Abstract
The objectives of this study were to collect and characterize epidemic meningococcal isolates from Ethiopia from 2002 to 2003 and to compare them to 21 strains recovered during the previous large epidemic of 1988 to 1989. Ninety-five patients in all age groups with clinical signs of meningitis and a turbid cerebrospinal fluid (CSF) sample were included in the study of isolates from 2002 to 2003. Seventy-one patients (74.7%) were confirmed as having Neisseria meningitidis either by culture (n = 40) or by porA PCR (n = 31) of their CSF. The overall case fatality rate (CFR) was 11.6%; the N. meningitidis-specific CFR was 4.2%. All 40 strains were fully susceptible to all antibiotics tested except sulfonamide, were serotyped as A:4/21:P1.20,9, and belonged to sequence type 7 (ST-7). The strains from 1988 to 1989 were also equally susceptible and were characterized as A:4/21:P1.20,9, but they belonged to ST-5. Antigenic characterization of the strains revealed differences in the repertoire of lipooligosaccharides and Opa proteins between the old and the recent strains. PCR analysis of the nine lgt genes revealed the presence of the lgtAHFG genes in both old and recent strains; lgtB was present in only some of the strains, but no correlation with sequence type was observed. Further analysis showed that in addition to their pgm alleles, the Ethiopian ST-5 and ST-7 strains also differed in their tbpB, opa, fetA, and lgtA genes. The occurrence of new antigenic structures in strains sharing the same serogroup, PorA, and PorB may help explain the replacement of ST-5 by ST-7 in the African meningitis belt.
Serogroup A Neisseria meningitidis is responsible for recurring epidemics of bacterial meningitis in the African meningitis belt (26). Although epidemics caused by serogroup W135 have recently arisen (54), most of the cases in the region are still caused by serogroup A meningococci (http://www.who.int/csr/don). Molecular epidemiological studies have shown that serogroup A strains are genotypically diverse, but specific complexes of related hypervirulent clones are responsible for a major part of the cases in the meningitis belt (8). Most recent epidemics have been caused by a clonal group introduced to the meningitis belt from Mecca, Saudi Arabia, in 1987; it was first identified using multilocus enzyme electrophoresis (MLEE) and was called subgroup III (8, 57). Serogroup A meningococci of subgroup III are very homogenous: basically, all of the meningococci express a serosubtype P1.20,9 PorA and a serotype 4/21 PorB (8). In spite of the capability of rapid genetic change through genomic rearrangements or uptake of foreign DNA (29), epidemic serogroup A strains of N. meningitidis mainly express a single PorA subtype that changes only slowly over time (44).
Using multilocus sequence typing (MLST), strains of subgroup III were found to belong to two main sequence types (STs), either ST-5 or ST-7, which differ in MLST solely by their allele at the pgm gene (31). The ST-5 clone was introduced in Africa in 1987; between 1988 and 1999, it reached all the countries of the meningitis belt, where it was responsible for numerous outbreaks. ST-7 was identified for the first time in sub-Saharan Africa in 1996, but since 2002, mostly ST-7 strains have been isolated in the region (35).
Further genetic analyses have shown that subgroup III strains may also differ at several other loci different from those analyzed by MLST as, e.g., loci encoding expressed surface epitopes (57). The replacement of ST-5 by ST-7 among subgroup III strains in the African continent in the mid-1990s reflects a significant genetic change (34), and there is interest in finding the immunologically relevant surface-exposed antigens that might have driven this shift (4).
Meningococcal meningitis in both endemic and epidemic forms has affected Ethiopia for over a century (7). Outbreaks and epidemics have been reported in 1935, the 1940s, the 1950s, 1964, 1976 to 1977, 1981 to 1983 (18), and 1988 to 1989 (19). Prior to 1988, the majority of epidemic cases occurred in the north, the northwest, and parts of the central regions of Ethiopia, which lie within the eastern end of the traditional meningitis belt (26). After the devastating epidemic of 1988 to 1989, however, this pattern changed, and the whole country has been affected by outbreaks (46), although increased awareness could also contribute to this observation. Epidemics were also reported in Ethiopia in the years 2001 to 2003. The number of cases and case fatality rates (CFRs) from 2000 to 2003 are given in Table 1. While the epidemics in 1981 and 1988 to 1989 struck with the magnitude of 40,000 to 50,000 cases (19, 46), these recent epidemics were much smaller; most cases occurred in the Amhara region and the Southern Nations, Nationalities, and Peoples' Region (SNNPR), respectively (http://www.who.int/csr/don) (Fig. 1).
TABLE 1.
Officially reported numbers of meningitis cases in Ethiopia and study areas from 2000 to 2003
| Yr | Whole countrya
|
North Gondar Zone, Amhara regionb
|
Sidama and Gedio Zones, SNNPRc
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | No. of deaths | CFRd (%) | No. of cases | No. of deaths | CFR (%) | No. vacce (%) | No. of cases | No. of deaths | CFR (%) | No. vacc (%) | |
| 2000 | 855 | 19 | 2.2 | 0 | 0 | 0 | 533 | 15 | 2.8 | 226,395 (18) | |
| 2001 | 6,266 | 311 | 5.0 | 384 | 26 | 7 | 473,300 (41) | 1,345 | 52 | 3.9 | 825,413 (58) |
| 2002 | 2,329 | 118 | 5.1 | 1,235 | 128 | 10.4 | 214,408 (26) | 346 | 0 | 0 | 377,794 (98) |
| 2003 | 3,540 | 166 | 4.7 | 0 | 0 | 0 | 1,010 | 44 | 4.4 | 279,561 (26) | |
Data source, http://www.who.int/csr/don.
Data sources, reference 33 and North Gondar Zone Health Bureau. The target population for vaccination was defined as those between 2 and 30 years of age in the affected subarea.
Data sources, SNNPR Regional Health Bureau, Central Statistical Authority, Addis Ababa, Ethiopia, and ORC Macro, Calverton, MD.
CFR is defined as the number of deaths attributed to meningitis per number of patients with meningitis.
No. vacc, number of individuals reported as vaccinated with serogroup A and serogroup C meningococcal polysaccharide vaccine as the epidemic was evolving.
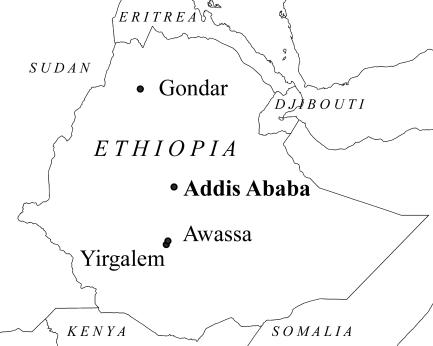
FIG. 1.
Map of Ethiopia, with cities of collaborating institutions indicated. The figure was prepared using ArcView 9.1 software (ESRI, Redlands, CA) and geographic data available from the European Joint Research Centre Digital Map Archive (http://dma.jrc.it).
The objectives of this study were to collect meningococcal strains from Ethiopia from 2002 to 2003, to characterize their genetic and antigenic variation, and to study their antibiotic susceptibility pattern. These strains were compared to strains isolated during the epidemic of 1988 to 1989 (19). Specifically, we studied these recent and older strains for variations in genes encoding the outer membrane (OM) proteins NadA, FetA, and TbpB and in genes associated with lipooligosaccharide (LOS) biosynthesis. NadA, FetA, and TbpB are surface-exposed phase-variable outer membrane proteins known to exert variation among meningococci and to induce antibodies following meningococcal disease (11, 29, 47). Our study revealed that all strains collected from patients in 2002 and 2003 were very homogenous and belonged to ST-7. The replacement of ST-5 by ST-7 occurred in Ethiopia between 1995 and 2000 and was accompanied by changes in tbpB, opa, fetA, and lgtA alleles in these strains.
(Part of this work was presented at the 14th International Pathogenic Neisseria Conference in Milwaukee, WI, September 2004.)
MATERIALS AND METHODS
Study area and population.
The study was conducted in the Sidama and Gedio Zones in the SNNPR and in the North Gondar Zone in the Amhara region of Ethiopia between April 2002 and December 2003. The Sidama and Gedio Zones are located in the lowlands of southern Ethiopia, within an area with a tropical climate. Most of the patients in the SNNPR were admitted to Yirgalem and Dilla hospitals and the Bushulo Major Health Center, all of which are located within 80 km south of Awassa (Fig. 1) and predominantly serve a rural community (approximately 3 million people). The North Gondar Zone is located in the highlands of northern Ethiopia and is part of the traditional meningitis belt (26). Most of the patients in the North Gondar Zone were admitted to Gondar University Hospital, which is located 750 km north of Addis Ababa and serves the population of the town of Gondar (Fig. 1) and the surrounding zones (approximately 4.5 million people). Patients were also admitted to Metema hospital, 160 km west of Gondar, and to health centers in remote villages.
Layout of the study and clinical diagnosis.
This prospective study included patients from the two meningitis seasons of 2001 to 2002 and 2002 to 2003. Suspected meningitis cases presenting at the study sites were included on the basis of the following criteria: (i) being clinically diagnosed with meningitis (53), (ii) having turbid cerebrospinal fluid (CSF), and (iii) being aged 6 months and older. In each region, patients fulfilling the inclusion criteria were recruited consecutively in the first year until at least eight patients were included in each of the following desired age groups: infants (6 months to up to 2 years of age), young children (2 to <6 years of age), older children and teenagers (6 to <15 years of age), young adults (15 to <20 years of age), and adults (20 years of age and above) for the purpose of serological analyzes. During the second season (2002 to 2003), recruitment focused on including children younger than 2 years of age, as too few were recruited during the first season. Consequently, the age distribution in this study does not necessarily reflect the true distribution among meningitis patients in Ethiopia.
The case definition for bacterial meningitis was made according to World Health Organization guidelines (53). Clinical data, history, information about meningococcal vaccination status, and other relevant parameters were entered into a case record form. Reports of sequelae and deaths attributed to the meningitis episode were only those observed during the admission period in the hospitals. Lumbar puncture for CSF sampling was carried out as a part of routine procedures, according to the decision of the doctor, and antibiotic treatment was started immediately thereafter, according to the treatment protocol of the respective institutions. Turbid CSF samples, remaining after local laboratory tests were performed, were collected in sterile test tubes and split into three aliquots. These were analyzed at (i) the microbiology laboratories at Gondar Medical Hospital or SNNPR Health Bureau in Awassa, (ii) the Armauer Hansen Research Institute (AHRI), and (iii) the Norwegian Institute of Public Health (NIPH), respectively, to maximize laboratory confirmation of the cases and for quality control of the laboratory procedures for culture in Ethiopia.
CSF samples and bacterial isolates.
Each aliquot of turbid CSF was inoculated into a Trans-Isolate (T-I) medium (3). Following transport to laboratories in Gondar and Awassa within 24 h, the vials were vented and incubated as described previously (41). T-I medium vial 1 was cultured in either Gondar or Awassa on Thayer Martin agar medium plates with a VCNT selective supplement (vancomycin [3.0 μg/ml], colistin methane sulfonate [7.5 μg/ml], nystatin [12.5 U/ml], and trimethoprim [5.0 μg/ml]) (Oxoid Ltd., Basingstoke, United Kingdom), while vials 2 and 3 were transported as soon as possible to AHRI and NIPH, respectively, for culture on either VCNT agar medium plates (AHRI) or chocolate agar plates with an LCAT selective supplement (lincomycin [0.5 μg/ml], colimycin [7.5 μg/ml], amphotericin B [1.0 μg/ml], and trimethoprim [5.0 μg/ml]) (NIPH). N. meningitidis isolates were identified by standard procedures (41) and serogrouped with antisera (Murex Biotech Ltd., Dartford, United Kingdom). Pure colonies were harvested into Greaves' solution (41) and frozen at −70°C. The remaining liquid phases of the culture-negative T-I vials at NIPH were boiled and stored frozen at −70°C for PCR analyses.
In addition, 21 strains collected in Addis Ababa and the town of Zewai (170 km south of Addis Ababa) in Ethiopia in 1988 and 1989 (19) and 3 strains collected in 2000 to 2001 in Ethiopia from the strain collection of the WHO Collaborating Centre for Reference and Research on Meningococci, Oslo, Norway, were included in the study for comparison (see Table 5). These strains had already been assigned to subgroup III based on the MLEE method (8). Strains 126E, M986, 44/76, Z1054 (47, 56, 57) (see Table 4), and 188/87 were obtained from the same collection for use as control strains in PCR analyses. Streptococcus pneumoniae ATCC 49619 and Escherichia coli ATCC 25922 were used for quality control purposes in antibiotic sensitivity testing as described previously (10).
TABLE 5.
Microvariation in subgroup III strains from Ethiopia analyzed in this studyc
| Yr | Origin | ST | Allele variant (no. of isolates with allele/no. of isolates analyzed)
|
||||
|---|---|---|---|---|---|---|---|
| pgm | tbpB | fetAa | lgtAa | opaBb | |||
| 2002-2003 | Gondar and SNNPR | 7 | 19 (40/40) | 55 (40/40) | 7 (8/8) | EL1 (8/8) | 92 (1/1) |
| 2000-2001 | Oromiya and Amhara regions | 7 | 19 (3/3) | 55 (3/3) | |||
| 1988-1989 | Addis Ababa or Zewai | 5 | 3 (21/21) | 1 (20/21) | 11 (6/6) | 11 (6/6) | 94 (2/2) |
| ET1 (1/21) | |||||||
Data are from isolates listed in Table 4.
Data are from isolates Eth 9, Eth 12, and Mk 686/02.
All characterized as A:4/21:P1.20,9 in dot blot.
TABLE 4.
Characterization of 14 serogroup A subgroup III-1 meningococcal strains collected in Ethiopia from 1988 to 1989 and 2002 to 2003
| Strain | Geographic origin, yr | ST | Major LOS | NadA
|
lgt-1
|
lgt-2
|
lgt-3
|
tbpB allele | fetA allele | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAba | Gene | (TAAA)nb | lgtAc | lgtB | lgtC | lgtD | lgtE | lgtZ | lgtH | lgtF | lgtG | ||||||
| Mk 499/03 | S, 2003 | 7 | L11 | + | + | 13 | + 5 | − | − | − | − | − | + | + | + | 55 | 7 |
| Mk 502/03 | S, 2003 | 7 | L10, L11 | − | + | 9 | + 5 | + | − | − | − | − | + | + | + | 55 | 7 |
| Mk 804/03 | S, 2003 | 7 | L10 | − | + | 9 | + 5 | + | − | − | − | − | + | + | + | 55 | 7 |
| Mk 365/02 | G, 2002 | 7 | L10, L11 | − | + | 12 | + 5 | + | − | − | − | − | + | + | + | 55 | 7 |
| Mk 686/02 | G,d 2002 | 7 | L11 | + | + | 11 | + 5 | − | − | − | − | − | + | + | + | 55 | 7 |
| Mk 689/02 | S,d 2002 | 7 | L10 | − | + | 12 | + 5 | + | − | − | − | − | + | + | + | 55 | 7 |
| Mk 691/02 | G, 2002 | 7 | L11 | + | + | 11 | + 5 | − | − | − | − | − | + | + | + | 55 | 7 |
| Mk 802/02 | S, 2002 | 7 | L10, L11 | − | + | 6 | + 5 | + | − | − | − | − | + | + | + | 55 | 7 |
| Eth 2 | A/Z,d 1989 | 5 | L11 | − | + | 9 | + 10 | − | − | − | − | − | + | + | + | 1 | 11 |
| Eth 9 | A/Z, 1989 | 5 | L8, L3,7,9, L11 | − | + | 9 | + 7 | + | − | − | − | − | + | + | + | 1 | 11 |
| Eth 12 | A/Z, 1989 | 5 | L10 | + | + | 8 | + 7 | + | − | − | − | − | + | + | + | ET1 | 11 |
| Eth 18 | A/Z, 1989 | 5 | L3,7,9 | + | + | 8 | + 11 | + | − | − | − | − | + | + | + | 1 | 11 |
| Eth 35 | A/Z, 1989 | 5 | L11 | + | + | 8 | + 10 | − | − | − | − | − | + | + | + | 1 | 11 |
| Eth 38 | A/Z, 1989 | 5 | “L13” | + | + | 8 | + 11 | + | − | − | − | − | + | + | + | 1 | 11 |
| 126Ee | Germany, 1964 | L1 | − | − | − | + | + | + | + | − | + | − | NDf | ND | |||
| M986e | USA | 11 | L3,7 | − | + | 12 | + | + | − | − | − | − | + | + | + | ND | ND |
| 44/76e | Norway, 1976 | 32 | L8, L3,7,9, L11 | − | + | + | − | − | + | − | − | + | + | ND | 1 | ||
| Z1054e | Finland | 5 | L11 | − | + | 9 | + | + | − | − | − | − | + | + | − | 1 | 7 |
+, clearly positive reaction with MAb; −, unclear or no reaction with MAb.
n, number of TAAA repeat motifs.
PCR results with number of guanine (G) residues in the homopolymeric tract. The gene is predicted to be switched “on” if the tract length is 5 or 11 Gs, while the gene is predicted to be “off” if the tract length is 7 or 10 Gs (5).
A/Z, Addis Ababa or Zewai; G, North Gondar Zone; S, Sidama and Gedio Zones; USA, United States.
Control strains.
ND, allele not determined.
Protein and LOS characterization.
Serotypes, serosubtypes, immunotypes, Opa protein repertoire, and OpcA and NadA protein expression were determined by dot blot with whole-cell preparations, as described previously (52). We used the monoclonal antibodies (MAbs) (with the designations in parentheses): P1.7 (MN14C11-6), P1.9 (MN5A10F), P1.20 (V502), P3.4 (15-1-P4), P3.21 (14-1-P21), L1 (223 D-8), L3,7 (MN15A8-1 and 9-1-L3,7,9), L8 (2-1-L8), L10 (14-1-L10), L11 (4C4), OpcA (279/5c), Opa5a (W320/15), Opa5f (AB419), Opa5h (U205), Opa5i (T116), NadA (1079-B6), and NspA (236 B-2) (references for MAbs and polyclonal sera used are given in reference 37). OM extracts were prepared using the LiCl/LiAc method (13) and stored at −20°C. The total content of proteins was determined using the Bio-Rad DC protein assay (Bio-Rad Inc., Richmond, CA). OM extracts were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by applying approximately 3 μg protein from each OM extract onto 12% gels, followed by Coomassie brilliant blue staining. LOS bands in the OM preparations were separated and visualized by applying approximately 120 ng protein from each OM extract onto a 16.5% tricine-SDS-PAGE (TSDS-PAGE) gel, followed by silver staining (28). To identify the positions of major antigens in the OM extracts, immunoblotting was carried out as described previously (51) by using the MAbs listed above or polyclonal antisera against the TdfH or Omp85 proteins.
Antibiotic sensitivity.
All 64 N. meningitidis strains from Ethiopia (21 from 1988 to 1989, 3 from 2000 to 2001, and 40 from 2002 to 2003) were tested by the Etest method (AB Biodisk, Solna, Sweden), according to the manufacturer's instructions, with chocolate Mueller-Hinton agar (20). Susceptibility to the following drugs was tested: penicillin G, ampicillin, ceftriaxone, chloramphenicol, ciprofloxacin, rifampin, and sulfamethoxazole. CLSI susceptibility criteria were applied (10).
MLST.
MLST was performed as described previously (31). Briefly, fragments of each of the seven genes abcZ, adk, aroE, gdh, pdhC, pgm, and fumC were amplified by PCR on a boiled bacterial cell suspension. Sequencing of the PCR products was performed on both strands using the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Sequencing reactions were run on an ABI Prism 377 instrument (Applied Biosystems) using 5% Long Ranger gels (FMC Bioproducts, Rockland, ME). Alleles and STs were assigned, as described previously (31), by querying the Neisseria MLST database (http://pubmlst.org/neisseria).
PCR for diagnosis of culture-negative CSF samples.
To detect meningococcal DNA in CSF, the porA gene was amplified using a nested PCR, as previously described (9), on a boiled liquid fraction of the inoculated T-I medium. CSF samples that were inconclusive due to inhibitors of the polymerase were retested after DNA purification with a QIAamp DNA mini kit (QIAGEN Inc., Valencia, CA). The amplified fragment of the porA gene was sequenced as described above. The deduced amino acid sequences of variable regions (VRs) 1 and 2 of the encoded PorA protein were assigned genosubtype names according to the N. meningitidis PorA variable region database (http://neisseria.org/nm/typing/pora). PCR amplification of the orf-2 gene, encoding the N. meningitidis serogroup A polysaccharide capsule, was done as described previously (45) for the CSF samples shown to contain the porA gene.
Genetic characterization of the strains.
The nadA gene was amplified using primers and PCR conditions as described previously (11). Similarly, the nine glycosyltransferase genes lgtA to lgtH and lgtZ were amplified as described previously (56). Selected PCR products of the nadA promoter region, lgtA, lgtB, lgtH, and lgtG were sequenced as described above. Additional primers were used for sequencing of lgtA (lgtA_M2 [5′-AGC GTT TCC AAG AAC AGG AC-3′] and P6 [56]) and lgtH (lgtH_M1 [5′-CGC CGT ATT TGA AGA TGA TG-3′] and P26 [56]). The fetA gene was amplified as described previously (47), and following sequencing of the variable region, the deduced amino acid sequences were assigned genosubtype names according to the N. meningitidis FetA variable region database (http://neisseria.org/nm/typing/feta). Analyses were based on the alignment of the ∼410-nucleotide-long sequences with the 81 currently available fetA sequences on this website. The tbpB gene was amplified using primers OTG6687 and OTG6689 (27). Following purification of the tbpB PCR product with the QIAquick purification kit (QIAGEN), macrorestriction fragments were obtained by digestion of the purified tbpB product using the restriction endonucleases ApoI and SspI (New England Biolabs Inc., Beverly, MA) according to the manufacturer's instructions. The fragments were thereafter separated on 2% agarose gels, stained with ethidium bromide, and compared with band patterns of similarly digested tbpB PCR products from a control strain, Z1054 (tbpB allele 1). The band patterns with restriction enzymes ApoI, HincII, and SspI of tbpB alleles 1, 38, 39, 55, and 59, which are the dominant alleles in subgroup III meningococci (57), were predicted using the software program NEBcutter (version 2.0; New England Biolabs Inc. [http://tools.neb.com/NEBcutter2]). The tbpB gene of strains with different restriction fragment patterns was sequenced using primers 3′Met2 and O1641 (29) for old strains and T55_F (5′-TGT TGA GTG CTT GTC TGG GC-3′) and T55_R (5′-TCC CCG GAA AAA GCA CTA TA-3′) for recent strains. The opaB gene was amplified using primers O464 and O3510 (2) and sequenced using primers OpaB_p1 (5′-TAT CGG TGT GCG CGT C-3′) and OpaB_p2 (5′-AAT GTC GGG TGT CGC GC-3′). The sizes of PCR products and macrorestriction fragments were determined using a 1-kb DNA ladder molecular weight marker (Invitrogen, Carlsbad, CA). MWG-Biotech, Ebersberg, Germany, synthesized all primers.
Statistical methods.
The clinical, phenotypic, and genetic strain data were analyzed using SPSS ver. 12.0.2 for Windows (SPSS Inc., Chicago, IL). The chi-square test or Fisher's exact test was used to analyze the differences in proportions of the various characteristics between patients confirmed or not confirmed as meningococcal cases (Table 2), proportions of lgtB-positive strains within the different LOS immunotypes, and dot blot data (Table 3). The nonparametric Mann-Whitney U test was used for comparisons of means of days of transport in T-I medium, ages of the patients, and duration of illness and stay at the hospital.
TABLE 2.
Demographic characteristics, clinical and laboratory findings at hospital admission, and end results for the 95 patients included in the study
| Characteristic | Value for groupa
|
|
|---|---|---|
| All patients | N. meningitidis meningitisb | |
| Demographic | ||
| Residence | ||
| North Gondar Zone | 23/95 (24) | 19/71 (27) |
| Sidama and Gedio Zones | 72/95 (76) | 52/71 (73) |
| Yr | ||
| 2002 | 51/95 (54) | 38/71 (54) |
| 2003 | 44/95 (46) | 33/71 (46) |
| Age (yr) | ||
| 0.5-<2 | 7/93 (8) | 3/70 (4) |
| 2-<6 | 12/93 (13) | 10/70 (14) |
| 6-<15 | 29/93 (31) | 25/70 (36) |
| 15-<20 | 15/93 (16) | 12/70 (17) |
| ≥20 | 30/93 (32) | 20/70 (29) |
| Alld | 16.0 (13.6-18.3), 14 | 15.0 (12.6-17.5), 14 |
| Sex | ||
| Male | 54/93 (58) | 38/70 (54) |
| Female | 39/93 (42) | 32/70 (46) |
| Preadmission history | ||
| Antibiotics given before admission | 10/90 (11) | 6/67 (9) |
| Previously vaccinated with serogroup A polysaccharide vaccine | 15/90 (17) | 11/67 (16) |
| Days of illness from first symptoms to examination at hospital (n = 92; range, 0-9)d | 2.9 (2.6-3.3), 3 | 2.9 (2.5-3.3), 2 |
| Clinical and laboratory findings at hospital admission | ||
| Nuchal rigidity | 89/89 (100) | 66/66 (100) |
| Back rigidity | 34/58 (59) | 23/41 (56) |
| Ecchymoses | 7/71 (10) | 5/56 (9) |
| Petechiae, ≥10 | 15/39 (38) | 11/28 (39) |
| Seizures | 12/91 (13) | 8/68 (12) |
| Shock | 4/90 (4) | 2/67 (3) |
| Coma | 14/91 (15) | 7/68 (10) |
| End resultc | ||
| Death | 11/95 (12) | 3/71 (4) |
| Sequelae | 3/95 (3) | 2/71 (3) |
| Days of stay at hospital (n = 47; range, 0-15)d | 6.5 (5.5-7.4), 7 | 6.3 (5.2-7.3), 7 |
Except where otherwise noted, values are no. of patients with characteristic/total no. of patients for whom data were available (% of reported cases).
Cases of N. meningitidis meningitis confirmed by either culture or PCR of CSF (n=71).
As observed during hospital admission.
Values are means (95% CIs), medians.
TABLE 3.
Phenotypic characteristics of 64 strains isolated in Ethiopia from 1988 to 2003
| Antigen | No. (%) of positive reactions for time perioda:
|
lgtB positiveb (n = 64) | |
|---|---|---|---|
| 2000-2003 (n = 43) | 1988-1989 (n = 21) | ||
| OpcA | 36 (84) | 10 (48) | |
| Opa type | |||
| 5a | 7 (16) | 4 (19) | |
| 5f | 13 (30) | 4 (19) | |
| 5h | 3 (7) | 0 | |
| 5i | 0 | 2 (10) | |
| 5af | 5 (12) | 6 (29) | |
| 5ah | 1 (2) | 0 | |
| 5fh | 3 (7) | 0 | |
| 5ai | 0 | 2 (10) | |
| NadA | 10 (23) | 8 (38) | |
| LOS types | |||
| L3,7,9 | 0 | 3 (14) | 3/3 (100) |
| L10 | 10 (23) | 4 (19) | 14/14 (100) |
| L11 | 26 (60) | 12 (57) | 4/38 (11) |
| L10, L11 | 6 (14) | 0 | 6/6 (100) |
| L8, L11 | 1 (2) | 0 | 1/1 (100) |
| L3,7,9, L8, L11 | 0 | 1 (5) | 1/1 (100) |
| L13 | 0 | 1 (5) | 1/1 (100) |
Only clearly positive reactions are reported as positive. Values in parentheses are percentages. Identities of MAbs used in dot blot testing of whole cells are listed in Materials and Methods.
Proportion of isolates of the different LOS immunotypes with positive reaction in lgtB-specific PCR. Values in parentheses are percentages.
Ethical clearance.
The study obtained ethical clearance from the AHRI/All Africa Leprosy TB and Rehabilitation Training Center Ethical Clearance Committee, the National Ethical Review Committee (Ethiopian Science and Technology Commission), and the Norwegian Regional Committee for Medical Research Ethics in Western Norway (REK III). Informed written consent was obtained from patients (above 18 years of age) or their parents or guardians (for those patients below 18 years of age or with a lack of consciousness) before enrollment in the study.
Nucleotide sequence accession numbers.
The sequences of the new alleles for tbpB, lgtA, and lgtH (two alleles) have been deposited in GenBank under the accession numbers DQ355978, DQ296151, and DQ296152 and DQ296153, respectively.
RESULTS
Patients.
Ninety-five patients between 6 months and 50 years of age were included in this study from April 2002 to June 2003 on the basis of clinical signs and macroscopic appearance of their CSF samples. Twenty-three patients were from Gondar, mainly in 2002, and 72 patients were from the SNNPR, mainly in 2003. The patients' demographic characteristics, clinical data, and the hospital laboratory investigation findings are shown in Table 2.
Laboratory confirmation of meningococcal meningitis.
Forty cases were confirmed by culture of N. meningitidis from CSF in at least one of the study laboratories. Most isolates were obtained from the SNNPR in 2003. In the current setting of hospitals with mobile study teams, meningococci from clinical CSF samples were able to survive in T-I medium for up to 67 days. The mean time between CSF inoculation in T-I medium in Ethiopia and isolation of meningococci in Norway (34.1 days; 95% confidence interval [CI], 25.8 to 42.5) was not statistically different (P = 0.840) from the mean time between inoculation and cultivation for those media that were culture negative (37.7 days; 95% CI, 30.0 to 45.4).
The culture-negative CSF samples from the remaining 55 patients were further tested by PCR. Of these samples, 31 were positive (56%) and 24 were negative (44%) in the nested porA PCR. Thus, in total, 71 patients (74.7%) had laboratory-confirmed meningococcal meningitis. Except for one patient confirmed as having Haemophilus influenzae serotype b infection by culture, the etiological agents in the CSF samples of the remaining 23 patients were not determined.
Comparison of the patients confirmed as having meningococcal meningitis with the other meningitis patients.
While 11 of the 95 meningitis cases (11.6%) resulted in death during hospital stay, only 3 of the fatal cases occurred in patients confirmed as having N. meningitidis in CSF by culture or PCR, resulting in a meningococcal meningitis-specific CFR of 4.2%. This contrasts with the significantly higher CFR among patients with nonmeningococcal meningitis (P = 0.0001) (Table 2). Three patients were reported as having sequelae during the hospital stay that could be attributed to the meningitis episode. Two of them, confirmed as meningococcal meningitis cases, had hearing abnormality; the third one suffered paresis of eye muscles. At least two more patients developed sequelae attributable to the meningitis episode after discharge from the hospital. These were identified among those patients contacted up to 1 year after the onset of disease during late-convalescent-phase blood sample collection. Both patients were confirmed as having N. meningitidis by PCR; one had hearing impairment, and the other had central nervous system complications. There was a significantly higher frequency of coma in patients with nonmeningococcal meningitis (P = 0.021) (Table 2). No other factors were significantly different between patients with or without demonstrable N. meningitidis in their CSF (Table 2). Vaccination status was self-reported by the patients, and data should thus not be used as an indication of vaccine efficacy.
Characterization of meningococcal strains and DNA from patients from 2002 to 2003.
All 40 strains were serogroup A, serotype 4/21, and serosubtype P1.20,9. When tested by MLST, they were assigned to ST-7, belonging to the ST-5 complex/subgroup III. PorA VR typing of the gene product from the 31 porA PCR-positive CSF samples revealed that all of them had meningococcal DNA from a P1.20,9 strain. Further PCR done on the same 31 CSF samples showed that 21 of the samples were also positive for the serogroup A capsule gene orf-2. Thus, all 71 patients had been infected by serosubtype P1.20,9 strains; 61 of these samples were also confirmed as being serogroup A strains.
Testing of antibiotic susceptibility revealed full sensitivity to penicillin G, ampicillin, ceftriaxone, chloramphenicol, ciprofloxacin, and rifampin, but all 40 strains were resistant to sulfamethoxazole (all with MICs ≥ 256 mg/liter) (10).
Phenotypic comparison of the strains from 2000 to 2003 with those from 1988 to 1989.
The 21 strains from 1988 to 1989 had all previously been characterized by MLEE as belonging to subgroup III and were serotyped as A:4/21:P1.20,9 (19). Antibiotic testing of these 21 strains and 3 strains from 2000 to 2001 revealed a susceptibility pattern identical to that of the strains from 2002 to 2003. To further study the phenotypic and genetic variation of the available strains from Ethiopia, we characterized all 64 strains from 1988 to 2003 for antigenic variation in their opacity proteins, their NadA proteins, and their LOS (Table 3).
Dot blotting showed that in contrast to the similarity in capsule serogroup, PorA, and PorB, the strains were less homogenous in their reactions with MAbs towards LOS and Opa proteins (Table 3). Except for OpcA and L3,7,9 reactions, there were no significant differences between old and recent strains, although a larger number of strains should be tested for conclusive results. OpcA was more frequently seen in recent strains than in old strains (P = 0.03). Opa5i was exclusively found in the old strains. Reaction with a MAb specific for the NadA protein was higher in recent strains than in old strains, although the difference was not significant (Table 3) (P = 0.246).
LOS types L11 and L10 were predominant among both the old and recent strains, with most of the strains showing an L11 reaction (Table 3). The L3,7,9 reaction was only seen in four of the old strains, and the difference was significant (P = 0.03). While only 1 of the old strains showed multiple LOS MAb reactions (strain Eth 9), 7 of the 43 recent strains did (Table 3). One strain from 1988 to 1989 (Eth 38) did not react with any of the anti-LOS MAbs used.
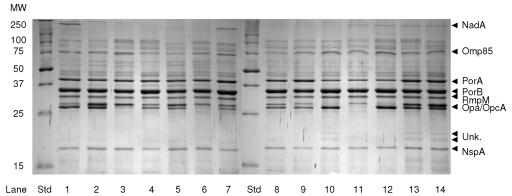
Overall, OM extracts of the strains were homogenous as judged by gel electrophoresis (Fig. 2 and 3). However, as in the dot blot analysis, differences were observed in expression levels of PorA and NadA and the band pattern of Opa proteins and LOS. Only three (8%) recent strains showed reduced amounts of the PorA protein in their OM extracts, and these strains also reacted weakly with the anti-PorA MAb in dot blotting. In general, the expression of PorB was higher than that of PorA. Some strains expressed high amounts of a protein of ∼270 kDa; which was identified as the NadA protein by immunoblot. The expression correlated well with the intensity of the reaction with a NadA-specific MAb in the dot blot. Some variation in migration was seen for a band at ∼70 kDa, which was probably the FetA protein, as seen by immunoblot analysis. TdfH, Omp85, PorB, RmpM, and NspA proteins were detected by immunoblotting and were present in similar amounts in all OM extracts. The amount and migration pattern of Opa proteins were highly variable. In some strains, two unidentified bands were present at approximately 20 to 25 kDa (unknown) (Fig. 2).
FIG. 2.
Coomassie brilliant blue-stained 12% SDS-PAGE gels with OM extracts from N. meningitidis isolates listed in Table 4. Lane 1, Mk 499/03; lane 2, Mk 502/03; lane 3, Mk 804/03; lane 4, Mk 365/02; lane 5, Mk 686/02; lane 6, Mk 689/02; lane 7, Mk 691/02; lane 8, Mk 802/02; lane 9, Eth 2; lane 10, Eth 9; lane 11, Eth 12; lane 12, Eth 18; lane 13, Eth 35; lane 14, Eth 38. Unk.; unknown protein bands; Std, standard; MW, molecular weight (in thousands).
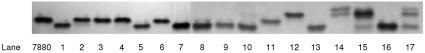
FIG. 3.
Silver-stained LOS bands of OM extracts separated in 16% TSDS-PAGE gels. 7880, L10 prototype strain (24). Lanes 1 to 14 contain OM extracts from same strains shown in Fig. 2. Lane 15, strain 44/76 (L3,7,9); lane 16, strain N 144/95 (L8); lane 17, strain Sudan 433/88 (L13) (42).
Visualization of LOS expression by silver staining revealed one of two bands (Fig. 3) in most strains: the upper band was confirmed as either L10 or L3,7 and the lower band was confirmed as either L11 or L8 by immunoblotting. OM extracts that reacted with both L10 and L11 MAbs in the dot blot had two bands but in different amounts. Strains reacting with the L3,7,9 MAb in the dot blot showed one major band, as confirmed by immunoblotting, where the MAbs MN15A8-1 and 9-1-L3,7,9 showed similar reactions. Strain Eth 38, which did not react with any of the tested anti-LOS MAbs, showed two bands on the TSDS-PAGE gel (Fig. 3). The LOS type in this strain could be L13 on the basis of electrophoretic migration by comparison with an L13 strain from Sudan (Fig. 3, lanes 14 and 17) (42), but it could also be of an immunotype for which MAbs were not available to us.
Genotypic comparison of the strains from 2002 to 2003 with those from 1988 to 1989.
Sequencing of the pgm locus from the strains isolated from 1988 to 1989 revealed allele 3, and thus, they were assigned to ST-5. The three strains from 2000 were also genotyped by MLST and were assigned to ST-7.
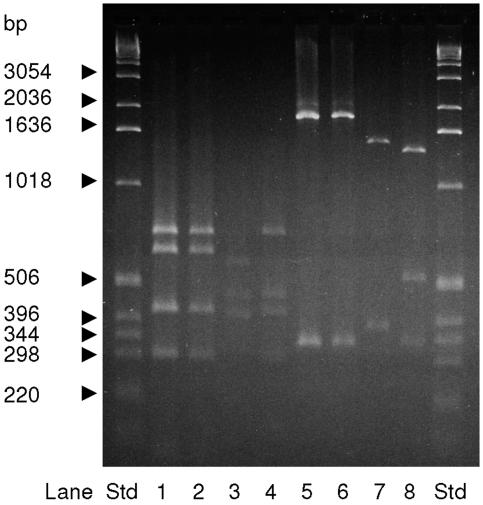
Based on results from previous studies on microheterogeneity in subgroup III strains (2, 57), the tbpB gene was chosen to be characterized for genetic variation. A tbpB PCR product of 2.1 kb, typical of the isotype II tbpB gene, was present in all strains. Restriction fragment length polymorphism analyses of the PCR product with ApoI, HincII, and SspI restriction enzymes showed that all 43 strains from 2000 to 2003 had the same restriction fragment band pattern, compatible with that expected for the tbpB55 allele. Of the 21 strains from 1988 to 1989, 20 presented with a restriction enzyme fragment band pattern similar to that of the tbpB1 control strain (Z1054), and one single strain (Eth 12) presented with a different band pattern comparable to that expected for allele 38 (Table 4 and Fig. 4). Sequencing of the tbpB gene in strains showing different tbpB restriction patterns confirmed the presence of tbpB1 and tbpB55, while the tbpB sequence of strain Eth 12 showed similarity to both tbpB38 and tbpB10 but probably represented a new allele.
FIG. 4.
Typical patterns of tbpB alleles previously found in subgroup III strains digested with the restriction enzyme ApoI (lanes 1 to 4) or SspI (lanes 5 to 8). Lanes 1 and 5, strain Z1054 (control allele 1); lanes 2 and 6, strain Eth 4 (allele 1); lanes 3 and 7, strain Eth 12 (new allele); lanes 4 and 8, strain Mk 502/03 (allele 55). Std, standard.
Fourteen strains representing different LOS types and different expression levels of PorA and NadA were selected for a more thorough comparison of genetic variation. PCR and sequencing of the fetA gene in these 14 strains showed that all encoded epitope F3-1, irrespective of whether they were ST-5 or ST-7 strains. Alignment of the fetA sequences, however, showed that while the eight ST-7 strains were identical to the fetA07 allele in the region sequenced, all six ST-5 strains had the fetA11 allele (Table 4).
PCR of the nadA gene showed that all 14 strains (Table 4) possessed a nadA gene with a similar size. Sequencing of the nadA gene from one strain (Eth 35) showed it to be of allele 3. Sequencing of the promoter area of the nadA gene revealed 6 to 13 copies of the TAAA repeat motif (Table 4). The number of repeats in the 14 strains correlated with the expression of NadA seen with MAb 1079-B6 in the dot blot: there was low or no reaction with 6, 9, and 12 TAAA repeats; moderate reaction with 11 repeats; and strong reaction with 8 and 13 repeat motifs.
PCR of the nine lgt genes showed two patterns in the 14 selected strains: pattern 1, with the presence of lgtABHFG, and pattern 2, with the presence of lgtAHFG (Table 4). On the basis of the organization of their lgt genes, the A:4/21:P1.20,9 strains appeared to belong to LOS genotypes 3 (VII-I-I) and 8 (VIII-I-I) (56). The presence or absence of lgtB was therefore analyzed for all strains, and the results are given in Table 3. There was a statistically significant association between the L11 immunotype and the lack of lgtB (P < 0.0001). Also, there was a significantly higher proportion of lgtB-positive strains originating from the SNNPR (18/27) than from Gondar (2/13) (P = 0.006), while the proportion was similar in old (10/21) and recent (21/43) strains. Seven of the eight L11 strains positive by lgtB PCR that were not included among the subset of 14 isolates in Table 4 were also analyzed for the presence of the other eight lgt genes. All genes showed pattern 1. Curiously, the ST-5 control strain, Z1054, isolated in Finland in the 1970s showed no presence of the lgtG gene in PCR, thus differing from the ST-5 Ethiopian strains (Table 4). Our PCR results for the reference strains were as previously reported (56), except for a positive PCR result for the lgtB gene in strain 126E (Table 4). Sequencing of this PCR product revealed a sequence that was not similar to any Neisseria lgt sequence in GenBank but that had 97% identity to gene NMA0505, encoding a putative ABC transport ATP-binding subunit.
Sequencing of the lgtB gene from three Ethiopian strains, one ST-7 (E6-02) and two ST-5 (Eth 9 and Eth 12) strains, showed that the gene fragment was identical to that of lgtB in strain Z2491 (56). We also explored the sequence variation in the genes lgtA and lgtG using two ST-5 (Eth 9 and Eth 12) and two ST-7 (Mk 686/02 and Mk 804/03) strains. For lgtA, the ST-5 strains were identical in the sequenced fragment to allele 11, while the ST-7 strains were identical to each other and showed high similarity with lgtA17 (502/523 nucleotides). This pattern was further confirmed for the 10 other Ethiopian strains listed in Table 4. All new strains possessed a homopolymeric tract of five guanine residues enabling a functional lgtA gene product, while old strains showed diverse tract lengths (Table 4). For the lgtG gene, the four sequences were identical and showed the highest sequence similarity (547/548 nucleotides identical) to the lgtG9 and lgtG10 alleles. Larger fragments must be sequenced in order to definitively identify the specific lgtG alleles these strains harbor. Different lengths of the homopolymeric cytosine tract were found; however, both ST-5 strains had a tract with 9 residues, and both ST-7 strains had a tract with 10 residues. With these lengths, the gene is predicted to be switched off (5). For the lgtH gene, we found that seven out of eight ST-7 strains and four out of six ST-5 strains harbored the lgtH3 allele. The remaining ST-7 strain was identical except for a single point mutation to lgtH3 (Mk 499/03), while the remaining two ST-5 strains were either identical (Eth 18) or identical except for a single point mutation (Eth 9) to the lgtH gene of strain Z2491.
DISCUSSION
Clinical data.
Of the 95 patients enrolled in the study, 71 had confirmed meningococcal disease by culture or PCR of the CSF. The CFRs found in this study were within the ranges of those reported overall in Ethiopia from 2002 to 2003 (Table 1). Among 132 children ≤14 years of age in Gondar from 1990 to 1994, the CFR for bacterial meningitis was 28%, while the N. meningitidis-specific CFR was 16% (15). CFRs for epidemic meningococcal disease in the meningitis belt range from 3 to 30% (6, 17, 26). They are probably underestimated due to the fact that septicemic patients might die before reaching the health facility (17, 25). The low N. meningitidis-specific CFR observed in our study (4.2%), with few of the patients confirmed with meningococcal disease presenting with ecchymoses or petechiae (Table 2), is most likely due to our inclusion criteria, which focused on the clinical signs of meningitis. The CFR among the 24 cases not confirmed as meningococcal disease was significantly higher. This fits with the observation that meningitis caused by, e.g., S. pneumoniae or H. influenzae, the other major agents of meningitis in Africa, is associated with high CFRs (39). Other microbes might also have been responsible for these unconfirmed cases.
Recovery of meningococci.
In this study, survival of meningococcal strains in CSF inoculated in T-I medium lasted for up to 67 days. Recovery of meningococci from the T-I medium seemed to depend more on factors other than the duration of transportation alone. The porA-specific PCR increased the percentage of patients confirmed as being positive for N. meningitidis from 42% to 75%, showing the benefit of PCR in ascertaining the burden of meningococcal meningitis. Although such a benefit is evident from numerous studies in the meningitis belt (21, 38, 45), even the most basic reagents and equipment remain scarce in hospital laboratories in Ethiopia; only a few reference laboratories in the meningitis belt can afford the relatively costly PCR method for routine testing. Long-term general-capacity building of regional microbiology laboratories and national production of transport medium, e.g., modified T-I medium (M. J. Hughes, M. A. Chang, G. W. Ajello, S. Diarra, F. Bougoudogo, S. E. Schmink, G. A. Barnett, P. L. Raghunathan, T. Popovic, and L. W. Mayer, Abstr. 14th Int. Pathogenic Neisseria Conf., abstr. 94, 2004), might be a more fruitful first step for improving diagnostics.
Characterization of recent strains.
Only genetically and antigenically very homogenous N. meningitidis strains of serogroup A, serotype 4/21, serosubtype P1.20,9, and ST-7 caused the meningitis epidemic in Ethiopia in 2002 and 2003. All strains proved susceptible to all tested antibiotics except sulfonamide, which is in line with other studies in the meningitis belt (12, 14, 19, 22, 38, 42). Resistance to sulfonamide in meningococcal isolates from Ethiopia was seen already in 1970 (55). Resistance to penicillin G and to chloramphenicol (43), which has emerged in other parts of the world in the last two decades, has not yet been documented in the meningitis belt (48). Although some Ethiopian meningococcal strains have been reported to be resistant to chloramphenicol (33), these isolates were not available for confirmation and further characterization.
Comparison of strains from 2000 to 2003 to those from 1988 to 1989.
The strains from the epidemic of 1988 to 1989 were all ST-5, while those collected from 2000 to 2003 were ST-7. ST-7 meningococci were first detected on the African continent in Algeria in 1995 (34, 57). Thus, the replacement of ST-5 with ST-7 in Ethiopia must have happened between 1995 and 2000. In a neighboring country, Sudan, ST-5 meningococci caused the epidemic of 1988 to 1989 (22), while the large epidemic of 1999, with over 33,000 cases, was caused by ST-7 (34). ST-7 has not yet caused epidemics of similar magnitude in Ethiopia. In recent years, additional STs within the ST-5 clonal complex have appeared in Africa: ST-580 in Burkina Faso in 1997 (34), ST-2144 in Sudan and ST-581 in Senegal in 1999, and ST-203 in Gambia and ST-2207 in Burundi in 2002. Also, strains of ST-2859 were reported in Niger and Burkina Faso in two consecutive seasons (2003 and 2004) (http://pubmlst.org/neisseria) (35, 36). The presence of multiple STs in the ST-5 complex might result from the selection of variants in response to changes in the immunity of the populations. This may be a challenge, and close surveillance is required to enable appropriate preventive measures to be initiated in time.
The significance of the phenotypic differences between the recent ST-5 complex strains and the older ones is difficult to judge, as phenotypic data of this kind only provide a “snapshot” of antigen expression under the given in vitro conditions. Still, the results of our dot blot analyses of the Ethiopian strains from 1988 to 1989 were consistent with those of subgroup III strains from Sudan in 1988 (42) and cases related to the Mecca outbreak of 1987 to 1988 (1); OpcA, Opa5a, Opa5f, and Opa5i, for example, were expressed in similar proportions (Table 3). In recent strains, however, the Opa reaction pattern showed the presence of Opa5a, Opa5f, and Opa5h. Some strains did not react with our panel of Opa MAbs, suggesting that new variants might have arisen. A relatively limited number of immunotypes was observed. Immunotypes L10 and L11 predominated, as previously observed in the “Mecca-related” and the Sudanese subgroup III strains (1, 42).
The protein patterns of the strains, as observed by SDS-PAGE, were very similar, with all strains showing the major proteins TdfH, Omp85, PorB, RmpM, Opa, and NspA (35). However, the expression of PorA, OpcA, and NadA and the repertoire of Opa proteins were variable. The level of NadA expression correlated with the number of TAAA repeats in the promoter region of the gene, as reported previously by Martin et al. (32). The significance of this variation for the ability of NadA to mediate adhesion and to induce an immune response remains unclear (32).
We further characterized allelic variation in the genes encoding two proteins with a high degree of variation, TbpB and FetA. TbpB is a surface-exposed protein thought to be important for immunity towards meningococci (29). In line with previous studies of subgroup III strains (57), we found that all the ST-7 strains harbored allele 55 (genocloud 8), while most ST-5 strains harbored allele 1 (genocloud 5). One ST-5 strain (Eth 12) recovered from the epidemic of 1988 to 1989, however, harbored a new allele, which could have been imported anew by DNA transformation from other neisseriae (29). The presence of the opaB94 allele in Eth 12 (Table 5) confirmed that the strain belonged to genocloud 5 (2, 57) and that the tbpB allele was imported independently in ST-5 in Ethiopia.
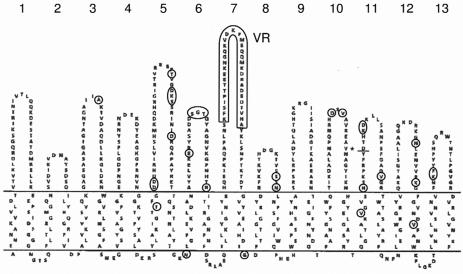
FetA is a hypervariable and phase-variable iron-regulated outer membrane protein to which antibodies are induced following disease or vaccination (47). Most of the variation is found in loop 7 of the proposed FetA topology model and allows for the designation of FetA epitope variants (Fig. 5) (47, 50). The six ST-5 and the eight ST-7 strains all had FetA epitope F3-1. However, the ST-5 strains had the fetA11 allele, while the ST-7 strains had the fetA07 allele. This is in agreement with the analysis of 10 subgroup III strains (47, 49), where fetA alleles 5, 7, 11, 54, and 55 were found among the ST-5 strains and the only ST-7 strain analyzed harbored allele 7. Thus, fetA might be considered another important indicator of microvariation in subgroup III strains. While fetA11 and fetA07 are encoding the same peptide variant, F3-1, they differ from one another in 32 amino acids outside the defined variable region. Most of these differences are located in areas likely to be surface exposed, more specifically, in loops 5, 6, 8, 10, 11, 12, and 13. FetA loops other than the defined main epitope could possess the ability to induce a functional immune response (50); one could speculate that this might be relevant for immune selection, and it should be a subject of further investigation. The full extent of fetA variation needs to be analyzed in a larger collection of ST-5 complex/subgroup III meningococci from multiple countries and time periods.
FIG. 5.
Topology of FetA from strain H44/76 in the outer membrane (based on a figure by Pettersson et al. [40]), with the top part showing the surface-exposed loops and their numbers and with the VR marked with a box. Regions where the fetA07 allele encodes different amino acids than the fetA11 allele are marked with circles; in the VR, the fetA07 and fetA11 alleles encode similar amino acid sequences. *, in this position, fetA07 encodes an insertion of two additional amino acids compared to fetA11.
LOS genotyping of the Ethiopian strains revealed a difference in the occurrence of lgtB. The difference was significantly associated with the geographic origin in the recent strains (P = 0.006); it was not associated with the time of collection (Table 3). lgtB was lacking in strains expressing the L11 LOS type alone (Table 3), implying that lgtB is involved in the biosynthesis of the non-L11 immunotypes. A lack of lgtB is known to result in the loss of the terminal galactose in the lacto-N-neotetraose structure in the oligosaccharide α-chain (16), whereby a strain cannot synthesize L3,7,9 (5). Deletion of the lgtB gene could occur through recombination between repeated DNA fragments at the flanking regions (56), a mechanism that could facilitate escape from the host response. Considering the LOS types (L3,7,9, L8, L10, L11, and L13) expressed in our strains, the observed LOS antigenic variation could also have been caused by the on/off switching of lgtA and lgtG mediated by variable homopolymeric repeat tracts (16), by allelic variation of the lgt genes, or by creation of new mosaic lgtH alleles due to intragenic recombination (56). In addition, the presence or absence of lpt-3, which is required for the synthesis of LOS types L1, L3, L7, and L8 (30), could have contributed to the observed variation. LOS genotyping by simply mapping the presence of the nine lgt genes (56) did not enable the epidemiologically relevant differentiation of the ST-5 complex strains from Ethiopia. However, our study identified different lgtA alleles in ST-5 and ST-7 strains, indicating that LOS biosynthesis-associated genes among ST-5 complex strains could be useful as additional markers of microevolution. However, the impact of allelic variation in lgtA on LOS antigenic structure or virulence is not clear.
Analysis of selected genes in subgroup III meningococci enabled the subdivision of the clonal complex into nine genoclouds (57). Our study showed that microheterogeneity in subgroup III strains occurred in additional genes, such as fetA and lgtA. The comparative proteomic approach, which recently was validated by tbpB analysis of strains from different genoclouds, has identified multiple proteins, which could be useful for resolving fine phylogenetic relationships of the strains (4). The evidence of further differences in antigenic structures among clones of the ST-5 complex, e.g., in TbpB and FetA, might explain the replacement of ST-5 by ST-7 in the African meningitis belt. Serological studies should be performed to test this hypothesis. Alternative hypotheses that could explain this replacement include short-lived or nonprotective immune response following disease, coinfections, or environmental changes.
The meningitis epidemics in northern and southern Ethiopia in 2002 and 2003 were caused by serogroup A N. meningitidis strains of ST-7, which were antigenically and genetically very homogeneous. In this epidemiological situation, polysaccharide conjugate vaccines (23), as well as outer membrane protein-based vaccines (37), could provide long-lasting immunological protection. An affordable conjugate vaccine against serogroup A meningococcal disease will hopefully be available for countries in the meningitis belt within the next decade. Prior to the introduction of these vaccines, country-specific estimates of meningococcal disease burden and serogroup determination of representative disease isolates are required to evaluate their potential impact, as these are the major factors for determining effectiveness, besides vaccine efficacy. Although only serogroup A meningococci were found in our study, serogroup W135 epidemics occurred in Burkina Faso in 2001 and 2002, and an outbreak of W135 meningococci was reported in a neighboring country, Sudan, in 2005 (http://www.who.int/csr/don). The Ethiopian health authorities should therefore enhance their laboratory-based surveillance network in order to detect potential meningococcal strain heterogeneity to be able to provide the appropriate vaccine in time.
Acknowledgments
We thank Elisabeth Fritzsønn, Torill Tangen, Berit Nyland, Anne-Marie Klem, Torill Alvestad, Jan Oksnes, and Girma Berhanu for their excellent technical assistance. Østein Ihle is thanked for helpful advice regarding genetic analyses. M. Achtman, C. E. Frasch, J. Kolberg, J. T. Poolman, P. C. Turner, and W. D. Zollinger are thanked for their generous gifts of MAbs or polyclonal sera. The institutions Yirgalem Hospital, Gondar College of Medical Science, North Gondar Zone Health Bureau, SNNPR Health Bureau, AHRI, and All Africa Leprosy TB and Rehabilitation Training Center are thanked for excellent support in this project. This publication made use of the Neisseria MLST site developed by K. Jolley and M. S. Chan and sited at the University of Oxford.
This work was supported in part by grant 146185/730 from the Research Council of Norway and a grant from the Norwegian “Pasteurlegatet.”
REFERENCES
- 1.Achtman, M., B. Kusecek, G. Morelli, K. Eickmann, J. F. Wang, B. Crowe, R. A. Wall, M. Hassan-King, P. S. Moore, and W. Zollinger. 1992. A comparison of the variable antigens expressed by clone IV-1 and subgroup III of Neisseria meningitidis serogroup A. J. Infect. Dis. 165:53-68. [DOI] [PubMed] [Google Scholar]
- 2.Achtman, M., A. van der Ende, P. Zhu, I. S. Koroleva, B. Kusecek, G. Morelli, I. G. Schuurman, N. Brieske, K. Zurth, N. N. Kostyukova, and A. E. Platonov. 2001. Molecular epidemiology of serogroup A meningitis in Moscow, 1969 to 1997. Emerg. Infect. Dis. 7:420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajello, G. W., J. C. Feeley, P. S. Hayes, A. L. Reingold, G. Bolan, C. V. Broome, and C. J. Phillips. 1984. Trans-Isolate medium: a new medium for primary culturing and transport of Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. J. Clin. Microbiol. 20:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardini, G., G. Renzone, M. Comanducci, R. Mini, S. Arena, C. D'Ambrosio, S. Bambini, L. Trabalzini, G. Grandi, P. Martelli, M. Achtman, A. Scaloni, G. Ratti, and A. Santucci. 2004. Proteome analysis of Neisseria meningitidis serogroup A. Proteomics 4:2893-2926. [DOI] [PubMed] [Google Scholar]
- 5.Berrington, A. W., Y. C. Tan, Y. Srikhanta, B. Kuipers, P. van der Ley, I. R. Peak, and M. P. Jennings. 2002. Phase variation in meningococcal lipooligosaccharide biosynthesis genes. FEMS Immunol. Med. Microbiol. 34:267-275. [DOI] [PubMed] [Google Scholar]
- 6.Campagne, G., A. Schuchat, S. Djibo, A. Ousseini, L. Cisse, and J. P. Chippaux. 1999. Epidemiology of bacterial meningitis in Niamey, Niger, 1981-96. Bull. W. H. O. 77:499-508. [PMC free article] [PubMed] [Google Scholar]
- 7.Castellani, A. 1939. La meningite cerebro-spinale endemique en Afrique orientale Italienne. Bull. Off. Int. Hyg. Publ. 31:455-456. [Google Scholar]
- 8.Caugant, D. A. 1998. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS 106:505-525. [PubMed] [Google Scholar]
- 9.Caugant, D. A., E. A. Høiby, L. O. Frøholm, and P. Brandtzaeg. 1996. Polymerase chain reaction for case ascertainment of meningococcal meningitis: application to the cerebrospinal fluids collected in the course of the Norwegian meningococcal serogroup B protection trial. Scand. J. Infect. Dis. 28:149-153. [DOI] [PubMed] [Google Scholar]
- 10.CLSI. 2005. Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement [M100-S15]. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 11.Comanducci, M., S. Bambini, B. Brunelli, J. Adu-Bobie, B. Arico, B. Capecchi, M. M. Giuliani, V. Masignani, L. Santini, S. Savino, D. M. Granoff, D. A. Caugant, M. Pizza, R. Rappuoli, and M. Mora. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe, B. A., R. A. Wall, B. Kusecek, B. Neumann, T. Olyhoek, H. Abdillahi, M. Hassan-King, B. M. Greenwood, J. T. Poolman, and M. Achtman. 1989. Clonal and variable properties of Neisseria meningitidis isolated from cases and carriers during and after an epidemic in The Gambia, West Africa. J. Infect. Dis. 159:686-700. [DOI] [PubMed] [Google Scholar]
- 13.Frasch, C. E., and L. F. Mocca. 1978. Heat-modifiable outer membrane proteins of Neisseria meningitidis and their organization within the membrane. J. Bacteriol. 136:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagneux, S., A. Hodgson, I. Ehrhard, G. Morelli, B. Genton, T. Smith, M. Tanner, F. Binka, M. Achtman, and G. Pluschke. 2000. Microheterogeneity of serogroup A (subgroup III) Neisseria meningitidis during an outbreak in northern Ghana. Trop. Med. Int. Health 5:280-287. [PubMed] [Google Scholar]
- 15.Gedlu, E., and S. I. Rahlenbeck. 1995. Pyogenic meningitis in children in north-western Ethiopia. Ann. Trop. Paediatr. 15:243-247. [DOI] [PubMed] [Google Scholar]
- 16.Gotschlich, E. C. 1994. Genetic locus for the biosynthesis of the variable portion of Neisseria gonorrhoeae lipooligosaccharide. J. Exp. Med. 180:2181-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwood, B. M., A. K. Bradley, A. W. Smith, and R. A. Wall. 1987. Mortality from meningococcal disease during an epidemic in The Gambia, West Africa. Trans. R. Soc. Trop. Med. Hyg. 81:536-538. [DOI] [PubMed] [Google Scholar]
- 18.Habte-Gabr, E., E. Tekle, and M. Mamo. 1984. Meningococcal meningitis in Ethiopia 1974-1983 and strategies of control. Ethiop. J. Health Dev. 1:47-63. [Google Scholar]
- 19.Haimanot, R. T., D. A. Caugant, D. Fekadu, G. Bjune, B. Belete, L. O. Frøholm, E. A. Høiby, E. Rosenqvist, R. K. Selander, and B. Bjorvatn. 1990. Characteristics of serogroup A Neisseria meningitidis responsible for an epidemic in Ethiopia, 1988-89. Scand. J. Infect. Dis. 22:171-174. [DOI] [PubMed] [Google Scholar]
- 20.Hughes, J. H., D. J. Biedenbach, M. E. Erwin, and R. N. Jones. 1993. Etest as susceptibility test and epidemiologic tool for evaluation of Neisseria meningitidis isolates. J. Clin. Microbiol. 31:3255-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issa, M., P. Mölling, A. Bäckman, M. Unemo, N. Sulaiman, and P. Olcén. 2003. PCR of cerebrospinal fluid for diagnosis of bacterial meningitis during meningococcal epidemics; an example from Sudan. Scand. J. Infect. Dis. 35:719-723. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsson, S., M. Issa, M. Unemo, A. Bäckman, P. Mölling, N. Sulaiman, and P. Olcén. 2003. Molecular characterisation of group A Neisseria meningitidis isolated in Sudan 1985-2001. APMIS 111:1060-1066. [DOI] [PubMed] [Google Scholar]
- 23.Jodar, L., F. M. LaForce, C. Ceccarini, T. Aguado, and D. M. Granoff. 2003. Meningococcal conjugate vaccine for Africa: a model for development of new vaccines for the poorest countries. Lancet 361:1902-1904. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. J., N. J. Phillips, B. W. Gibson, J. M. Griffiss, and R. Yamasaki. 1994. Meningococcal group A lipooligosaccharides (LOS): preliminary structural studies and characterization of serotype-associated and conserved LOS epitopes. Infect. Immun. 62:1566-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kristos, T. G., and L. Muhe. 1993. Epidemic meningococcal meningitis in children. A retrospective analysis of cases admitted to ESCH (1988). Ethiop. Med. J. 31:9-14. [PubMed] [Google Scholar]
- 26.Lapeyssonnie, L. 1963. Cerebrospinal meningitis in Africa. Bull. W. H. O. 28:3-114. [PMC free article] [PubMed] [Google Scholar]
- 27.Legrain, M., B. Rokbi, D. Villeval, and E. Jacobs. 1998. Characterization of genetic exchanges between various highly divergent tbpBs, having occurred in Neisseria meningitidis. Gene 208:51-59. [DOI] [PubMed] [Google Scholar]
- 28.Lesse, A. J., A. A. Campagnari, W. E. Bittner, and M. A. Apicella. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 126:109-117. [DOI] [PubMed] [Google Scholar]
- 29.Linz, B., M. Schenker, P. Zhu, and M. Achtman. 2000. Frequent interspecific genetic exchange between commensal neisseriae and Neisseria meningitidis. Mol. Microbiol. 36:1049-1058. [DOI] [PubMed] [Google Scholar]
- 30.Mackinnon, F. G., A. D. Cox, J. S. Plested, C. M. Tang, K. Makepeace, P. A. Coull, J. C. Wright, R. Chalmers, D. W. Hood, J. C. Richards, and E. R. Moxon. 2002. Identification of a gene (lpt-3) required for the addition of phosphoethanolamine to the lipopolysaccharide inner core of Neisseria meningitidis and its role in mediating susceptibility to bactericidal killing and opsonophagocytosis. Mol. Microbiol. 43:931-943. [DOI] [PubMed] [Google Scholar]
- 31.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, P., K. Makepeace, S. A. Hill, D. W. Hood, and E. R. Moxon. 2005. Microsatellite instability regulates transcription factor binding and gene expression. Proc. Natl. Acad. Sci. USA 102:3800-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mengistu, G., K. Mitiku, and W. Teferi. 2003. Analysis and reporting of meningococcal meningitis epidemic in north Gondar 2001-2002. Ethiop. Med. J. 41:319-331. [PubMed] [Google Scholar]
- 34.Nicolas, P., L. Decousset, V. Riglet, P. Castelli, R. Stor, and G. Blanchet. 2001. Clonal expansion of sequence type (ST-)5 and emergence of ST-7 in serogroup A meningococci, Africa. Emerg. Infect. Dis. 7:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicolas, P., G. Norheim, E. Garnotel, S. Djibo, and D. A. Caugant. 2005. Molecular epidemiology of Neisseria meningitidis isolated in the African meningitis belt between 1988 and 2003 shows dominance of sequence type 5 (ST-5) and ST-11 complexes. J. Clin. Microbiol. 43:5129-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Njanpop-Lafourcade, B. M., I. Parent du Chatelet, O. Sanou, J. M. Alonso, and M. K. Taha. 2005. The establishment of Neisseria meningitidis serogroup W135 of the clonal complex ET-37/ST-11 as an epidemic clone and the persistence of serogroup A isolates in Burkina Faso. Microbes Infect. 7:645-649. [DOI] [PubMed] [Google Scholar]
- 37.Norheim, G., A. Aase, D. A. Caugant, E. A. Høiby, E. Fritzsønn, T. Tangen, P. Kristiansen, U. Heggelund, and E. Rosenqvist. 2005. Development and characterisation of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis. Vaccine 23:3762-3774. [DOI] [PubMed] [Google Scholar]
- 38.Parent du Chatelet, I., Y. Traore, B. D. Gessner, A. Antignac, B. Naccro, B. M. Njanpop-Lafourcade, M. S. Ouedraogo, S. R. Tiendrebeogo, E. Varon, and M. K. Taha. 2005. Bacterial meningitis in Burkina Faso: surveillance using field-based polymerase chain reaction testing. Clin. Infect. Dis. 40:17-25. [DOI] [PubMed] [Google Scholar]
- 39.Peltola, H. 2001. Burden of meningitis and other severe bacterial infections of children in Africa: implications for prevention. Clin. Infect. Dis. 32:64-75. [DOI] [PubMed] [Google Scholar]
- 40.Pettersson, A., A. Maas, D. van Wassenaar, P. van der Ley, and J. Tommassen. 1995. Molecular characterization of FrpB, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect. Immun. 63:4181-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popovic, T., G. W. Ajello, R. Racklam, D. A. Caugant, P. Nicolas, B. A. Perkins, N. E. Rosenstein, and E. Tikhomirov. 1999. Laboratory manual for the diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. Document no. WHO/CDS/CSR/EDC/99.7. World Health Organization, Geneva, Switzerland.
- 42.Salih, M. A., D. Danielsson, A. Bäckman, D. A. Caugant, M. Achtman, and P. Olcén. 1990. Characterization of epidemic and nonepidemic Neisseria meningitidis serogroup A strains from Sudan and Sweden. J. Clin. Microbiol. 28:1711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shultz, T. R., J. W. Tapsall, P. A. White, C. S. Ryan, D. Lyras, J. I. Rood, E. Binotto, and C. J. Richardson. 2003. Chloramphenicol-resistant Neisseria meningitidis containing catP isolated in Australia. J. Antimicrob. Chemother. 52:856-859. [DOI] [PubMed] [Google Scholar]
- 44.Suker, J., I. M. Feavers, M. Achtman, G. Morelli, J. F. Wang, and M. C. Maiden. 1994. The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol. Microbiol. 12:253-265. [DOI] [PubMed] [Google Scholar]
- 45.Taha, M. K. 2000. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J. Clin. Microbiol. 38:855-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tedla, T. 1993. Meningococcal meningitis, p. 285-293. In H. Kloos and A. Zein (ed.), Health and disease in Ethiopia. Westview Press, Boulder, Colo.
- 47.Thompson, E. A., I. M. Feavers, and M. C. Maiden. 2003. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 149:1849-1858. [DOI] [PubMed] [Google Scholar]
- 48.Tondella, M. L., N. E. Rosenstein, L. W. Mayer, F. C. Tenover, S. A. Stocker, M. W. Reeves, and T. Popovic. 2001. Lack of evidence for chloramphenicol resistance in Neisseria meningitidis, Africa. Emerg. Infect. Dis. 7:163-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urwin, R., J. E. Russell, E. A. Thompson, E. C. Holmes, I. M. Feavers, and M. C. Maiden. 2004. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect. Immun. 72:5955-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Ley, P., J. van der Biezen, R. Sutmuller, P. Hoogerhout, and J. T. Poolman. 1996. Sequence variability of FrpB, a major iron-regulated outer-membrane protein in the pathogenic neisseriae. Microbiology 142:3269-3274. [DOI] [PubMed] [Google Scholar]
- 51.Wedege, E. 2001. Immunoblot analysis of sera from patients and vaccinees, p. 275-288. In A. J. Pollard and M. C. Maiden (ed.), Methods in molecular medicine: meningococcal vaccines. Methods and protocols. Humana Press, Totowa, N.J. [DOI] [PubMed]
- 52.Wedege, E., E. A. Høiby, E. Rosenqvist, and L. O. Frøholm. 1990. Serotyping and subtyping of Neisseria meningitidis isolates by co-agglutination, dot-blotting and ELISA. J. Med. Microbiol. 31:195-201. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. 1998. Control of epidemic meningococcal disease. WHO practical guidelines. Document no. WHO/EMC/BAC/98.3. World Health Organization, Geneva, Switzerland.
- 54.World Health Organization. 2002. Meningococcal disease, serogroup W135, Burkina Faso: preliminary report 2002. Wkly. Epidemiol. Rec. 77:152-155. [PubMed] [Google Scholar]
- 55.Wright, L. J., and J. J. Plorde. 1970. Group-A sulfadiazine-resistant Neisseria meningitidis in Ethiopia. Lancet ii:1033. [DOI] [PubMed] [Google Scholar]
- 56.Zhu, P., M. J. Klutch, M. C. Bash, R. S. Tsang, L. K. Ng, and C. M. Tsai. 2002. Genetic diversity of three lgt loci for biosynthesis of lipooligosaccharide (LOS) in Neisseria species. Microbiology 148:1833-1844. [DOI] [PubMed] [Google Scholar]
- 57.Zhu, P., A. van der Ende, D. Falush, N. Brieske, G. Morelli, B. Linz, T. Popovic, I. G. Schuurman, R. A. Adegbola, K. Zurth, S. Gagneux, A. E. Platonov, J. Y. Riou, D. A. Caugant, P. Nicolas, and M. Achtman. 2001. Fit genotypes and escape variants of subgroup III Neisseria meningitidis during three pandemics of epidemic meningitis. Proc. Natl. Acad. Sci. USA 98:5234-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]