Abstract
Streptococcus pneumoniae strains which fail to produce a polysaccharide capsule are commonly isolated from carriage and disease contexts. Here we use a multilocus approach to distinguish genuine nontypeable pneumococci from closely related nontypeable streptococcal isolates in a data set of 121 untypeable pneumococci from nasopharyngeal swabs and middle ear fluid of Finnish children and demonstrate that 70 of these belong to a pneumococcal lineage which has lost its capsular locus. Strains of this relatively old lineage include sequence types 344, 448, and 449. Comparison with the multilocus sequence typing database shows that strains of this lineage have spread intercontinentally and have been isolated from carriage, mucosal, and invasive disease. Furthermore we note a particular association of this nontypeable lineage with outbreaks of conjunctivitis. The diversification and geographic spread of this lineage suggest that loss of capsule is not inconsistent with long-term persistence and raise questions about the capsule's role in pneumococcal transmission.
The pneumococcus (Streptococcus pneumoniae) causes a spectrum of infections ranging from mild and self-limiting disease (e.g., acute otitis media) (3) and conjunctivitis (8) to acute invasive manifestations associated with significant mortality (e.g., meningitis) (17). The vast majority of pneumococci are carried asymptomatically in the nasopharynx and disease is a relatively rare outcome of colonization. One of the factors most consistently associated with pneumococcal virulence is the polysaccharide capsule. This exhibits a high degree of antigenic variation, with 90 serotypes described, and is the protective antigen in conjugate vaccines, which include capsular polysaccharides from the serotypes most frequently isolated from invasive disease in the targeted pediatric population (2). The capsule exhibits antiphagocytic properties thought to be associated with the ability to persist in blood, and there is some evidence that certain serotypes may be associated with poor outcome (12).
Despite the importance of the capsule for virulence, nontypeable variants of the pneumococcus are not infrequently isolated from disease and carriage, particularly in association with epidemics of conjunctivitis among healthy young people. The study of these isolates has been complicated by difficulties in distinguishing between true unencapsulated pneumococci and closely related streptococci of the mitis group which also colonize the oropharynx (21). However, the use of the sequences obtained from multilocus sequence typing allows species identification and the resolution of similar but distinct populations (9) and has enabled genuine nontypeable pneumococci to be confidently identified and resolved from very closely related but distinct streptococcal isolates (10).
There are several possible mechanisms by which nontypeable pneumococci may be generated. First, and most simply, the expression of capsule may be down-regulated. This is frequently observed during long passage in the laboratory and may be advantageous in certain biological situations (20). Alternatively, the cps region, which contains the genes encoding the enzymes responsible for capsule biosynthesis, may have been disrupted, leading to a failure to produce capsule (11). Finally, the strain in question may express a capsule which has yet to be described.
We have characterized by multilocus sequence typing a collection of genuine nontypeable pneumococci isolated from carriage and acute otitis media among children in Finland. We show several examples of isolates of normally serotypeable strains, including those assigned to major internationally distributed clones, which have apparently downregulated their capsule, and one in which the cps region appears to have undergone significant disruption. However, the major nontypeable strains belonged to a lineage of exclusively nontypeable pneumococci that has a defective capsular locus, has spread intercontinentally, is isolated from carriage and invasive disease, and shows a particular association with outbreaks of conjunctivitis in a variety of settings.
MATERIALS AND METHODS
Strains.
A data set of 121 nontypeable presumptive pneumococci was obtained from the Finnish otitis media (6, 13, 14, 18) studies of pneumococcal disease and carriage conducted in Finland. Isolates were obtained from either middle ear fluid of children with acute otitis media or nasopharyngeal swabs of healthy children or children with acute otitis media. All strains were optochin sensitive (6 μg, Biodisk PDM Diagnostic Disks, Sweden). Following multilocus sequence typing (10), 70 of the 121 isolates were assigned as genuine pneumococci by the clustering of the concatenated sequences of the multilocus sequence typing loci (excluding ddl) on a minimum evolution (ME) tree within those of the reference set of serotypeable pneumococci (10). The other isolates clustered apart from the pneumococcal reference set among a more diverse set of strains that are not considered to be authentic pneumococci. Details of the 70 isolates assigned as nontypeable pneumococci are summarized in Table 1. Serotyping was carried out using the Quellung reaction with sera from the Statens Seruminstitut (Copenhagen, Denmark).
TABLE 1.
Strainsa
| Strain | No. of strains carrying:
|
ST | Serotype? | wzg | Site of isolationb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| aroE | gdh | gki | recP | spi | xpt | ddl | |||||
| IOPR 5877 | 1 | 5 | 4 | 5 | 5 | 3 | 8 | 15 | (14) | + | NP |
| IOPR 868 | 5 | 12 | 29 | 12 | 9 | 39 | 18 | 100 | 33 | + | NP |
| IOPR 1295 | 5 | 12 | 29 | 12 | 9 | 39 | 18 | 100 | 33 | + | NP |
| IOPR 5853 | 5 | 12 | 29 | 12 | 9 | 39 | 18 | 100 | 33 | + | NP |
| IOKOR 818 | 5 | 12 | 29 | 12 | 9 | 39 | 18 | 100 | 33 | + | NP |
| IOPR 3524 | 5 | 12 | 29 | 12 | 9 | 39 | 18 | 100 | 33 | + | NP |
| IOPR 1128 | 5 | 12 | 29 | 12 | 9 | 39 | 18 | 100 | 33 | + | MEF |
| IOPR 1387 | 7 | 5 | 8 | 5 | 10 | 6 | 14 | 138 | (6B) | − | NP |
| IOPR 3071 | 7 | 11 | 10 | 1 | 6 | 8 | 14 | 162 | 9V | + | NP |
| IOPR 3942 | 7 | 11 | 10 | 1 | 6 | 8 | 14 | 162 | 9V | + | NP |
| IOPR 3399 | 8 | 13 | 14 | 4 | 17 | 4 | 14 | 199 | (19A) | + | NP |
| IOPR 5878 | 15 | 8 | 8 | 18 | 15 | 1 | 31 | 235 | 20 | + | NP |
| IOPR 1329 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | NP | |
| IOPR 1746 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | NP | |
| IOPR 2052 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | NP | |
| IOPR 2257 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | NP | |
| IOPR 4124 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | NP | |
| IOPR 4125 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | NP | |
| IOPR 4573 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | NP | |
| IOPR 4848 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | NP | |
| IOPR 2302 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | MEF | |
| IOPR 6449 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | MEF | |
| IOPR 1734 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | MEF | |
| IOPR 4169 | 8 | 37 | 9 | 29 | 2 | 12 | 53 | 344 | − | MEF | |
| IOPR 1422 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | NP | |
| IOPR 5944 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | NP | |
| IOPR 5966 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | NP | |
| IOKOR 1051 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | NP | |
| IOKOR 1858 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | NP | |
| IOKOR 768 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | NP | |
| IOPR 3595 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | NP | |
| IOPR 6073 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | MEF | |
| IOKOR 294 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | MEF | |
| IOKOR 543 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | MEF | |
| IOPR 129 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | MEF | |
| IOPR 2740 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | MEF | |
| IOPR 2851 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | MEF | |
| IOPR 48 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | MEF | |
| IOPR 5580 | 8 | 5 | 2 | 27 | 2 | 11 | 71 | 448 | − | MEF | |
| IOPR 1586 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 1793 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 2368 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 4036 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 5745 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 5844 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 2687 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 2866 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 3014 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 3065 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 3223 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 4131 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 4184 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 4293 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 4847 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 5027 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | NP | |
| IOPR 1496 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | MEF | |
| IOPR 3933 | 8 | 37 | 9 | 29 | 2 | 47 | 5 | 449 | − | MEF | |
| IOKOR 1054 | 7 | 5 | 1 | 1 | 6 | 31 | 9 | 492 | 6B | + | NP |
| IOKOR 492 | 13 | 8 | 65 | 1 | 60 | 16 | 6 | 508 | + | NP | |
| IOKOR 609 | 13 | 8 | 65 | 1 | 60 | 16 | 6 | 508 | + | NP | |
| IOKOR 707 | 13 | 8 | 65 | 1 | 60 | 16 | 6 | 508 | + | NP | |
| IOKOR 314 | 1 | 1 | 4 | 1 | 18 | 16 | 17 | 520 | 22F | + | NP |
| IOPR 5599 | 1 | 1 | 4 | 1 | 18 | 16 | 17 | 520 | 22F | + | NP |
| IOKOR 328 | 1 | 1 | 4 | 1 | 18 | 16 | 17 | 520 | 22F | + | MEF |
| IOKOR 345 | 1 | 1 | 4 | 1 | 18 | 16 | 17 | 520 | 22F | + | MEF |
| IOPR 5268 | 8 | 37 | 9 | 29 | 2 | 47 | 59 | 1054 | − | NP | |
| IOPR 2619 | 7 | 5 | 8 | 5 | 87 | 6 | 14 | 1055 | (6B) | + | NP |
| IOPR 4849 | 8 | 5 | 2 | 27 | 2 | 136 | 71 | 1229 | − | MEF | |
| IOPR 2837 | 2 | 5 | 29 | 16 | 42 | 3 | 146 | 1248 | 33 | + | NP |
| IOPR 193 | 8 | 5 | 2 | 27 | 9 | 11 | 71 | 1290 | − | MEF | |
Allelic profiles, sequence types, serotypes (if present), and the presence or absence of the wzg gene are shown. Where initially untypeable strains were subsequently found to express capsule, this is shown. For those cases where, based on previous submissions to the MLST database, a serotype is expected, that serotype is shown in parentheses.
NP, nasopharyngeal swab; MEF, middle ear fluid.
DNA isolation and sequencing.
Genomic DNA was isolated using QIAGEN DNeasy kits (QIAGEN Ltd., Crawley, United Kingdom). Internal fragments of multilocus sequence typing loci were PCR amplified (Taq polymerase and 10× buffer from QIAGEN), 50 nM deoxynucleoside triphosphates (Geneamp; Applied Biosystems, Foster City, CA) using the primers and PCR conditions described previously (5). The PCR products were precipitated with 20% polyethylene glycol 8000-2.5 M NaCl (Sigma), and the fragments were sequenced on both strands using the same primers and BigDye II terminators (Applied Biosystems). The products of the sequencing reactions were precipitated with 185 mM sodium acetate in 70% ethanol and were resuspended in 10 μl HiDi formamide (Applied Biosystems) and loaded onto an ABI Prism 3700 sequencer. Sequences were analyzed using STARS (obtainable from www.mlst.net), a modified Staden interface developed by Man-Suen Chan for use with multilocus sequence typing projects. Alleles were assigned by comparing the sequences to those in the pneumococcal multilocus sequence typing database (http://spneumoniae.mlst.net).
Presence of the capsular locus.
To test whether nontypeable strains contained a cps locus, we used PCR with primers specific for the conserved wzg (cpsA) gene (wzg-up, 5′ATCCTTGTCAGCTCTGTGTC, and wzg-down, 5′TCACTTGCAACTACATGAAC), with an annealing temperature of 55°C in an MJ-Research PTC-200 DNA engine. Results were visualized using agarose gel electrophoresis and a positive test for wzg was shown by amplification of a single product of the predicted size (481 bp).
Phylogenetics.
For comparison with nontypeable isolates, a reference set of 39 serotypeable pneumococci was chosen to define the diversity found within the species. To construct this pneumococcal reference data set, which is described elsewhere (10), the entire multilocus sequence typing database was divided into nonoverlapping groups of related strains using the program eBURST with the default setting for the group definition (sharing of six out of seven loci). The reference set includes examples of the predicted founding genotypes of the major clonal complexes identified by eBURST and also isolates of some of the major internationally disseminated antibiotic-resistant clones (details of this reference set are available at http://spneumoniae.mlst.net/).
For all nontypeable isolates and the pneumococcal reference set, the sequences of the multilocus sequence typing loci (with the exception of ddl) were concatenated in frame and used to construct an ME tree in MEGA 3.0 with the Kimura two-parameter model and using all nucleotide sites. Support for the nodes on the tree was assessed by 1,000 bootstrap resamplings, and the tree was rooted using a nontypeable isolate that has been assigned as closely related to, but distinct from, authentic pneumococci (NT 27, described by Hanage et al.) (10). In order to explore relationships between the nontypeable pneumococci described in this paper and those identified by other researchers, the concatenated sequences of all 1,702 sequence types (STs) in the entire multilocus sequence typing database (as of 31 March 2005) were used to construct an ME tree, as described above.
RESULTS
Diversity of nontypeable pneumococci in Finland and comparison with the multilocus sequence typing database.
The allelic profiles and STs of the isolates described here are shown in Table 1. The three most common strains among the 70 nontypeable isolates assigned as pneumococci were STs 449, 448, and 344 (19, 15, and 11 isolates, respectively). There were also six isolates of ST100, which initially was found to be nontypeable, but three isolates of this ST are in the pneumococcal multilocus sequence typing database and all are serotype 33F; the ST100 isolates from Finland were therefore reexamined and were shown to express a serogroup 33 capsule. Other STs represented by multiple isolates were 520 (four), 508 (three), and 162 (two). The last is a single-locus variant and the likely immediate ancestor of the Spain9V-3 penicillin-resistant clone, and both isolates were rechecked and shown to express serotype 9V capsule. Several of the nontypeable isolates belonged to known STs that are represented in the multilocus sequence typing database by serotypeable isolates, including isolates of the prevalent STs 15, 138, and 199 (Table 1).
Distinct lineage of nontypeable pneumococci.
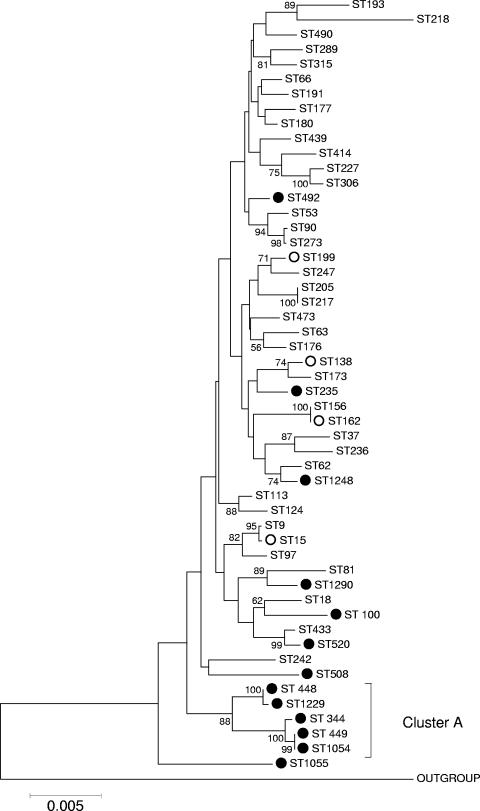
Figure 1 shows an ME tree constructed from the concatenated sequences of the multilocus sequence typing loci (excepting ddl; this locus is excluded due to the presence in many penicillin-resistant strains of highly divergent alleles resulting from its location near a penicillin-binding protein gene) (4) of the reference set of serotypeable pneumococci and the nontypeable pneumococcal STs described in this work. The position on the tree of those STs associated with nontypeable isolates is shown by the solid circles.
FIG. 1.
ME tree of nontypeable pneumococci. The concatenated sequences of the STs identified among the 70 nontypeable pneumococcal isolates from Finland and those of the serotypeable strains of the pneumococcal reference set (10) were used to construct an ME tree, as described in Materials and Methods. Bootstrap confidence levels greater than 60% are shown next to the node in question. The positions of nontypeable pneumococcal STs are indicated with circles. Solid circles are nontypeable STs in the Finnish collection; open circles were STs in the Finnish nontypeable collection that also were represented in the pneumococcal reference set. A related S. mitis group isolate that has been shown to be closely related to but distinct from authentic pneumococci (10) was used as an outgroup.
The majority of nodes on the tree show low bootstrap support, as is expected in a highly recombinogenic species such as the pneumococcus, where phylogenetic information between anything other than relatively closely related strains is likely to have been obscured by recombination (6). However, a high level of support (88%) is indicated for a cluster containing STs 344, 448, 449, 1054, and 1229, all of which were found in this work to be exclusively nontypeable (indicated in Fig. 1 as cluster A). Using a dendrogram constructed from differences in allelic profiles, or eBURST, this cluster was not readily identified because STs within it commonly share fewer than six of seven alleles (see below). The remainder of nontypeable strains were scattered across the ME tree with no indication of other nontypeable lineages, although ST 1290 is predicted to be related to cluster A on the basis of its allelic profile, as it is a single-locus variant of ST 448.
Relationship of cluster A to previously described strains.
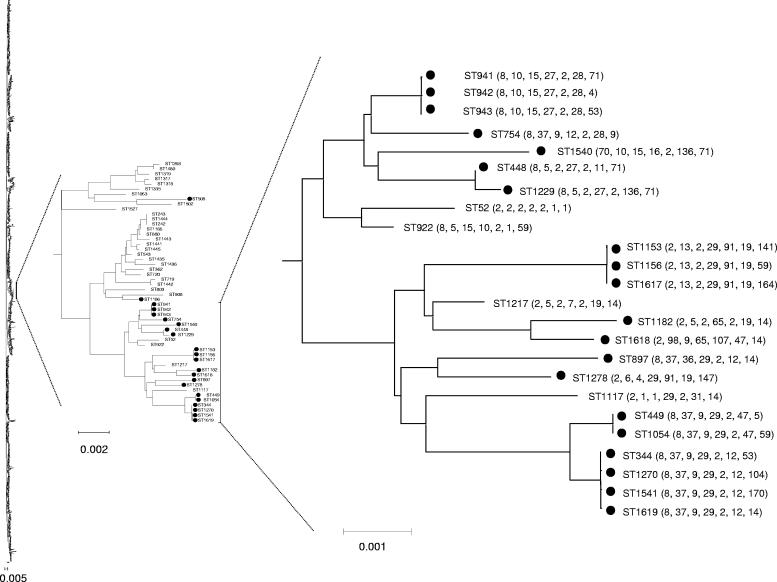
Isolates of the two most common STs in the present study, STs 344 and 448, have been previously reported in the multilocus sequence typing database. In order to find out whether the STs falling into cluster A are part of a larger nontypeable pneumococcal lineage, we produced an ME tree from the concatenated sequences of the Finnish nontypeable STs combined with all of the STs in the pneumococcal multilocus sequence typing database. As in Fig. 1, all members of cluster A were found to be part of a single lineage, shown in Fig. 2, along with a number of other STs not represented in the Finnish sample of nontypeable pneumococci, a high proportion of which (23 of 27, 85%) were nontypeable.
FIG. 2.
ME tree of all STs in the multilocus sequence typing database and the nontypeable pneumococci. The concatenated sequences of the nontypeable pneumococcal STs and all STs in the multilocus sequence typing databases were used to construct an ME tree. The tree of all STs present in the database as of 31 March 2005 is shown at left, and the part of the large tree that includes STs within cluster A is shown together with its sister clade. Those STs which have been reported as nontypeable in the multilocus sequence typing database are indicated with solid circles. The association of nontypeable strains with the clade containing cluster A strains is clear and this group of strains is shown at right with the allelic profiles of STs shown after the ST number. The serotypes associated with those STs expressing capsule were as follows: STs 52 and 1217, 19F; ST 922, 17F; and ST 1117, 13. Some isolates are identical on the ME tree but differ in allelic profile at ddl as this locus is not used in the sequence concatenation.
In the rest of the pneumococcal multilocus sequence typing database, nontypeable isolates account for less than 2.5% of strains. The clustering of these STs suggests that they are descended from a common ancestor and are thus also part of the same relatively old lineage which is unusual in being largely or exclusively represented by nontypeable isolates. Four serotypeable STs from the multilocus sequence typing database also clustered with these strains. Whether these are genuinely related to the STs of cluster A or are spuriously grouped with this predominantly nontypeable lineage as a result of recombination is unknown.
Analysis of the relatedness of nontypeable pneumococcal isolates using the sharing of alleles at the multilocus sequence typing loci also supports the view that the STs in cluster A are members of a single lineage. ST 344 and ST 449 differ at only two of the seven multilocus sequence typing loci and there is little doubt that they are descended from a recent common ancestor. ST 448 does not cluster with ST 344 and ST 449 on a tree constructed using the pairwise differences in their allelic profiles (data not shown) but the presence in all three STs of identical alleles at aroE and spi supports the view derived from the ME tree that they may belong to the same lineage. Interrogation of the multilocus sequence typing database showed that isolates of all seven STs that shared alleles at three or more multilocus sequence typing loci with ST 344 and/or ST 449 were nontypeable. Similarly, the three STs that shared four or more alleles with ST 448 were nontypeable, although some of the STs that shared only three loci were serotypeable.
Absence of wzg in nontypeable isolates.
To test whether the Finnish nontypeable pneumococcal isolates had down-regulated capsule expression or appeared to lack cps genes, PCR was carried out using primers specific for the conserved wzg (cpsA) gene. All nontypeable isolates that were identical, or very similar, in allelic profile to serotypeable isolates in the multilocus sequence typing database were positive for wzg, with the exception of one of the isolates of ST 138 (IOPR 1387). However, for those STs falling within cluster A of Fig. 1, all isolates tested were negative for wzg, as was the case for ST 1290.
DISCUSSION
The capsule of the pneumococcus is commonly regarded as both a major virulence factor and a significant target for acquired immunity. Evidence for the first assertion comes from its antiphagocytic properties which are likely to be important in bacteremia, studies of virulence in animal models, and the relative rarity of nontypeable isolates in invasive disease (19) and for the second from the excellent serotype-specific efficacy against invasive disease of vaccines directed against protein-conjugated capsular polysaccharide (2). Despite this, a capsule is not necessary to effectively colonize the nasopharynx, as clearly shown by the frequent isolation of nontypeable pneumococci from this niche, and indeed evidence suggests that lack of capsular expression may be an advantage for carriage in some situations (20). Here we demonstrate the existence of a lineage of pneumococci (cluster A) which lacks capsule, has spread widely, and has a clear ability to cause disease.
The pneumococcus undergoes recombination at high frequency and therefore the details of the ME tree shown in Fig. 1 should be treated with suspicion (7). However, strains that have diverged relatively recently may still be discerned on a tree based on concatenated sequences and the STs within cluster A are grouped together with surprisingly good bootstrap support, and include STs 449, 448, and 334. There is additional support for the proposal that these three STs are descended from a common ancestor as they share alleles at both aroE and spi. Isolates of these predominant STs were all nontypeable and lacked the conserved capsular gene wzg. A further strain, ST 1290, which was isolated once in this study, groups with ST 81 on the tree shown in Fig. 1, but based on its allelic profile (it is a single locus variant of ST 448) it should be grouped within cluster A. The absence of the wzg gene in this isolate (see Table 1 and below) provides further evidence that it is in fact part of the cluster A lineage. The single locus that differs between ST 1290 and ST 448 (spi) differs at 16 nucleotide sites and the introduction by recombination of a relatively divergent spi allele has presumably markedly changed the position of ST 1290 on the tree.
STs 448 and 449 have previously been reported in studies of pneumococcal carriage from Oxford (16), Finland (9), and Switzerland (11). Moreover, ST 448 was reported as the cause of an outbreak of conjunctivitis affecting 698 college students in the United States (15). This association with conjunctivitis was also noted for ST 344 and STs 941, 942, and 943 (one), which in an analysis of the entire multilocus sequence typing database are also placed within cluster A (Fig. 2). Records in the multilocus sequence typing database show that nontypeable isolates of ST 344 has been reported from two cases of invasive disease: one in Norway and the other in Australia. Finally, outbreaks of conjunctivitis in the United States separated by more than 20 years were found to be caused by pneumococcal strains that were identical by pulsed-field gel electrophoresis and which are assigned to ST 488 (15), providing further evidence that these strains are far from short lived within the population.
Interestingly, the great majority of isolates in the multilocus sequence typing database that fall within cluster A on an ME tree are nontypeable, as are all of the isolates in the database that share four or more alleles with STs 344, 448, and 449. The few STs that are within cluster A on the ME tree and are serotypeable may be positioned anomalously on the tree as a result of recombination or may have regained a capsule by recombination. The fact that these isolates are not closely related to the major nontypeable STs in their allelic profiles suggests anomalous clustering on the ME tree. Cluster A strains therefore appear to be a relatively diverse set of STs, descended from a common ancestor (a lineage), that do not express a capsule and lack the capsular gene wzg. Most of the other nontypeable pneumococcal isolates from Finland were closely related to STs that are normally represented by serotypeable isolates and these differed from cluster A isolates in having the wzg gene.
Previously, Hathaway et al. (11) demonstrated disruption of the cps locus in nontypeable pneumococcal strains which were highly prevalent in Switzerland, including isolates of STs 344 and 448. The STs of strains with disrupted cps loci were either identical or similar to those identified in this work as cluster A (because the new alleles from the Swiss isolates were not logged with the multilocus sequence typing database it was not possible to include them in this study). In particular, isolates with STs 344 and 448 were found with distinct but similar genotypes at the cps locus, providing further evidence that these strains are related although they only share alleles at two loci. We can suggest therefore that the ancestor of cluster A lost its cps locus through recombination (perhaps with a related streptococcal species as suggested by Hathaway et al.) but that this has not prevented its subsequent successful spread and diversification.
Normal pneumococcal transmission is to be distinguished from disease, in which capsule has a relatively well defined role. However disease cannot be considered to be important in transmission because it is such a rare consequence of colonization. Nontypeable strains are pervasive components of carried populations. The present work demonstrates that nonencapsulated pneumococcal variants arise naturally, both through down regulation and loss of the cps locus, and that such variants and not just transient strains, destined to be rapidly lost from the population, but may be successful and persist, becoming geographically widespread, and may cause disease.
REFERENCES
- 1.Berron, S., A. Fenoll, M. Ortega, N. Arellano, and J. Casal. 2005. Analysis of the genetic structure of nontypeable pneumococcal strains isolated from conjunctiva. J. Clin. Microbiol. 43:1694-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 3.Block, S. L. 1997. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr. Infect. Dis. J. 16:449-456. [DOI] [PubMed] [Google Scholar]
- 4.Enright, M. C., and B. G. Spratt. 1999. Extensive variation in the ddl gene of penicillin-resistant Streptococcus pneumoniae results from a hitchhiking effect driven by the penicillin-binding protein 2b gene. Mol. Biol. Evol. 16:1687-1695. [DOI] [PubMed] [Google Scholar]
- 5.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 6.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Käyhty, P. Karma, R. Kohberger, G. Siber, and P. H. Mäkelä. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 7.Feil, E. J., E. C. Holmes, D. E. Bessen, M. S. Chan, N. P. Day, M. C. Enright, R. Goldstein, D. W. Hood, A. Kalia, C. E. Moore, J. Zhou, and B. G. Spratt. 2001. Recombination within natural populations of pathogenic bacteria: short-term empirical estimates and long-term phylogenetic consequences. Proc. Natl. Acad. Sci. USA 98:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gigliotti, F., W. T. Williams, F. G. Hayden, J. O. Hendley, J. Benjamin, M. Dickens, C. Gleason, V. A. Perriello, and J. Wood. 1981. Etiology of acute conjunctivitis in children. J. Pediatr. 98:531-536. [DOI] [PubMed] [Google Scholar]
- 9.Hanage, W. P., K. Auranen, R. Syrjanen, E. Herva, P. H. Makela, T. Kilpi, and B. G. Spratt. 2004. Ability of pneumococcal serotypes and clones to cause acute otitis media: implications for the prevention of otitis media by conjugate vaccines. Infect. Immun. 72:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanage, W. P., T. Kaijalainen, E. Herva, A. Saukkoriipi, R. Syrjänen, and B. G. Spratt. 2005. Using multilocus sequence data to define the pneumococcus. J. Bacteriol. 187:6223-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hathaway, L. J., P. Stutzmann Meier, P. Battig, S. Aebi, and K. Muhlemann. 2004. A homologue of aliB is found in the capsule region of nonencapsulated Streptococcus pneumoniae. J. Bacteriol. 186:3721-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausdorff, W. P., D. R. Feikin, and K. P. Klugman. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83-93. [DOI] [PubMed] [Google Scholar]
- 13.Kilpi, T., H. Åhman, J. Jokinen, K. S. Lankinen, A. Palmu, H. Savolainen, M. Grönholm, M. Leinonen, T. Hovi, J. Eskola, H. Käyhty, N. Bohidar, J. C. Sadoff, and P. H. Mäkelä. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 37:1155-1164. [DOI] [PubMed] [Google Scholar]
- 14.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjänen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 15.Martin, M., J. H. Turco, M. E. Zegans, R. R. Facklam, S. Sodha, J. A. Elliott, J. H. Pryor, B. Beall, D. D. Erdman, Y. Y. Baumgartner, P. A. Sanchez, J. D. Schwartzman, J. Montero, A. Schuchat, and C. G. Whitney. 2003. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N. Engl. J. Med. 348:1112-1121. [DOI] [PubMed] [Google Scholar]
- 16.Meats, E., A. B. Brueggemann, M. C. Enright, K. Sleeman, D. T. Griffiths, D. W. Crook, and B. G. Spratt. 2003. Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae. J. Clin. Microbiol. 41:386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuchat, A., K. Robinson, J. D. Wenger, L. H. Harrison, M. Farley, A. L. Reingold, L. Lefkowitz, and B. A. Perkins. 1997. Bacterial meningitis in the United States in 1995. N. Engl. J. Med. 337:970-976. [DOI] [PubMed] [Google Scholar]
- 18.Syrjänen, R. K., T. M. Kilpi, T. H. Kaijalainen, E. E. Herva, and A. K. Takala. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J Infect. Dis. 184:451-459. [DOI] [PubMed] [Google Scholar]
- 19.Watson, D. A., and D. M. Musher. 1999. A brief history of the pneumococcus in biomedical research. Semin. Respir. Infect. 14:198-208. [PubMed] [Google Scholar]
- 20.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whatmore, A. M., A. Efstratiou, A. P. Pickerill, K. Broughton, G. Woodard, D. Sturgeon, R. George, and C. G. Dowson. 2000. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 68:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]