Abstract
Clostridium perfringens strains (type A) isolated from an integrated poultry operation were subtyped using repetitive-element PCR with Dt primers. Isolates were obtained from fecal, egg shell, fluff, and carcass rinse samples as part of a previously reported temporally linked epidemiological survey. A total of 48 isolates of C. perfringens were obtained from different stages of the broiler chicken production chain from two separate breeder farms that supplied a single hatchery that in turn provided chicks to a single grow-out farm whose flocks were processed at a single plant. All 48 isolates were typeable (100% typeability) by repetitive-element PCR with Dt primers. This subtyping method was highly reproducible and discriminatory. By repetitive-element PCR with Dt primers, isolates were classified into four major branches with 12 subgroups or clades. The Simpson's index of discrimination was calculated to be 0.96 for groupings of >95% correlation. Toxin gene profiles of the isolates indicated that all of the isolates were C. perfringens alpha-toxin gene positive and 46 of 48 isolates were beta2-toxin gene positive. All strains were negative for beta- and epsilon-toxin genes. Repetitive sequence-based PCR was found to be a technically practical and reproducible means of subtyping C. perfringens libraries from specific epidemiological or production environment settings.
Clostridium perfringens plays a significant role in food-borne human disease as well as non-food-borne human, animal, and poultry disease (34). C. perfringens enteritis negatively impacts the integrated system of poultry production (1, 6, 7, 23-25, 48, 58). A common commensal inhabitant of the healthy broiler chicken gut microflora, C. perfringens is frequently found in the feces of livestock and poultry at high levels (51). However, its overgrowth in fowl can be considered an imbalance of the gut ecosystem at the microbial level resulting in gastrointestinal dysbacteriosis and necrotic enteritis (58). Poultry necrotic enteritis is associated with predisposing factors (9) such as Eimeria spp. infections (coccidiosis); the incorporation of dietary fish meal, rye, barley, and wheat as major feed components; and the withdrawal of dietary subtherapeutic antibiotic growth promotants (AGPs). While the impact of AGP withdrawal on chicken health, welfare, and production efficiency has been studied to a limited degree (4), it is known that the exclusion of ionophore coccidiostat antibiotics, which are generally anticlostridial, from the broiler chicken diet has resulted in higher rates of necrotic enteritis in broiler chickens raised in AGP-free settings (8, 9).
C. perfringens types are classified based on major toxin production into types A, B, C, D, or E, depending on the toxin profile (3, 32, 35, 39). The genotype of cpa (alpha-toxin or phospholipase C) with cpe (Clostridium perfringens enterotoxin) is generally associated with human food-borne disease strains. Human disease-associated food isolates of C. perfringens generally have a chromosomally located cpe gene, while the cpe locus of non-food-borne isolates is episomally or plasmid located (5, 49). Miwa et al. (36), for example, reported that 84% of chicken meat tested was found to be C. perfringens positive, while only 12% of the chicken meat was cpe positive. Likewise, Sparks et al. (49) reported that 2 to 5% of C. perfringens isolates examined harbored the cpe gene and produced C. perfringens enterotoxin. Wen and McClane (59) observed that 1.4% of C. perfringens isolates from retail foods were cpe gene positive. Among libraries composed of only human food-borne disease outbreak-related C. perfringens strains, about 70% were cpe gene positive (29). The relative scarcity of cpe-positive strains of C. perfringens among nonoutbreak strains in food isolate libraries has been reported previously (42). From a survey of 131 food samples, Lin and Labbe (25) found that none of the 40 C. perfringens isolates obtained harbored cpe genes, whereas all were cpa positive.
A basic premise underlying microbial subtyping is that strains obtained or isolated from an epidemiologically related cluster arise from a common precursor or clone and that, subsequently, these strains share common characteristics that distinguish them from epidemiologically unrelated strains classified as the same species (41). Since it is entirely possible to obtain identical subtypes from totally unrelated sources regardless of the subtyping method, it is imperative that subtyping data for source tracking only be evaluated against a known epidemiologic backdrop or context; otherwise, these data have little or no meaning (60). Subtyping protocols are evaluated on performance parameters including typeability, discrimination, and practical concerns, e.g., ease of use, cost, etc. (16, 38). Several subtyping methods have been used for Clostridium spp. and C. perfringens that included a serotyping scheme for C. perfringens (11, 15). It has been previously reported that not all isolates were agglutinable in the serotyping scheme when it was used on C. perfringens food-borne disease isolates (63). The amplified fragment length polymorphism (AFLP) technique was utilized to subtype 35 isolates of C. perfringens from seven suspected outbreaks (33), which illustrated that different subtypes were associated with each different outbreak source. Interestingly, these same AFLP subtypes were nearly completely congruent with the serotype-based characterization. A multilocus enzyme electrophoresis typing scheme was previously used for typing of human and animal isolates of C. perfringens (40, 53). Pulsed-field gel electrophoresis (PFGE) has been used for subtyping of Clostridium spp. including C. perfringens (10, 13, 17, 18, 22, 29, 31, 37, 43-46); however, less-than-complete typeability has been reported. For example, Maslanka et al. (31) utilized PFGE (SmaI digestion) for subtyping of C. perfringens food-borne disease outbreak strains and found that of 62 C. perfringens isolates tested, 8% were untypeable by PFGE. The inconsistent typeability of clostridia by PFGE is widely thought to be due to the presence of active nucleases and recalcitrance to cell wall lysis (2). Ribotyping has been applied to subtyping of Clostridium spp. from human food-borne disease (14) and poultry operations (7).
Repetitive DNA sequences are ubiquitous in prokaryotic microbial genomes (30). The discovery of repetitive extragenic palindromic (REP) elements in the genomes of Escherichia coli and Salmonella enterica serovar Typhimurium (30) subsequently led to the discovery of the presence of many highly conserved repetitive, intergenic DNA sequence regions in several diverse members of the Bacteria, Archaea, and Eucarya taxa (30). At between 33 and 40 bp in length, REP elements occupy about 1% of the source bacterial genomes in E. coli and Salmonella, representing 500 to 1,000 copies per genome. Another enterobacterium-derived repetitive element, enterobacterial repetitive intergenic consensus (ERIC), was counted at about 30 to 150 copies per genome and ranges from 124 bp to 127 bp in size. The first such repetitive intergenic sequences for gram-positive species, termed BOX elements (52), were found in Streptococcus pneumoniae. Collectively, PCR assays targeting these highly conserved sequences for use in strain differentiation or subtyping are known as repetitive-element PCR (26, 27, 56, 57, 62). Under standard conditions of high stringency, repetitive-element PCR appears not to be strictly random priming, since its primers (∼18-mers) amplify regions conserved in bacterial and archaeal genomes. On the other hand, it is not impossible that amplification of repetitive sequences associated with gram-negative bacteria, e.g., REP and ERIC, in gram-positive strains results in patterns more attributable to a random amplification. This is especially true in lower-stringency procedures using low primer annealing temperatures where less-specific primer binding results in amplification. However, as in the example documented in this current set of experiments with C. perfringens as well as in previously reported examples using Streptococcus and Listeria spp. (19), subtyping was accomplished at a relatively high annealing temperature with discrimination, reproducibility, and rapidity.
Subtype variation in C. perfringens isolates from poultry has been documented using PFGE and AFLP (10, 37) as well as automated ribotyping (7). Herein, we present experimental studies in which we utilized repetitive-element PCR to subtype C. perfringens isolates from the poultry production environment taken from each of the main steps of broiler meat production, namely, the hatchery, breeder, grow-out farm, and processing stages, and discuss our findings as an alternative means to subtype this organism. We also include a comparison of repetitive-element PCR subtyping with automated ribotyping of this same isolate set (7) from a previous study that suggested the transmission of subtypes between and among the different production stages.
(Portions of this research have been previously presented [G. R. Siragusa, et al., Abstr. Gen. Meet. Am. Soc. Microbiol., abstr. P-73, 2002].)
MATERIALS AND METHODS
Bacterial isolates and growth conditions.
Source descriptions, month of isolation (all within the same year), and laboratory codes of Clostridium perfringens strains are provided in Table 1. All isolates were obtained and characterized, as presented previously (6, 7), from a national poultry epidemiological survey conducted by the Poultry Microbiological Safety Research Unit of the ARS, USDA. Isolates were propagated from cooked meat medium by streaking onto tryptic soy agar plates or tryptic soy agar with 5% defibrinated sheep blood (Difco Laboratories, Detroit, MI, and REMEL Laboratories, Lenexa, KS) for single, isolated colonies. Plates were incubated overnight in anaerobic jars containing AnaeroGen sachets (Oxoid, Hampshire, England) at 37°C. Single colonies were selected and streaked for maximal growth onto tryptic soy agar or sheep blood agar plates and incubated under anaerobic conditions overnight at 37°C. The resulting colonies were swabbed into 2.0 ml phosphate-buffered saline, pH 7.4, for cell suspensions to obtain genomic DNA as described below.
TABLE 1.
C. perfringens isolates used in this study
| Isolate | Sample day and montha | Sample source | Sampling site | Geneb
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| C. perfringens 16S rRNA | cpe | cpa | cpb | cpb2 | etx | ||||
| CP-6 | 3 Feb | Breeder 1 | Feces | + | − | + | − | + | − |
| CP-7 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-8 | 3 Feb | Breeder 1 | Feces | + | − | + | − | + | − |
| CP-9 | 3 Feb | Breeder 1 | Feces | + | − | + | − | + | − |
| CP-10 | 3 Feb | Breeder 1 | Feces | + | − | + | − | − | − |
| CP-11 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-12 | 3 Feb | Breeder 1 | Feces | + | − | + | − | − | − |
| CP-13 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-14 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-15 | 3 Feb | Breeder 1 | Feces | + | − | + | − | + | − |
| CP-16 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-17 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-18 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-19 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-20 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-21 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-22 | 27 Mar | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-23 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-24 | 4 Apr | Processing plant | Carcass rinse | + | − | + | − | + | − |
| CP-25 | 14 Mar | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-26 | 14 Mar | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-27 | 24 Feb | Hatchery | Fluff | + | − | + | − | + | − |
| CP-28 | 24 Feb | Hatchery | Fluff | + | − | + | − | + | − |
| CP-29 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-30 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-31 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-32 | 24 Feb | Hatchery | Fluff | + | − | + | − | + | − |
| CP-33 | 24 Feb | Hatchery | Fluff | + | − | + | − | + | − |
| CP-34 | 27 Mar | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-35 | 28 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-36 | 24 Feb | Hatchery | Shells | + | − | + | − | + | − |
| CP-37 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-38 | 4 Apr | Processing plant | Carcass rinse | + | − | + | − | + | − |
| CP-39 | 4 Apr | Processing plant | Carcass rinse | + | − | + | − | + | − |
| CP-40 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-41 | 4 Apr | Processing plant | Carcass rinse | + | − | + | − | + | − |
| CP-42 | 4 Apr | Processing plant | Carcass rinse | + | − | + | − | + | − |
| CP-43 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-44 | 4 Apr | Processing plant | Carcass rinse | + | − | + | − | + | − |
| CP-45 | 24 Feb | Hatchery | Shells | + | − | + | − | + | − |
| CP-46 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-47 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-48 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-49 | 4 Apr | Processing plant | Carcass rinse | + | − | + | − | + | − |
| CP-50 | 4 Apr | Processing plant | Carcass rinse | + | − | + | − | + | − |
| CP-51 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
| CP-52 | 3 Feb | Grow-out farm | Feces | + | − | + | − | + | − |
| CP-53 | 18 Feb | Breeder 2 | Feces | + | − | + | − | + | − |
Feb, February; Mar, March.
+ indicates the presence and − indicates the absence of the indicated gene.
DNA isolation and purification.
DNA was isolated using the UltraClean microbial genomic DNA isolation kit (MoBio Laboratories Inc., Solana Beach, CA) according to the manufacturer's instructions. The resulting DNA size was checked by electrophoresis in 1% (wt/vol) SeaKem LE agarose (BioWhittaker Molecular Applications, Rockland, ME) minigels prepared and run with 0.5× Tris-borate-EDTA buffer at a constant voltage of 120 V. DNA concentrations were determined spectrophotometrically using a GeneQuant RNA/DNA calculator (Amersham Pharmacia Biotech, Piscataway, NJ).
Gene profiling.
Using DNA samples obtained as described above, all isolates were genotypically congruent with the taxon of C. perfringens (61). Toxin profiling and C. perfringens species-specific 16S rRNA-DNA analysis were conducted as previously described (35, 61). C. perfringens toxin designations are as follows: cpa, alpha-toxin; cpb, beta-toxin; cpb2, beta2 toxin; cpe, C. perfringens enterotoxin; etx, epsilon-toxin.
Repetitive sequence-based PCR subtyping of C. perfringens DNA.
Repetitive sequence-based PCR was performed using the RepPro repetitive sequence-based PCR DNA fingerprinting kit with Uprime Dt primer sets according to the manufacturer's recommendations (Bacterial Barcodes, Inc., Athens, GA) (54). For all repetitive-element PCR assays, Amplitaq DNA polymerase (ABI, Inc., Foster City, CA) was used. Thermocycling procedures were performed with a PTC-225 Peltier thermal cycler (MJ Research, Waltham, MA) as follows: an initial denaturation step at 95°C for 2 min, a denaturation step at 94°C for 3 s and 92°C for 30 s, an annealing step at 40°C for 1 min, and an extension step at 65°C for 8 min, for 31 total cycles (denaturation to extension), followed by a final extension step at 65°C for 8 min. Amplicons from the repetitive-element PCR were then separated by electrophoresis on 1.5% SeaKem LE agarose (BioWhittaker Molecular Applications) gels (25 cm by 25 cm; Bio-Rad Sub Cell model 192 with buffer recirculation) prepared in running buffer (1× Tris-acetate-EDTA buffer) containing 3.0 μg/ml of ethidium bromide at a constant voltage of 120 V. Run times were approximately 6 h. Amplicon samples (10 μl) were premixed with 2 μl of loading buffer, and the entire mixture volume was loaded into wells. DNA standards of ∼200 to 5,000 bp (Spectral Genomics/Bacterial BarCodes, Inc., Houston, TX) were run concomitantly. Gels were photographed with a UV transilluminator (BioChemi Imager System; UVP, Inc., Upland, CA [or MultiImage light cabinet and FluorChem v1.02a software; Alpha Inotech Corp., San Leandro, CA]). Unless noted, all chemicals and buffer components were obtained from Sigma Chemical Co. (St. Louis, MO). All repetitive-element PCR analyses were performed in duplicate from each genomic DNA sample. Duplicate analyses were indistinguishable, and phylogenetic trees constructed using single determinations (patterns) are presented herein this paper.
RiboPrint subtyping of C. perfringens isolates.
RiboPrint patterns were obtained from a previous study (6, 7). Ribotype analyses of these isolates were performed using an automated system, the DuPont (Wilmington, DE) Qualicon RiboPrinter, with EcoRI digestion. RiboPrint patterns were downloaded into the BioNumerics analysis software and analyzed as described below.
Pattern analysis.
Resulting repetitive-element PCR and RiboPrint patterns were analyzed by using BioNumerics software (BioSystematica, Devon, United Kingdom). All images were normalized by alignment to the positive control and molecular weight markers included in each gel. Dendrograms were constructed using the unweighted-pair group method with arithmetic mean feature.
Discriminatory index.
Simpson's discriminatory index (Ds), as previously presented by Hunter and Gaston (16), and as utilized according to the method described previously by Snelling et al. (47), was calculated from groupings or observed subtypes with >95% correlation (Pearson coefficient) using the following equation: 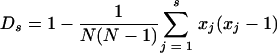 , where N is the total number of strains in the sample population being examined, s is the total number of types described, and xj is the number of isolates belonging to the jth subtype (16). This estimates the probability that two unrelated and different isolates or strains will be grouped as different subtypes by a specific typing method.
, where N is the total number of strains in the sample population being examined, s is the total number of types described, and xj is the number of isolates belonging to the jth subtype (16). This estimates the probability that two unrelated and different isolates or strains will be grouped as different subtypes by a specific typing method.
RESULTS
All C. perfringens isolates obtained in this study were collectively part of a vertically integrated commercial poultry production continuum. Two geographically distinct breeder farms supplied eggs to a common hatchery. The resulting hatchlings chicks proceeded to a common grow-out farm and finally to a single common processing plant. Of the 48 C. perfringens strains, all were positive for the 16S rRNA-DNA C. perfringens species-specific signature marker and alpha-toxin (cpa) and negative for beta-toxin (cpb) and epsilon-toxin (etx) genes (Table 1). All isolates but two isolates, CP-10 and CP-12, were positive for the beta2-toxin (cpb2). All isolates were cpe negative. Based on phenotype and gene profiling, the isolates studied here were classified as type A C. perfringens strains.
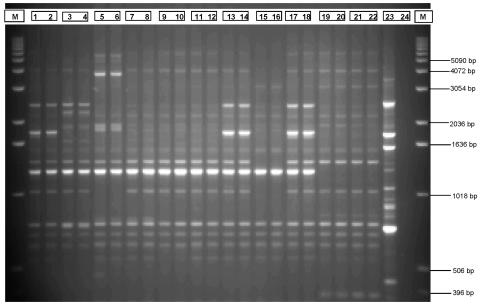
Using both methods of molecular subtyping, repetitive-element PCR with Dt primers and EcoRI ribotyping, all C. perfringens isolates were typeable. All isolated DNA samples analyzed by repetitive-element PCR with Dt primers were high-molecular-weight intact DNA with little or no visible smearing on agarose check gels (data not shown). Duplicate reactions and resulting patterns of repetitive-element PCR with Dt primers were highly reproducible and virtually indistinguishable (Fig. 1).
FIG. 1.
Examples of typical duplicate patterns obtained using repetitive-element PCR with Dt primers for C. perfringens isolates obtained from a poultry production continuum. Lanes (lane numbers are followed by isolates): M, DNA molecular mass reference ladder; 1 and 2, CP-29; 3 and 4, CP-30; 5 and 6, CP-31; 7 and 8, CP-32; 9 and 10, CP-33; 11 and 12, CP-34; 13 and 14, CP-35; 15 and 16, CP-36; 17 and 18, CP-37; 19 and 20, CP-38; 21 and 22, CP-17; 23, positive control; 24, negative control. Refer to Table 1 for isolate source information.
The calculated Simpson's indices of discrimination and observed groupings were similar for both methods considered at the 95% Pearson correlation coefficient level. A greater disparity in these numerical indices was observed at lower levels of correlation (Table 2). For the purposes of discussion and data interpretation, a line drawn at the 80% correlation level threshold was used to assign second-level subtype groups (Fig. 2 and 3, indicated by capital letters). Based on our observations, major branches of each dendrogram were assigned a roman numeral. Each typing method resulted in four such major groups (groups I to IV) (Fig. 2 and 3).
TABLE 2.
Observed subtypes and Simpson's index of discriminatory power at two correlation levels for repetitive-element PCR with Dt primers and ribotyping (EcoRI) of poultry-associated C. perfringens isolates
| Method | Correlation level (%) | Observed subtype | Dsa |
|---|---|---|---|
| Repetitive-element PCR with Dt primers | 95 | 21 | 0.961 |
| 90 | 19 | 0.938 | |
| 80 | 12 | —b | |
| 70 | 9 | — | |
| 60 | 7 | — | |
| EcoRI ribotyping | 95 | 23 | 0.960 |
| 90 | 14 | 0.873 | |
| 80 | 8 | — | |
| 70 | 5 | — | |
| 60 | 4 | — |
See Materials and Methods.
—, not determined.
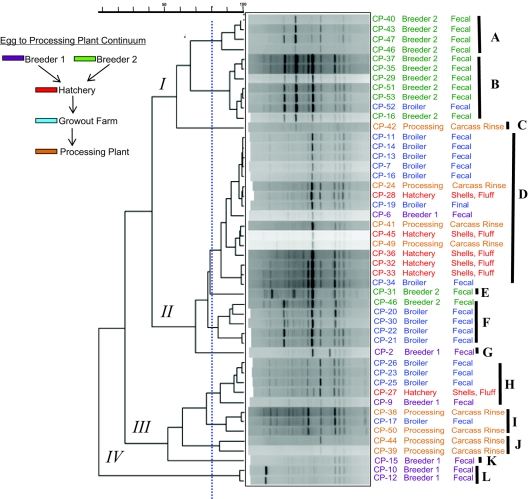
FIG. 2.
Dendrogram of pattern subtypes of C. perfringens isolates obtained from a poultry production continuum using repetitive element PCR with Dt primers. Isolate number, source, and source material are indicated next to each pattern. The dashed vertical line indicates the 80% correlation level, and roman numerals and capital letters are visually observed major and minor branch groups based on approximately 50% and 80% correlation cutoff levels, respectively.
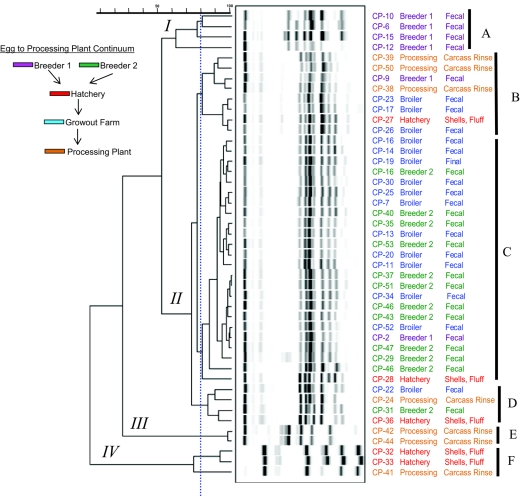
FIG. 3.
Dendrogram of EcoRI RiboPrint patterns of C. perfringens isolates obtained from a poultry production continuum. Isolate number, source, and source material are indicated next to each pattern. The dashed vertical line indicates the 80% correlation level, and roman numerals and capital letters are visually observed major and minor branch groups based on approximately 50% and 80% correlation cutoff levels, respectively.
By the Simpson's numerical descriptor, the two subtyping methods performed alike; however, the similarity ends at that point. Typing based on repetitive-element PCR with Dt primers resulted in a strong partitioning of isolates from breeder farm 2 (breeder 2) into clades I-A and -B (Fig. 2). Only two breeder 2 isolates were placed outside of major branch grouping I. The remaining two breeder 2 isolates grouped into two different clades, II-E and II-F. Isolates from the breeder 1 source did not cluster as closely as did those from breeder 2 and were distributed through clades II, III, and IV. Two unique and highly similar patterns obtained by repetitive-element PCR with Dt primers forming clade IV-L were also the only constituents of major branch IV, and these were the only two isolates found to be beta2-toxin negative (CP-10 and CP-12). One notable difference in patterns obtained by repetitive-element PCR with Dt primers from clade IV-L is the presence of an approximately >5-kb dense band that was absent from the other isolate patterns.
Broiler farm fecal C. perfringens isolates representing the grow-out phase of production were grouped into clades I-B, II-D, II-F, III-H, and III-I (Fig. 2). Grow-out farm strains clustered mainly in clades II-D, II-F, and III-H. Hatchery-derived isolates clustered in group II-D, with the only other isolate grouping into clade III-H. Processing-plant-derived isolates were distributed across clades I-C, II-D, III-I, and III-J, with the most isolates categorized into clade II-D followed by clades III-I-J and II-C. Interestingly, clade II-D contains only a single isolate from a breeder flock (breeder 1) and is otherwise dominated by hatchery farm- and processor-derived isolate patterns, suggesting an environmental or independently acquired isolate.
Subtyping by automated EcoRI ribotyping also accomplished 100% typeability with the current set of C. perfringens isolates, resulting in the patterns and groupings presented in Fig. 3. Overall, band densities and subsequently derived digitized images were more discreet using the RiboPrinter automated apparatus compared to the patterns obtained by repetitive-element PCR with Dt primers that were obtained by standard large-format gels and image capture. As with repetitive-element PCR with Dt primers, ribotype analysis resulted in four major branches, branches I to IV. Six second-level groupings, A to F (based on clades of patterns with approximately 80% correlation), were discerned with ribotyping. Breeder 1 isolates clustered into clade I-A, with isolate CP-12 apparently more distantly related to the other three members of the group. Isolates from breeder farm 2 and most grow-out broiler farm isolates were placed within the same clade, clade II-C (Fig. 3), which is in contrast to subtyping by repetitive-element PCR with Dt primers, which separated the majority of breeder 2 strains from the broiler isolates at the major 50% correlation level. Within clade II-C, these patterns were highly correlated (>95%).
DISCUSSION
Unlike reports of PFGE subtyping of clostridia, where not every isolate was typeable, all isolates in this experiment were typeable by repetitive-element PCR. Patterns obtained by repetitive-element PCR with Dt primers resulted in approximately 10 bands of between 0.3 and 5 kb in size. In our laboratory, and despite using agarose gels, this method was remarkably reproducible in duplicated whole procedures (i.e., DNA purification, repetitive-element PCR, and gel resolution) on the same isolates subtyped at different times. A similar finding was recently reported by Kang and Dunne (21) for both gram-positive and gram-negative bacteria subtyped by repetitive-element PCR, in which repetitive sequence-based PCR patterns were retained with >90% similarity over 3 days of continuous incubation or following 15 daily sequential subcultures for both gram-positive and gram-negative species tested with ERIC or RW3A repetitive-element PCR primers.
The C. perfringens isolates used in this study were all obtained from healthy, conventionally reared (i.e., fed nontherapeutic growth-promoting antibiotics) poultry and their environment. The broiler-breeder isolates represent the beginning of the broiler chicken production cycle and might be expected to harbor subtypes that are subsequently distributed into the subsequent production segments. However, we must caution that making broad ecological inferences about this organism is tenuous at best, since it is a ubiquitous bacterium of the agricultural environment capable of surviving in a sporulated state in soil and water without the need of a live host. C. perfringens is a commensal inhabitant of the broiler chicken gut and can exist as populations containing several subtypes within the same healthy bird or fewer subtypes in the diseased or necrotic enteritis state (10, 37). It is widely held that several factors dictate either the subclinical or nonfatal C. perfringens subtype diversity and disease status in broiler chickens and their grow-out environs. These factors include the use of anticoccidials, the use of AGPs, and litter quality (8, 9, 20, 28). Using PFGE and AFLP, Engstrom et al. (10) subtyped C. perfringens isolates from poultry and reported that the clustering of isolates correlated to flock, farm, and individual birds/tissue samples. They also observed subtype intermingling whether the isolates were from a bird either in a diseased state (necrotic enteritis or C. perfringens-related hepatitis) or healthy both with and without ionophorous antibiotic feed regimens.
It is of great interest that 46 of 48 isolates (95%) in this culture collection were beta2-toxin gene positive as determined using a well-documented PCR assay (35). Previously, it was reported from Swedish broiler chicken farms (10) that only 2 of 53 isolates (∼4%) were beta2-toxin gene positive. The later isolates were all derived from various flocks of birds, and the two beta2-toxin gene-positive samples were derived from a single farm and from birds with C. perfringens-associated hepatitis. In the current study, the two negative strains, CP-10 and CP-12, clustered in a single branch clade, clade IV-L, as determined by repetitive sequence-based PCR with Dt primers (Fig. 2). These strains were obtained from broiler chicken fecal samples taken from the same breeder farm. The relevance of this finding as well as beta2-toxin gene sequence variability is currently the subject of more detailed investigation by this laboratory. Within the dendrogram generated by EcoRI ribotyping, these two isolates clustered as members of a clade composed of all breeder farm 1 fecal isolates, clade I-A (Fig. 3).
The basic procedures of repetitive-element PCR for microbial subtyping have previously been detailed by Louws et al. (27). Gel-based resolution of large DNA amplicons presents an assortment of potential pitfalls that could directly impact the final subtype pattern. These include not only intrinsic factors such as gel material, buffer composition, and temperature but also time parameters and image capture quality, all of which vary not only between laboratories but between operators (27). The impact of gel-to-gel variation should be eliminated in the newest iteration of the technology, which employs microfluidic chips in place of agarose gels (12), thereby merging data generation and data analysis into a single platform.
Previously, subtyping of the current set of C. perfringens isolates by automated ribotyping (7) accomplished complete typeability with a pattern of subtype distribution considerably different than that accomplished by repetitive-element PCR with Dt primers. Compared to repetitive-element PCR with Dt primers, automated ribotyping resulted in similar Simpson's discrimination indices (Table 2) when calculated at the 95% correlation level. However, at the 90% correlation level, repetitive-element PCR with Dt primers demonstrated greater discrimination than the alternate method. The most notable difference between the two methods was the coclustering of grow-out farm isolates with breeder 2 isolates (Fig. 2) by automated ribotyping. Breeder 2 isolates were categorized into largely a single clade by repetitive-element PCR with Dt primers. This difference could possibly be due to the fundamental and characteristic biological differences between isolates as manifested in repetitive-element PCR with Dt primers. The relevance of these groupings is still open for interpretation owing to the relatively small number of isolates. It has not escaped our attention that the primers used in these experiments (54) were based on sequences originally identified in gram-negative bacteria that were then modified into degenerate primers with an inosine content and that it is not impossible that the amplified elements manifested by repetitive-element PCR with Dt primers are not actually repetitive elements but amplicons from randomly primed sequences. We did not use low annealing temperatures, on the order of 25°C, but a higher temperature, 40°C, that would require a higher fidelity for amplification. Other investigators have utilized repetitive-element PCR with Dt primers for subtyping of clostridia, namely, Clostridium difficile and Clostridium botulinum. In the case of C. difficile, subtyping data were comparable to those generated by PFGE and were more discriminatory than ribotyping (50). For the case of C. botulinum, randomly amplified polymorphic DNA (RAPD) with specified random primers and repetitive-element PCR with Dt primers were comparable, yet RAPD was more discriminatory, on a level comparable to that of PFGE, due to the larger number of resulting bands generated and resolved. Repetitive sequence-based PCR (REP and ERIC) primers were used to subtype Listeria monocytogenes (19), resulting in a high level of discrimination among human, animal, and food-borne isolates. Collectively, the aforementioned study as well as the current study provide evidence of consistent amplification among gram-positive bacteria with the same primers, indicating a high probability that these species harbor repetitive elements that are conserved. Hyytiä et al. (17) subtyped C. botulinum group I and group II isolates using RAPD and repetitive-element PCR (primers REP1R-Dt and REP2R-Dt) and determined different subtypes based on typically six to eight bands of between 400 and 2,200 bp in size. It was concluded that under the annealing temperature conditions used, repetitive sequence-based PCR was actually more similar to RAPD than amplifying of REP elements as a basis of discrimination. Those same workers concluded that PFGE and RAPD were more discriminatory than repetitive-element PCR; however, as reported here and in numerous other publications, the typeability of C. perfringens by PFGE is variable. Spigaglia and Mastrantonio (50) previously reported the presence of repetitive DNA elements in C. difficile, and while repetitive-element PCR using Uprime Dt primer sets facilitated 100% typeability, 4 of 34 isolates (12%) were not typeable by PFGE. Of those patterns obtained, repetitive-element PCR and PFGE resulted in a generally similar discrimination and grouping.
Several criteria must be taken into account when evaluating microbial subtyping (38, 55, 60). The highly conserved nature of repetitive sequences among the Bacteria and Archaea and their distribution throughout the genome make them attractive targets for strain differentiation. Repetitive elements are not limited to a single gene locus or restriction site, which are subject to insertions, deletions, and substitutions, imparting subtype variation that might bear no taxonomic significance. While not considered as discriminatory as PFGE, comparatively, repetitive-element PCR offers many distinct advantages as a subtyping method (12, 38). For the case of the clostridia, repetitive-element PCR offers higher typeability and reproducibility, lower cost, rapidity, and, more recently, semiautomation from the replacement of slab gels with microfluidic device resolution of a fluorescently labeled amplicon (12).
One time-consuming step of repetitive-element PCR is the preparation of template isolate DNA. While repetitive-element PCR subtyping has been attempted using whole-cell samples instead of isolated semipurified DNA (47), that report indicated typeability of less than 100%. The current isolate set was fully typeable by repetitive-element PCR when purified DNA was utilized. Important to the reproducibility of repetitive-element PCR as well as RAPD subtyping is consistent concentration and relative intactness (i.e., high molecular weight) of template DNA. Currently, the aforementioned reasons are the main reasons why purified DNA-to-subtype, rather than direct whole-cell-to-subtype, procedures are the methods of choice (41, 52). Reliable whole-cell-to-subtype methodology, obviating the need for DNA purification, would represent a much-needed advancement in the field of DNA-based molecular subtyping.
In summary, we have demonstrated the use of repetitive-element PCR with Dt primers for the subtyping of Clostridium perfringens isolates. Given that repetitive-element PCR has crossed the semiautomation threshold (11), it offers shared databasing along with a high degree of interlaboratory reproducibility. The method proved to be relatively discriminatory and robust. Like other subtyping schemes, repetitive-element PCR can be the basis of more extensive electronic databases, which are useful in the study of epidemiological relationships and for intervention strategies such as vaccine strain selection. Genotypic heterogeneity was observed within an isolate set derived from all major stages of broiler chicken production. Currently, our laboratory is exploring that very heterogeneity by applying semiautomated repetitive-element PCR to a larger set of dysbacteriosis-related C. perfringens isolates as a means to elucidate those subtypes of C. perfringens of greater relative importance in the process of clostridial overgrowth.
Acknowledgments
We acknowledge Johnna Garrish for expert technical laboratory support.
This research was funded by the Agricultural Research Service of the USDA.
REFERENCES
- 1.Bean, N., and P. M. Griffin. 1990. Food borne disease outbreaks in the United States, 1973-1987; pathogens, vehicles and trends. J. Food Prot. 53:804-817. [DOI] [PubMed] [Google Scholar]
- 2.Blaschek, H. P., and M. A. Klacik. 1984. Role of DNase in recovery of plasmid DNA from Clostridium perfringens. Appl. Environ Microbiol. 48:178-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brynestad, S., and P. E. Granum. 2002. Clostridium perfringens and food borne infections. Int. J. Food Microbiol. 74:195-202. [DOI] [PubMed] [Google Scholar]
- 4.Casewell, M., C. Friis, E. Marco, P. McMullin, and I. Phillips. 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 52:159-161. [DOI] [PubMed] [Google Scholar]
- 5.Collie, R. E., J. F. Kokai-Kun, and B. A. McClane. 1998. Phenotypic characterization of enterotoxigenic Clostridium perfringens isolates from non-food borne human gastrointestinal diseases. Anaerobe 4(2):69-79. [DOI] [PubMed] [Google Scholar]
- 6.Craven, S. E. 2001. Occurrence of Clostridium perfringens in the broiler chicken processing plant determined by recovery in iron milk medium. J. Food Prot. 64:1956-1960. [DOI] [PubMed] [Google Scholar]
- 7.Craven, S. E., N. A. Cox, J. S. Bailey, and D. E. Cosby. 2003. Incidence and tracking of Clostridium perfringens through an integrated broiler chicken operation. Avian Dis. 47:707-711. [DOI] [PubMed] [Google Scholar]
- 8.Elwinger, K., E. Berndtson, B. Engstrom, O. Fossum, and L. Waldenstedt. 1998. Effect of antibiotic growth promoters and anticoccidials on growth of Clostridium perfringens in the caeca and on performance of broiler chickens. Acta Vet. Scand. 39:433-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elwinger, K., C. Schneitz, E. Berndtson, O. Fossum, B. Teglof, and B. Engstom. 1992. Factors affecting the incidence of necrotic enteritis, caecal carriage of Clostridium perfringens and bird performance in broiler chicks. Acta Vet. Scand. 33:369-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engstrom, B. E., C. Fermer, A. Lindberg, E. Saarinen, V. Baverud, and A. Gunnarsson. 2003. Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry. Vet. Microbiol. 94:225-235. [DOI] [PubMed] [Google Scholar]
- 11.Gross, T. P., L. B. Kamara, C. L. Hatheway, P. Powers, J. P. Libonati, S. M. Harmon, and E. Israel. 1989. Clostridium perfringens food poisoning: use of serotyping in an outbreak. J. Clin. Microbiol. 27:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healy, M., J. Huong, T. Bittner, M. Lising, S. Frye, S. Raza, R. Schrock, J. Manry, A. Renwick, R. Nieto, C. Woods, J. Versalovic, and J. R. Lupski. 2005. Microbial DNA typing by automated repetitive-sequence-based PCR. J. Clin. Microbiol. 43:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hielm, S., J. Bjorkroth, E. Hyytia, and H. Korkeala. 1998. Prevalence of Clostridium botulinum in Finnish trout farms: pulsed-field gel electrophoresis typing reveals extensive genetic diversity among type E isolates. Appl. Environ. Microbiol. 64:4161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hielm, S., J. Bjorkroth, E. Hyytia, and H. Korkeala. 1999. Ribotyping as an identification tool for Clostridium botulinum strains causing human botulism. Int. J. Food Microbiol. 47:121-131. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, J. A., P. C. B. Turnbull, and M. F. Stringer. 1976. A serotyping scheme for C. welchii (C. perfringens) type A, and studies on the type-specific antigens. J. Med. Microbiol. 9:475-485. [DOI] [PubMed] [Google Scholar]
- 16.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyytia, E., J. Bjorkroth, S. Hielm, and H. Korkeala. 1999. Characterisation of Clostridium botulinum groups I and II by randomly amplified polymorphic DNA analysis and repetitive element sequence-based PCR. Int. J. Food Microbiol. 48:179-189. [DOI] [PubMed] [Google Scholar]
- 18.Hyytiä, E., S. Hielm, J. Bjorkroth, and H. Korkeala. 1999. Biodiversity of Clostridium botulinum type E strains isolated from fish and fishery products. Appl. Environ. Microbiol. 65:2057-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jersek, B., E. Tcherneva, N. Rijpens, and L. Herman. 1996. Repetitive element sequence-based PCR for species and strain discrimination in the genus Listeria. Lett. Appl. Microbiol. 23:55-60. [DOI] [PubMed] [Google Scholar]
- 20.Kaldhusdal, M., M. Hofshagen, A. Lovland, H. Langstrand, and K. Redhead. 1999. Necrotic enteritis challenge models with broiler chickens raised on litter: evaluation of preconditions, Clostridium perfringens strains and outcome variables. FEMS Immunol. Med. Microbiol. 24:337-343. [DOI] [PubMed] [Google Scholar]
- 21.Kang, H. P., and W. M. Dunne. 2003. Stability of repetitive-sequence PCR patterns with respect to culture age and subculture frequency. J. Clin. Microbiol. 41:2694-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilic, U., B. Schalch, and A. Stolle. 2002. Ribotyping of Clostridium perfringens from industrially produced ground meat. Lett. Appl. Microbiol. 34:238-243. [DOI] [PubMed] [Google Scholar]
- 23.Labbe, R. G. 2000. C. perfringens, p. 1110-1135. In B. Lund, T. B. Parker, and G. Gould (ed.), The microbiological safety and quality of food. Aspen Publishers, Gaithersburg, Md.
- 24.Labbe, R. G., and V. K. Juneja. 2002. Clostridium perfringens, p. 119-126. In D. O. Cliver and H. P. Riemann (ed.), Food borne diseases, 2nd ed. Elsevier Science Ltd., New York, N.Y.
- 25.Lin, Y. T., and R. Labbe. 2003. Enterotoxigenicity and genetic relatedness of Clostridium perfringens isolates from retail foods in the United States. Appl. Environ. Microbiol. 69:1642-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louws, F. J., D. W. Fulbright, C. T. Stephens, and F. J. de Bruijn. 1994. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl. Environ. Microbiol. 60:2286-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louws, F. J., J. L. W. Pacemaker, and F. J. de Bruijn. 1999. The three Ds of PCR-based genomic analysis of phytobacteria: diversity, detection and disease diagnosis. Annu. Rev. Phytopathol. 37:81-125. [DOI] [PubMed] [Google Scholar]
- 28.Lovland, A., and M. Kaldhusdal. 2001. Severely impaired performance in broiler flocks with high incidence of Clostridium perfringens-associated hepatitis. Avian Pathol. 32:527-534. [DOI] [PubMed] [Google Scholar]
- 29.Lukinmaa, S., E. Takkunen, and A. Siitonen. 2002. Molecular epidemiology of Clostridium perfringens related to food-borne outbreaks of disease in Finland from 1984 to 1999. Appl. Environ. Microbiol. 68:3744-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lupski, J. R., and G. M. Weinstock. 1992. Short, interspersed repetitive DNA sequences in prokaryotic genomes. J. Bacteriol. 174:4525-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maslanka, S. E., J. G. Kerr, G. Williams, J. M. Barbaree, L. A. Carson, J. M. Miller, and B. Swaminathan. 1999. Molecular subtyping of Clostridium perfringens by pulsed-field gel electrophoresis to facilitate food-borne-disease outbreak investigations. J. Clin. Microbiol. 37:2209-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClane, B. A., D. M. Lyerly, J. S. Moncrief, and T. D. Wilkins. 2000. Enterotoxic clostridia: Clostridium perfringens type A and Clostridium difficile, p. 551-562. In V. A. Fischetti, et al. (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 33.McLauchlin, J., G. Ripabelli, M. M. Brett, and E. J. Threlfall. 2000. Amplified fragment length polymorphism (AFLP) analysis of Clostridium perfringens for epidemiological typing. Int. J. Food Microbiol. 56:21-28. [DOI] [PubMed] [Google Scholar]
- 34.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meer, R. R., and J. G. Songer. 1997. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 58:702-705. [PubMed] [Google Scholar]
- 36.Miwa, N., T. Nishina, S. Kubo, M. Atsumi, and H. Honda. 1998. Amount of enterotoxigenic Clostridium perfringens in meat detected by nested PCR. Int. J. Food Microbiol. 42:195-200. [DOI] [PubMed] [Google Scholar]
- 37.Nauerby, B., K. Pedersen, and M. Madsen. 2003. Analysis by pulsed-field gel electrophoresis of the genetic diversity among Clostridium perfringens isolates from chickens. Vet. Microbiol. 94:257-266. [DOI] [PubMed] [Google Scholar]
- 38.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petit, L., M. Gibert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104-110. [DOI] [PubMed] [Google Scholar]
- 40.Pons, J. L., M. L. Combe, and G. Leluan. 1994. Multilocus enzyme typing of human and animal strains of C. perfringens. FEMS Microbiol. Lett. 121:25-30. [DOI] [PubMed] [Google Scholar]
- 41.Power, E. G. M. 1996. RAPD typing in microbiology—a technical review. J. Hosp. Infect. 34:247-265. [DOI] [PubMed] [Google Scholar]
- 42.Ridell, J., J. Bjorkroth, H. Eisgruber, B. Schalch, A. Stolle, and H. Korkeala. 1998. Prevalence of the enterotoxin gene and clonality of Clostridium perfringens strains associated with food-poisoning outbreaks. J. Food Prot. 61:240-243. [DOI] [PubMed] [Google Scholar]
- 43.Samore, M. H., M. Kristjansson, L. Venjataraman, P. C. DeGirolami, and R. D. Arbeit. 1994. Comparison of arbitrarily primed polymerase chain reaction, restriction enzyme analysis, and pulsed-field gel electrophoresis for typing Clostridium difficile strains. J. Clin. Microbiol. 32:1963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schalch, B., L. Bader, H. P. Schau, R. Bergmann, A. Rometsch, G. Maydl, and S. Kessler. 2003. Molecular typing of Clostridium perfringens from a food-borne disease outbreak in a nursing home: ribotyping versus pulsed-field gel electrophoresis. J. Clin. Microbiol. 41:892-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schalch, B., J. Bjorkroth, H. Eisgruber, H. Korkeala, and A. Stolle. 1997. Ribotyping for strain characterization of Clostridium perfringens isolates from food poisoning cases and outbreaks. Appl. Environ. Microbiol. 63:3992-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schalch, B., B. Sperner, H. Eisgruber, and A. Stolle. 1999. Molecular methods for the analysis of Clostridium perfringens relevant to food hygiene. FEMS Immunol. Med. Microbiol. 24:281-286. [DOI] [PubMed] [Google Scholar]
- 47.Snelling, A. M., P. Gerner-Smidt, P. M. Hawkey, J. Heritage, P. Parnell, C. Porter, A. R. Bodenham, and T. Inglis. 1996. Validation of use of whole-cell repetitive extragenic palindromic sequence-based PCR (REP-PCR) for typing strains belonging to the Acinetobacter calcoaceticus-Acinetobacter baumannii complex and application of the method to the investigation of a hospital outbreak. J. Clin. Microbiol. 34:1193-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sparks, S. G., R. J. Carman, M. R. Sarker, and B. A. McClane. 2001. Genotyping of enterotoxigenic Clostridium perfringens fecal isolates associated with antibiotic-associated diarrhea and food poisoning in North America. J. Clin. Microbiol. 39:883-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spigaglia, P., and P. Mastrantonio. 2003. Evaluation of repetitive element sequence-based PCR as a molecular typing method for Clostridium difficile. J. Clin. Microbiol. 41:2454-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tschirdewahn, B., S. Notermans, K. Wernars, and F. Untermann. 1991. The presence of enterotoxigenic Clostridium perfringens strains in faeces of various animals. Int. J. Food Microbiol. 14:175-178. [DOI] [PubMed] [Google Scholar]
- 52.Tyler, K. D., G. Wang, S. D. Tyler, and W. M. Johnson. 1997. Factors affecting reliability and reproducibility of amplification-based DNA fingerprinting of representative bacterial pathogens. J. Clin. Microbiol. 35:339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urwin, R., and M. C. Maiden. 2003. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 11:479-487. [DOI] [PubMed] [Google Scholar]
- 54.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Versalovic, J., M. Schneider, F. J. de Bruijn, and J. R. Lupski. 1994. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Met. Mol. Cell Probes 5:25-40. [Google Scholar]
- 56.Versalovic, J., and J. Lupski. 1998. Interspersed repetitive sequences in bacterial genomes, p. 38-47. In F. J. de Bruijn, J. R. Lupski, and G. M. Weinstock (ed.), Bacterial genomes—physical structure and analysis. Chapman & Hall, London, United Kingdom.
- 57.Versalovic, J., F. J. de Bruijn, and J. R. Lupski. 1998. Repetitive sequence-based PCR (rep-PCR) DNA fingerprinting of bacterial genomes, p. 38-48. In F. J. de Bruijn, J. R. Lupski, and G. M. Weinstock (ed.), Bacterial genomes—physical structure and analysis. Chapman & Hall, London, United Kingdom.
- 58.Wages, D. P., and K. Opengart. 2003. Necrotic enteritis, p. 781-783. In Y. M. Siaf (ed.), Diseases of poultry, 11th ed. Iowa State University Press, Ames, Iowa.
- 59.Wen, Q., and B. A. McClane. 2004. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl. Environ. Microbiol. 70:2685-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiedmann, M. 2002. Subtyping of bacterial food borne pathogens. Nutr. Rev. 60:201-208. [DOI] [PubMed] [Google Scholar]
- 61.Wise, M. G., and G. R. Siragusa. 2005. Quantitative detection of Clostridium perfringens in the broiler fowl gastrointestinal tract by real-time PCR. Appl. Environ. Microbiol. 71:3911-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woods, C. R., J. Versalovic, T. Koeuth, and J. R. Lupski. 1993. Whole-cell repetitive element sequence-based polymerase chain reaction allows rapid assessment of clonal relationships of bacterial isolates. J. Clin. Microbiol. 31:1927-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoo, H. S., S. U. Lee, K. Y. Park, and Y. H. Park. 1997. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J. Clin. Microbiol. 35:228-232. [DOI] [PMC free article] [PubMed] [Google Scholar]