALP) obtained from the patients (both responder and nonresponder) before and after treatment to check the statistical significance. A P value of <0.05 was considered to be significant. For both groups, the values calculated for all enzymes (ALT, AST, and ALP) before treatment reflected considerable significance compared with normal levels, whereas there was no statistical evidence to prove that the values after treatment were significantly different from the normal control levels.
Influence of genotypes.
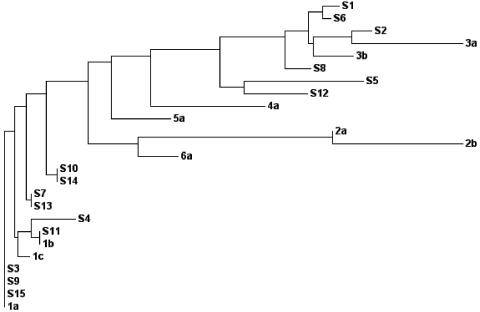
In order to study the influence of the genotype in sustained virological response to interferon treatment, part of the HCV 5′ UTR sequences (nucleotides 70 to 310) of all 15 samples were compared with those of the known HCV genotypes 1, 2, 3, 4, 5, and 6, along with their subtypes. For the phylogenetic analysis, the results were represented as a phylogenetic tree (Fig. 2). The results suggest that 9/15 were type 1 and 6/15 were type 3. Additionally, the percent homology (a score table was obtained from the same phylogram analysis) was considered in assigning the subtype for each sample (data not shown). The results showed the presence of types 1a (6/15), 1b (3/15), and 3b (6/15) in our sample population (Table 2). Interestingly, we have observed that a majority (three of four) of the fast responders belonged to type 3b, whereas the slow responders were predominantly of type 1a (four of six). As expected, in the nonresponder group, a majority (four of five) were found to be genotype 1.
FIG. 2.
Phylogenetic analysis of the Indian isolates using the 5′ UTR sequences. Shown is a phylogenetic tree demonstrating the genetic relationships of different patient samples with standard HCV isolates based on the nucleotide identity of the 5′ UTR (nucleotides 70 to 310). The values of genetic distances between isolates are as follows: S1, 0.00278; S6, 0.00139; S2, 0.00313; 3a, 0.01771; 3b, 0.00635; S8, 0.00415; S5, 0.01904; S12, 0.01013; 4a, 0.01825; 5a, 0.00939; 2a, 0.00000; 2b, 0.02083; 6a, 0.00638; S10, 0.00000; S14, 0.00000; S7, 0.00000; S13, 0.00000; S4, 0.00706; S11, 0.00000; 1b, 0.00000; 1c, 0.00196; S3, 0.00000; S9, 0.00000; S15, 0.00000; and 1a, 0.00000.
Analysis of mutations in the ISDR region of nonstructural protein 5A.
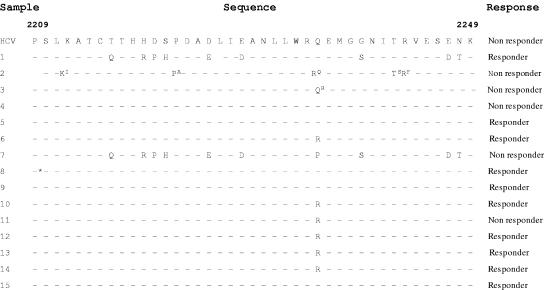
Earlier studies revealed that the NS5A protein binds and represses the IFN-induced PKR. The PKR-interacting domain of NS5A spans amino acid positions 2209 to 2249 and encompasses the ISDR. Mutations in this region are believed to partially account for different patient responses to treatment (14). Considering the importance of this region, we looked for mutations within the region in different patient samples and compared the sequence with a representative nonresponder sequence (prototype J). It was found to be highly conserved except for some mutations, which were highly variable. Multiple mutations were found (9/41) in the ISDR of sample 1, which was a fast responder. However, sample 7 showed 10/41 mutations but still failed to respond to interferon treatment (Fig. 3). The mutation frequency in the rest of the samples was 1 or null, but there was no clear correlation with the response pattern. To get a clearer picture, we investigated whether the virus had undergone mutation in the ISDR region during treatment. However, the ISDR sequences of only two cases out of five nonresponders showed variations after treatment (samples 2 and 3), whereas in the other three cases, the sequences were found to be identical after treatment (samples 4, 7, and 11). Thus, the results were again not clear enough to predict the effect of mutations in the ISDR on the outcome of interferon treatment.
FIG.3.
Mutations within ISDR. Shown is the alignment of amino acid residues 2209 to 2249 of the ISDR of the NS5A protein obtained from the samples and compared with the reference prototype J (nonresponder) sequence. The amino acids that are shown as superscript represent the mutations at the respective positions observed after interferon treatment in the same nonresponder sample. In sample 2, the sequences were compared (both AT and BT) from amino acids 2210 to 2249. The asterisk indicates insertion of two R residues in sample 8 (before treatment).
Analysis of the mutations in the PePHD region of the E2 envelope protein.
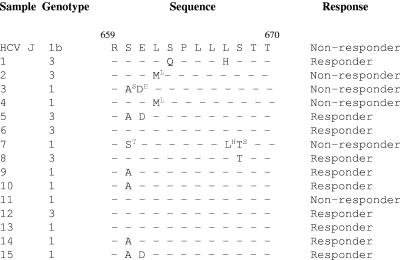
The PePHD region of the envelope protein was recently reported to be an important determinant in predicting the interferon response in HCV-infected patients (23). Thus, we were further interested in analyzing the mutations that affected the PePHD region and in studying its possible impact on the interferon response (3). For this, 12-amino-acid domain sequences (659 [position 1 {P1}] to 670 [P12]) of the PePHD regions from 15 patient samples (before treatment) were compared with the nonresponder prototype (prototype J) sequence (Fig. 4). Although, the number of mutations (one or two) did not vary significantly between responders and the nonresponder, certain positions (e.g., serine660 [P2]) were found to be highly susceptible to mutation and might play a role in determining the type of response to interferon therapy. In our study, in six samples (samples 3, 5, 9, 10, 14, and 15), the amino acid at position 660, serine, was mutated to alanine. Interestingly, this mutation in a majority of cases (five of six) occurred more frequently in responders than in nonresponders (one of six). Glutamic acid at position 661 (P3) was also found to be mutated to aspartic acid in three samples (samples 3, 5, and 15), and among these three samples, two belong to the responder group. At position 662 (P4), leucine was mutated to methionine in two samples, which belong to the nonresponder group.
FIG.4.
Mutations within PePHD. Shown is the alignment of amino acid residues 659 to 670 of the PePHD region of the E2 envelope protein obtained from patient samples and compared with the reference prototype J (nonresponder) sequence. The superscript amino acid residues represent mutations observed after interferon treatment in the same nonresponder sample.
Further investigation of the mutations in this region after therapy showed some interesting variations. The residue at position 662 (P4), methionine, had reverted to leucine (the same as prototype J) after treatment of the patient (samples 2 and 4). Similarly, in sample 7, threonine668 (P10) was found to revert to serine (the same as prototype J) during the course of treatment. In fact, serine at this position was found to be relatively conserved in all samples. These point mutations might have direct or indirect consequences for the phosphorylation of the PePHD region during HCV infection and might influence responses during interferon therapy.
Quasispecies analysis.
Viral quasispecies variation in the otherwise-conserved 5′ UTR during treatment with interferon could also be an important determinant of the response. In order to investigate the influence of mutations in the 5′ UTR in a single individual during treatment, the sequences of the 5′ UTRs of the nonresponder samples (before and after treatment) were compared with known HCV prototypes.
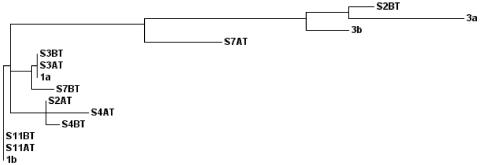
Among five nonresponders that were analyzed, four belonged to type 1 and one belonged to type 3 before treatment. The 5′ UTRs of four nonresponders who belonged to type 1 had conserved sequences with very few mutations compared with sequences obtained after treatment. However, in sample 2, an interesting pattern was observed in which the genotype was determined to be type 3 before treatment, but during treatment, mutations caused it to change to type 1 (Fig. 5). These mutations in sample 2 were found to be distributed throughout its 5′ UTR, but none of them were located in conserved regions, which have been shown to be important for viral translation by internal initiation (data not shown).
FIG. 5.
Analysis of quasispecies variations. Shown is a phylogenetic tree of the 5′ UTR quasispecieses in the five nonresponder samples studied BT and AT. The values for genetic distance between isolates are as follows: S2BT, 0.00381; 3a, 0.01702; 3b, 0.00624; S7AT, 0.01178; S2AT, 0.00214; S4AT, 0.00214; S4BT, 0.00206; S3BT, 0.00000; S3AT, 0.00000; 1a, 0.00000; S7BT, 0.00333; S11BT, 0.00000; S11AT, 0.00000; and 1b, 0.00000.
DISCUSSION
Several reports have demonstrated possible correlations between the mutations in the PePHD and ISDR and the interferon responsiveness of HCV-infected patients worldwide. Here, we have analyzed the clinical significance of the mutations in the ISDR and the PePHD region to the response to therapy among HCV-infected patients in India. In this study, out of 15 patients, six samples belonged to type 1a, three samples were type 1b, and six samples were type 3b.While 10 patients (predominantly type 3) showed complete response to therapy, 5 of them did not respond (predominantly type 1). Thus, the study group was comprised of both responders and nonresponders and also included the representative prevalent genotypes (types 1 and 3) in India.
However, the differences in the genotype did not seem to influence the pretreatment viral load, since the viral-RNA load ranged from 5.2 to 6.09 log10 IU/ml for samples having genotype 3, whereas it was 5.36 to 6.13 log10 IU/ml for samples that belonged to type 1. Interestingly, in this study, we found that the initial viral loads in a majority of responders (7/10) were lower than those of the nonresponders. In the responders, the viral load was found to be steadily decreasing to below the detectable limit after 24 weeks of treatment, along with the enzyme level. However, in the nonresponders, the initial viral load was relatively high, and during treatment the viral load was found to fluctuate, albeit marginally (data not shown), and it was not reduced significantly after 24 weeks of therapy, although in some cases (samples 3, 4, 7, and 11) the enzyme levels were found to return to normal level.
In sample 1, we found nine mutations in the ISDR, and the patient showed fast response to interferon therapy. On the other hand, in sample 7, 10 mutations in the ISDR were noticed, but the patient did not respond to interferon at all. Other samples showed at most one or two mutations that did not correlate with the response pattern. In fact, earlier studies also demonstrated that the correlation between mutations in the ISDR and interferon responsiveness was observed only with the J group (an HCV 1b isolate in Japan) (14), but similar correlations were not observed in studies conducted in Europe and United States (11).
However, the mutations in the PePHD region could provide better correlations of the response to interferon therapy. In our study, the frequency of mutations was found to be highest (six samples) at position 660 (S→A), and a majority of these patients responded well to interferon therapy. Additionally, a mutation at position 661 (E→D) was also found to be associated with response (two out of three samples) to some extent. Interestingly, the histidine at position 667, which was shown earlier to be associated with response (2), was found to be present in sample 1 (from a complete responder) and might influence the response to interferon treatment.
Mutations observed at positions 662 and 663 were not associated with response, whereas a mutation at position 668 could be associated with response, which is consistent with earlier studies (2, 18). Additionally, in some cases, we have observed reversion of a mutation after treatment (samples 2 and 4, M662 to L662; sample 3, A660 to S660 and D661 to E661; and sample 7, T668 to S668), which matched the prototype J sequence and might have contributed to resistance to interferon therapy.
The 5′ UTR of HCV probably plays a role in the ability of HCV variants to replicate in infected patients and affect the response pattern during interferon therapy. The results of our study showed that the 5′ UTR was highly conserved in most of the cases (four of five), and no significant sequence heterogeneity was observed after treatment. However, in sample 2 (one of five), several nucleotide changes were observed after treatment, which resulted in a change in its genotype from type 3b to type 1a. In the same sample, although it initially responded to the therapy, the viral load showed progressive increase after the fourth week of treatment (data not shown), suggesting that the mutations in the 5′ UTR might have facilitated replication of the virus in the infected patient. When these mutations were plotted onto the putative secondary structure of the HCV 5′ UTR, a majority of them were located in the SLIII region. However, most of the quasispecies variations of sample 2 after treatment were found to be in the apparently nonconserved segment of SLIII, as we reported earlier (1), and were not likely to affect the structure of the 5′ UTR RNA (data not shown).
Acknowledgments
We gratefully acknowledge our laboratory members for their help and discussions. We sincerely thank D. Raghunath for helpful discussion.
The work is supported by research grants to S.D. from the Sir Dorabji Tata Centre for Research in Tropical Diseases, India.
REFERENCES
- 1.Bhattacharyya, S., K. Mapa, S. Prabhavathi, S. R. Sudhamani, P. K. Menon, K. P. John, C. Shivaram, S. Amarnath, and S. Das. 2004. Phylogenetic conservation of the stem-loop III structure of the 5′ untranslated region of hepatitis C virus RNA among natural variants in samples collected from Southern India. Arch. Virol. 149:1015-1026. [DOI] [PubMed] [Google Scholar]
- 2.Catherine, G., M. Lambele, A. Moreau, P. Veillon, F. Lunel, and A. Goudeau. 2005. Mutations with in the hepatitis C virus genotype 1b E2-PePHD domain do not correlate with treatment outcome. J. Clin. Microbiol. 43:750-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chayama, K., F. Suzuki, A. Tsubota, M. Kobayashi, Y. Arase, S. Saitoh, Y. Suzuki, N. Murashima, K. Ikeda, N. Takahashi, M. Kinoshita, and H. Kumada. 2000. Association of amino acid sequence in the PKR-eIF2 phosphorylation homology domain and response to interferon therapy. Hepatology 32:1138-1144. [DOI] [PubMed] [Google Scholar]
- 4.Choo, Q. L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury, A., A. Santra, S. Chaudhuri, G. K. Dhali, S. Chaudhuri, S. G. Maity, T. N. Naik, S. K. Bhattacharya, and D. N. Mazumder. 2003. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology 37:802-809. [DOI] [PubMed] [Google Scholar]
- 6.Francesco, D. R. 1999. Molecular virology of the hepatitis C virus. J. Hepatol. 31:47-53. [DOI] [PubMed] [Google Scholar]
- 7.Fried, M. W., and S. J. Hadziyannis. 2004. Treatment of chronic hepatitis C infection with peginterferons plus ribavirin. Semin. Liver Dis. 24:47-54. [DOI] [PubMed] [Google Scholar]
- 8.Gale, M. J., C. M. Blakely, B. Kwieciszewski, S. L. Tan, M. Dosset, N. M. Tang, M. J. Korth, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural protein 5A: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18:5208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagedorn, C. H., and C. M. Rice. 2000. Hepatitis C virus. Curr. Top. Microbiol. Immunol. 242:85-111. [DOI] [PubMed] [Google Scholar]
- 10.Hazari, S., S. K. Panda, S. D. Gupta, Y. Batra, R. Singh, and S. K. Acharya. 2004. Treatment of hepatitis C virus infection in patients of northern India. J. Gastroenterol. Hepatol. 19:1058-1065. [DOI] [PubMed] [Google Scholar]
- 11.Hofgärtner, W. T., S. J. Polyak, D. G. Sullivan, R. L. Carithers, Jr., and D. R. Gretch. 1997. Mutations in the NS5A gene of hepatitis C virus in North American patients infected with HCV genotype 1a or 1b. J. Med. Virol. 53:118-126. [DOI] [PubMed] [Google Scholar]
- 12.Lake-Bakaar, G. 2003. Current and future therapy for chronic hepatitis C virus liver disease. Curr. Drug Targets Infect. Disord. 3:247-253. [DOI] [PubMed] [Google Scholar]
- 13.Lole, K. S., J. A. Jha, S. P. Shrotri, B. N. Tandon, V. G. Prasad, and V. A. Arankalle. 2003. Comparison of hepatitis C virus genotyping by 5′ noncoding region- and core-based reverse transcriptase PCR assay with sequencing and use of the assay for determining subtype distribution in India. J. Clin. Microbiol. 41:5240-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano, I., Y. Fukuda, Y. Katano, S. Nakano, T. Kumada, and T. Hayakawa. 1999. Why is the interferon sensitivity-determining region (ISDR) system useful in Japan? J. Hepatol. 30:1014-1022. [DOI] [PubMed] [Google Scholar]
- 15.Nousbaum, J. B. 1998. Genomic subtypes of hepatitis C virus: epidemiology, diagnosis and clinical consequences. Bull. Soc. Pathol. Exot. 91:29-33. [PubMed] [Google Scholar]
- 16.Panigrahi, A. K., J. Roca, S. K. Aacharya, S. Jameel, and S. K. Panda. 1996. Genotype determination of hepatitis C virus from northern India: identification of a new subtype. J. Med. Virol. 48:191-198. [DOI] [PubMed] [Google Scholar]
- 17.Pawlotsky, J. M. 2003. The nature of interferon-alpha resistance in hepatitis C virus infection. Curr. Opin. Infect. Dis. 16:587-592. [DOI] [PubMed] [Google Scholar]
- 18.Sarrazin, C., I. Kornetzky, B., Ruster, J. H. Lee, B. Kronenberger, K. Bruch, W. K. Roth, and S. Zeuzem. 2000. Mutations within the E2 and NS5A protein in patients infected with hepatitis C virus type 3a and correlation with treatment response. Hepatology 31:1360-1361. [DOI] [PubMed] [Google Scholar]
- 19.Simmonds, P. 2004. Genetic diversity and evolution of hepatitis C virus—15 years on. J. Gen. Virol. 85:3173-3188. [DOI] [PubMed] [Google Scholar]
- 20.Singh, S., V. Malhotra, and S. K. Sarin. 2004. Distribution of hepatitis C virus genotypes in patients with chronic hepatitis C infection in India. Indian J. Med. Res. 119:145-148. [PubMed] [Google Scholar]
- 21.Sloer, M., M. Pellerin, C. E. Malnou, D. Dhumeaux, K. M. Kean, and J. M. Pawlotsky. 2002. Quasispecies heterogeneity and constraints on the evolution of the 5′noncoding region of hepatitis C virus (HCV): relationship with HCV resistance to interferon α therapy. Virology 298:160-173. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki, R., T. Suzuki, K. Ishii, Y. Matsuura, and T. Miyamura. 1999. Processing and functions of hepatitis C virus proteins. Intervirology 42:145-152. [DOI] [PubMed] [Google Scholar]
- 23.Taylor, D. R., S. T. Shi, P. R. Romano, G. N. Barber, and M. M. C. Lai. 1999. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 285:107-110. [DOI] [PubMed] [Google Scholar]
- 24.Valliammai, T., S. P. Thyagarajan, A. J. Zuckerman, and T. J. Harrison. 1995. Diversity of genotypes of hepatitis C virus in southern India. J. Gen. Virol. 76:711-716. [DOI] [PubMed] [Google Scholar]
- 25.Wolf, P. H., S. Zeuzem, and C. Sarrazin. 2005. Hepatitis C virus resistance mechanisms to interferon α-based antiviral therapy. J. Clin. Virol. 32:86-91. [DOI] [PubMed] [Google Scholar]
- 26.Zeuzem, S., M. Diago, E. Gane, K. R. Reddy, P. Pockros, D. Prati, M. Shiffman, P. Farci, N. Gitlin, C. B. O'Brien, F. Lamour, P. Lardelli, and the PEGASYS Study NR16071 Investigator Group. 2005. Peginterferon alfa-2a (40 kilodaltons) and ribavirin in patients with chronic hepatitis C and normal aminotransferase levels. Gastroenterology 128:1531-1532. [DOI] [PubMed] [Google Scholar]