Abstract
Rhino-orbitocerebral mucormycosis (ROCM) caused by more common zygomycetes (e.g., Mucor) is known to cause rapidly fatal infections in immunocompromised patients. Apophysomyces elegans is an emerging zygomycete that has been reported to cause invasive cutaneous and rhino-orbitocerebral infections in immunocompetent individuals. Limited data exist describing the syndrome of ROCM caused by A. elegans. We describe a recent case and performed a comprehensive literature review to delineate the clinical characteristics of ROCM caused by A. elegans. Our case is a 50-year-old man with diabetes mellitus who presented with facial pain and right eye proptosis. Endoscopic sinus sampling revealed A. elegans. He was treated with liposomal amphotericin B and multiple debridements, with no disease on 1.5-year follow-up examination. Seven cases were identified on literature review, including the present case. Most patients (86%) were male, with a mean age of 40 years. Most patients (71%) did not have predisposing medical conditions. Three patients had predisposing head trauma. All presented with facial and/or periorbital pain. All had magnetic resonance imaging or computed tomography of the head showing intraorbital and/or sinus inflammation. Diagnosis was confirmed by histopathology and deep tissue culture in all cases. All patients required eye exenteration and extensive surgical debridement, in addition to intravenous amphotericin B. Six of the seven patients (86%) recovered. ROCM caused by A. elegans is rarely reported in the literature. Most such infections occurred in immunocompetent patients, often after facial trauma. Survival in ROCM caused by A. elegans is favorable in reported cases, with prompt surgical debridement and antifungal therapy.
Mucormycosis is a rare necrotizing infection caused by fungi within the class Zygomycetes and the order Mucorales (7). These fungi can cause serious and rapidly fatal infections, particularly in the immunocompromised, such as poorly controlled diabetics with ketoacidosis (17). The genera reported to cause invasive infection include Absidia, Mucor, Rhizomucor, Rhizopus, Apophysomyces, Saksenaea, Cunninghamella, Cokeromyces, and Syncephalastrum, with the first four being the most commonly reported pathogens (7). Apophysomyces elegans is an emerging pathogen that, unlike the other members of Mucorales, has been reported to cause invasive cutaneous and rhino-orbitocerebral infections in immunocompetent individuals (37). In rhino-orbitocerebral infections, inhalation is the natural route of infection. However, traumatic inoculation has been described, particularly with Apophysomyces elegans (17).
In 2001, Garcia-Covarrubias et al. reported a review of the literature of mucormycosis attributable to Apophysomyces elegans (17). At that time, there were 21 reported cases of mucormycosis caused by A. elegans since 1985, only 4 of which were rhino-orbitocerebral mucormycosis (ROCM) (2-4, 8-10, 13, 17, 19-22, 24-28, 31, 36-37). Since then, there have been 13 more reported cases of Apophysomyces elegans, mostly cutaneous infections, 8 of which were from India and 2 of which were cases of ROCM (1, 5, 7, 15, 23, 29). We report an additional case of ROCM caused by A. elegans and review the literature specifically for ROCM caused by A. elegans to better understand the characteristics of this rare disease.
CASE REPORT
A 50-year-old white male with a history of diet-controlled diabetes mellitus, allergic rhinitis, and recurrent sinusitis about once every 2 to 3 years, with the last infection about 1 year ago, presented with a 2-week history of right maxillary facial pain. He denied trauma and did not have fevers or chills. His pain worsened over the subsequent week, and he developed diplopia, severe right-sided periorbital pain and swelling, right eye ptosis, and right-sided facial drooping and numbness. He presented to the local emergency room. A computed tomography (CT) scan of the head and sinuses was unremarkable. He was discharged on therapy with prednisone (30 mg daily) and acyclovir for possible Bell's palsy.
The following day, he awoke with complete blindness in the right eye, as well as increasing pain, swelling, and numbness in the right upper face. He presented again to the emergency room. Magnetic resonance imaging (MRI) of the head showed sphenoid and ethmoid sinusitis associated with a mass or phlegmon posterior to the right globe with inflammatory changes of the extraocular muscles. Upon admission to the hospital, laboratory studies showed leukocytosis (18.5 × 109/liter) and the following chemistry values: serum glucose, 297 mg/dl; serum bicarbonate, 17 mmol/liter, serum hemoglobin A1c, 11.4%; serum creatinine, 0.9 mg/dl; and urinary ketones, 2+. He underwent a right endoscopic total ethmoidectomy, maxillary enterostomy, and sphenoidectomy. Tissue cultures grew Staphylococcus aureus and coagulase-negative staphylococci. Fungal stains obtained at the hospital were negative. Therapy was initiated with cefazolin and rifampin. A few days later, he developed worsening orbital pain and proptosis. A repeat MRI of the head showed increasing intraorbital inflammation and proptosis with tenting of the posterior globe.
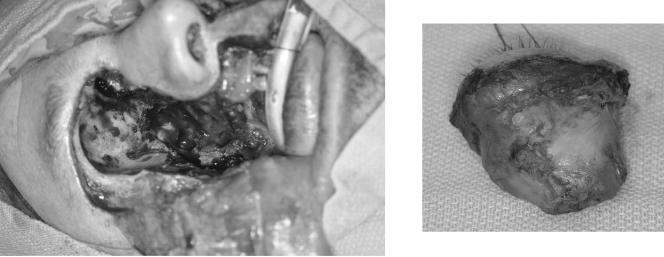
He was then transferred to our institution (the Mayo Clinic). The social history was significant for previous exposure to multiple dusts (wood, farm, etc.), as he was a construction inspector and materials tester. There were no risk factors for human immunodeficiency virus or tuberculosis. Physical examination was significant for right eye proptosis with erythema, tenderness, and swelling (Fig. 1). The remainder of the physical examination was unremarkable. Laboratory studies upon transfer were significant for a hemoglobin A1c of 10.2%, blood sugar levels of 120 to 170 mg/dl with no ketones, a normal leukocyte count (8.9 × 109/liter), and a serum creatinine level of 1.5 mg/dl. CT and MRI of the head showed right eye proptosis with inflammatory changes in the periorbital, extraconal, and intraconal fat consistent with orbital cellulitis; scattered gas in the intraconal fat; subperiosteal phlegmon and/or abscess; and an opacified right maxillary sinus (Fig. 2). Magnetic resonance venography of the head was negative for thrombosis. The patient underwent right endoscopic sphenoid sinus sampling. Tissue histopathology showed aseptate hyphae consistent with mucormycosis.
FIG. 1.
Note the significant right eye proptosis.
FIG. 2.
Note the right eye proptosis and retrobulbar inflammation seen on axial fluid-attenuated inversion recovery (FLAIR) image of the MRI of the head, with inflammatory changes extending posteriorly and superiorly to involve the right cavernous sinus.
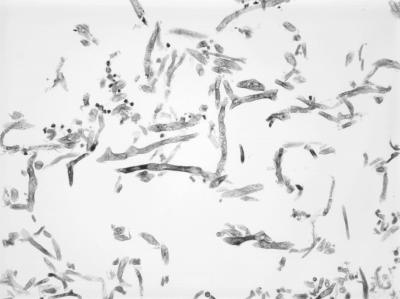
The patient then underwent right eye exenteration, maxillectomy, sphenoidotomy, and multiple aggressive debridements (Fig. 3). He was started on amphotericin B liposomal complex (Ambisome) at 5 mg/kg of body weight intravenously (i.v.) every 24 h, cefepime, and metronidazole. A tissue culture grew Apophysomyces elegans (Fig. 4). The organism showed sparsely septate hyphae and unbranched sporangiophores, along with a characteristic funnel-shaped apophysis. Sporangia were pyriform and contained numerous smooth-walled, oblong sporangiospores (14).
FIG. 3.
Note the radical surgical debridement and orbital exenteration, performed to achieve disease control. (Right) The base of the right eye was found to be necrotic.
FIG. 4.
On histopathologic examination, the right orbital tissue was found to contain a fungus ball with nonseptate hyphae with right-angle branching, consistent with mucormycosis.
The patient underwent several debridements until intraoperative findings, as well as pathology and microbiology studies, showed no residual infection or necrosis. His hospital course was complicated by amphotericin B-associated renal failure, but his creatinine levels stabilized and he was discharged on amphotericin B therapy. One month after hospital discharge, or 2.5 months after initial diagnosis and institution of therapy, liposomal amphotericin B therapy was discontinued due to another significant increase in serum creatinine levels (to 5.2 mg/dl). Follow-up clinical examination showed no evidence of persistent fungal disease.
MATERIALS AND METHODS
Our retrospective review focused on patients with ROCM caused by A. elegans. ROCM was defined as infection originating in the paranasal sinuses and presenting in contiguous structures of the orbit, nose, palate, face, or brain. The diagnosis was accepted only if it was confirmed by histologic examination or by a deep tissue culture positive for A. elegans.
We searched the MEDLINE database for articles published in the English-language literature since 1966 using mucormycosis and Apophysomyces as keywords or text words. Where applicable, we reviewed references cited in the above reports and studies. Incomplete case reports with limited clinical information (such as those reported in the context of a broader analysis) (6) were not included in our analysis. Care was taken to ensure that the same patient was not included twice in our analysis by review of demographics and clinical presentation of all reported cases.
RESULTS
Six reported cases met our criteria for ROCM caused by A. elegans, making a total of seven cases, including our case.
Demographics, predisposing conditions, and presentation.
Table 1 summarizes the age and sex, predisposing conditions, presenting symptoms, physical findings, and radiographic manifestations for each patient. Six of the seven patients were male. The mean age was 40 years (range, 19 to 59 years). Four of the seven patients had no predisposing conditions, and only one had diabetes mellitus. Three of the seven patients had presenting symptoms of facial or orbital pain following head trauma. The remaining four had presenting symptoms of facial and/or periorbital pain and swelling. Three patients had fever as a presenting sign. On physical examination, five of the seven patients had orbital proptosis with erythema and swelling; the other two patients had a friable mass in the nasal cavity and congested nasal turbinates with pus draining from sinus tracts. All seven patients had a CT of the head or sinuses performed, and two had an MRI of the head and/or orbits performed. The radiographic manifestations seen on these imaging studies are summarized in Table 1; notably, in the present report, the initial CT of the sinuses was unremarkable, but subsequent MRI of the head showed the severe intraorbital inflammation and proptosis.
TABLE 1.
Summary of patients with rhino-orbitocerebral mucormycosis caused by A. elegans
| Reference or source | Age (yr)/sex | Predisposing condition(s) | Presenting symptoms; preceding surgery or trauma | Physical findings | Radiographic manifestations |
|---|---|---|---|---|---|
| 31 | 19/M | None | Pain in upper palate and decreased vision in left eye after fall from tractor, striking left temple on ground | Dark necrotic tissue on left upper palate, left eye proptosis | CT of head/sinuses: opacification of left maxillary sinus and swelling of left periorbital soft tissue |
| 9 | 52/M | Myelofibrosis | Fever, right facial swelling | Right facial swelling, right eye chemosis, whitish friable mass in right nasal cavity | CT of sinuses: soft tissue shadow in maxillary antrum and ethmoid sinuses; preseptal orbital cellulitis |
| 2 | 54/M | Chronic sinusitis | Fever, nasal congestion, frontal headache, left cheek swelling | Large congested inferior turbinates, swollen maxillary vestibule, pus draining from sinus tracts on labial surface of upper alveolar ridge | CT of sinuses: extensive mucoperiosteal thickening of both maxillary sinuses with bony resorption of left maxillary sinus |
| 17 | 24/M | None | Closed-head trauma with Le Fort II complex facial fracture after motorcycle collision | Right periorbital ecchymosis and proptosis with full-thickness skin necrosis of right frontotemporal region and cheek; corneal opacification | CT of head: opacification of paranasal sinuses |
| 15 | 59/M | None | Sharp, deep retro-orbital pain and fever following high-pressure water jet injury to right inner canthus while cleaning air conditioner filter | Marked right-sided periorbital edema, erythema, and proptosis | CT of orbits: marked proptosis and chemosis; extensive mucosal thickening in right ethmoid and maxillary sinuses; MRI of orbits: right proptosis, infiltrated orbital fat, enlarged extraocular muscles, extending into right maxillary antrum, nasal cavity, sphenoid sinus, infratemporal fossa |
| 7 | 20/F | None | Pain, redness, swelling, and protrusion of right eye, rhinitis, headache, fever | Right eye protrusion, erythema, and swelling | CT of head: mass extending across nasal septum, displacing right orbital tissue with retro-orbital extension |
| This study | 50/M | Diabetes mellitus, allergic rhinitis, and previous sinusitis | Right maxillary facial and periorbital pain and swelling, diplopia, right eye ptosis, and right facial droop and numbness | Right eye proptosis with erythema, tenderness, and swelling | CT sinuses: unremarkable; MRI of head: intraorbital inflammation and proptosis with tenting of posterior globe |
Therapy and outcome.
Table 2 summarizes the antimicrobial and surgical management, complications, and outcomes for each patient. Prior to the diagnosis of ROCM, two of the seven patients had received empirical therapy with prednisone (20 to 30 mg daily). After diagnosis, three patients received empirical therapy with i.v. broad-spectrum antibiotics, and two patients received empirical oral antibiotic therapy. All patients were treated with i.v. amphotericin B (three patients were reported to have received the liposomal formulation); doses ranged from 1 to 5 mg/kg/day, and duration ranged from 5 days to approximately 70 days. All patients (100%) required eye exenteration and extensive surgical debridement for disease control. One patient was additionally treated with hyperbaric oxygen (HBO2) and granulocyte colony-stimulating factor (G-CSF). In three of the seven cases, acute renal failure (ARF) associated with amphotericin B therapy was reported. Three cases had no complications of therapy reported. Only one of the seven patients died; this patient had myelofibrosis and thrombocytopenia. The remaining six patients recovered; the reported relapse-free follow-up period ranged from 1 month to 2 years.
TABLE 2.
Treatment and outcome of patients with rhino-orbitocerebral mucormycosis caused by A. elegansa
| Reference or source | Immunosuppressive treatment reported prior to diagnosis | Antimicrobial treatment (dose; duration) | Surgical and other treatments | Complications reported | Outcome | Length of followup |
|---|---|---|---|---|---|---|
| 31 | None | AMB (total dose, 3.5 g; 1.5 mg/kg/day), ceftazidime, clindamycin | Left eye enucleation, palatectomy, bilateral maxillectomy, multiple aggressive debridement | None | Cure | 1 yr after presentation |
| 9 | None | AMB (1 mg/kg/day; ∼10 days) | Orbital exenteration, debridement of sinuses | Thrombocytopenia, generalized oozing and bleeding from wound | Death | 15 days after sx onset |
| 2 | Prednisone (20 mg daily for 3 days, then tapered over 3 days) | AMB (1 mg/kg/day; 5 days), i.v. clindamycin | Bilateral Caldwell-Luc procedures | ARF secondary to AMB, anemia | Cure | 2 yr after initial surgery |
| 17 | None | Liposomal systemic and local AMB (total dose, 27.5 g; first course, ∼14 days; additional “brief” course for relapse), tobramycin, penicillin | Orbital exenteration, multiple surgical debridements, HBO2 (2.5 atm for 90 min twice daily; 14 days), G-CSF (600 μg; given for relapse) | Mild hypokalemia, ARF secondary to AMB | Cure (after additional therapy for initial relapse 18 days after discontinuing liposomal AMB) | 4 mo after hospital discharge (8 mo after the injury) |
| 15 | None | Liposomal AMB (5 mg/ kg/day; total dose, 14.7 g; 42 days) | Right orbital exenteration, radical debridement, two subsequent debridements | None | Cure | 12 mo after presentation |
| 7 | None | AMB (total dose, 1.5 g; 1 mo) | Local debridement and exenteration of right orbit | None | Cure | 1 mo after presentation |
| This study | Prednisone (30 mg daily for 10 days) | Liposomal AMB (5 mg/kg/day; ∼70 days), cefepime, metronidazole | Right eye exenteration, maxillectomy, sphenoidotomy, multiple aggressive debridements | ARF secondary to AMB, hypoxia | Cure | 1.5 yr after presentation |
i.v., intravenous; AMB, amphotericin B; TMP/SMX, trimethoprim/sulfamethoxazole; sx, symptom; ARF, acute renal failure; HBO2, hyperbaric oxygen therapy; atm, atmosphere absolutes; min, minutes; G-CSF, granulocyte colony stimulating factor.
DISCUSSION
We present the first review of ROCM caused by A. elegans. Unlike ROCM caused by the more common genera of Mucoraceae (Rhizopus, Mucor, and Absidia), ROCM due to A. elegans appears to occur in immunocompetent patients. The majority of patients had no predisposing conditions. Of the three who did, only one had a serious underlying hematologic disorder (myelofibrosis); the other two had only sinusitis and non-insulin-dependent diabetes mellitus. In addition, almost half of the cases occurred following facial or head trauma.
The most common presenting signs and symptoms of ROCM caused by A. elegans are similar to the classic features of ROCM caused by other organisms. These include fever, headache, sinusitis, facial or orbital pain and swelling, orbital cellulitis, external ophthalmoplegia, decreased vision or blindness, proptosis, chemosis, nasal congestion, mucosal ulceration and/or necrosis, and central retinal artery occlusion. Radiographic findings are also similar and include proptosis, sinus thickening or opacification, and intraorbital inflammation (1, 5, 7, 15, 23, 29). Morphological features of large sparsely septate or nonseptate hyphae with right-angle branching, often with angioinvasion, on histopathologic examination suggest the etiology (32). Specific identification of the etiologic agent requires isolation of the organism by culture (20).
Prompt and aggressive surgical debridement, as well as administration of antifungals, is required for treatment. In patients with a predisposing condition, treatment should also include correction of the medical problem, if possible (e.g., correction of ketoacidosis). Agents of mucormycosis are relatively refractory to medical treatment; higher than usual doses of amphotericin B, ranging from 1.0 to 1.5 mg/kg/day, have been recommended (32). Lipid formulations may allow administration of very large amounts of amphotericin B over a short period and thus may offer an opportunity for more effective treatment. However, this is only a theoretical consideration at present and remains to be validated (32).
None of the currently available azoles (ketoconazole, fluconazole, itraconazole, and voriconazole) are effective in treating mucormycosis (32). None of the echinocandins, either currently available or in development (caspofungin, anidulafungin, and micafungin), are active against zygomycetes (32). Dannaoui et al. (12) recently studied the in vitro and in vivo efficacy of amphotericin B (given intraperitoneally at doses of 0.5 to 4.5 mg/kg/day) in a nonneutropenic murine model of disseminated zygomycosis. For the in vivo experiments, none of the 120 mice infected with A. elegans treated with amphotericin B died, compared with all of those in the control group given glucose. Walsh et al. (35) recently evaluated the efficacy and safety of amphotericin B lipid complex in 556 patients with invasive fungal infection in an open-label emergency-use study of patients refractory to or intolerant of conventional antifungal therapy. Out of 24 patients with invasive zygomycosis infections, 17 (71%) had a complete or partial response. Out of six patients specifically with zygomycosis sinus infections, four (67%) had a complete or partial response.
Posaconazole, a new triazole that is currently in phase III trials, has been reported to have good activity against some strains that cause mucormycosis (11, 18, 33-34). Tobon et al. (34) reported a case of an immunocompromised diabetic patient who developed a severe mediastinal fungal infection due to Rhizopus species postoperatively after a dual heart-kidney transplantation. No improvement was noted following 6 weeks of amphotericin B therapy, but treatment with posaconazole resulted in dramatic clinical improvement as early as 7 days into treatment, with continued improvement during 23 weeks of treatment and no adverse events attributable to posaconazole. Posaconazole was active in vivo against three strains of Mucor in an immunosuppressed-mouse model of disseminated infection, (33), as well as against a Rhizopus species and an Absidia species (11). Posaconazole oral suspension at a dose of 800 mg/day was recently studied in a multicenter open-label clinical trial in patients with central nervous system (CNS) fungal infections who had refractory disease or who were intolerant of standard antifungal therapy (30). A total of 39 out of 330 subjects were evaluable for efficacy. Successful outcomes were observed in 14 of 29 (48%) subjects with cryptococcal meningitis and 5 of 10 (50%) of subjects with CNS infections due to other fungal pathogens; one patient had an infection with A. elegans plus Basidiomycetes sp. This patient was among the five subjects who did not have a successful outcome, but these subjects had advanced disease and severe underlying medical conditions (e.g., solid organ or allogeneic bone marrow transplantation or lymphoma) when posaconazole therapy was initiated. The authors concluded that posaconazole has clinical activity against CNS fungal infections (with success rate overall approaching 50%) and may provide a valuable alternative in patients failing existing antifungal agents. Because of amphotericin B-associated nephrotoxicity reported in almost half the cases of ROCM due to A. elegans, further studies of posaconazole activity in vitro and in vivo against Apophysomyces species are warranted. Ravuconazole is another new triazole in clinical development. However, it is not active against zygomycetes (16).
HBO2 and G-CSF have been used in one reported case of ROCM due to A. elegans (17). It is postulated that HBO2 increases tissue oxygen levels, boosts leukocyte-killing capacity, and may have an additive effect in combination with amphotericin B (2). However, the reports of benefit from this modality involve small numbers of patients, are observational, and are uncontrolled in nature. Additionally, the rationale why this approach should work in treating organisms that are obligate aerobes is unclear. G-CSF may also boost polymorphonuclear fungicidal activity and was used in the same case (17). However, whether that translates into an improved clinical outcome, particularly in those patients who are not granulocytopenic, is unknown.
There is no general consensus on the optimal duration of antifungal therapy in ROCM due to A. elegans. Treatment courses ranging from 5 days to 70 days have been reported. An all-too-frequent treatment-limiting factor is amphotericin B-induced renal failure. In general, surgical debridement and systemic antifungal therapy should be continued until follow-up evaluations show no residual fungal infection or necrosis.
The prognosis of ROCM has improved greatly. Mortality was 88% in 1961 and now ranges from 15 to 34% (15). Because ROCM caused by A. elegans is rare, the rate of mortality is not known; however, among the seven reported cases, only one patient (14%) died.
Although ROCM caused by A. elegans is rare, it is probably underrepresented in the medical literature because of publication bias, thereby limiting the generalizability of our findings. The retrospective nature of our study has unavoidable inherent limitations, including differences in completeness of reported data. To minimize this, we limited our series to those reported cases with sufficient data and to those identified through medical literature search engines.
Conclusion.
Unlike other members of this group of fungi, A. elegans is a zygomycete that is reported to cause invasive infection in immunocompetent patients. Infections with A. elegans are usually the result of traumatic inoculation. Similar to other cases of mucormycosis, angioinvasion, thrombosis, and necrotic lesions are the hallmark features. Prompt and aggressive surgical debridement and therapy with an amphotericin B formulation are necessary for successful treatment. Posaconazole therapy may be an effective alternative to the use of amphotericin B in these patients. Survival in ROCM caused by A. elegans is favorable in the seven reported cases.
Acknowledgments
We thank Gordon F. Gibbs of the Department of Radiology at Mayo Clinic for assistance with Fig. 2, Scott McLean of the Department of Otorhinolaryngology at Mayo Clinic for kindly providing intraoperative photographs for Fig. 3, and Marie-Christine Aubry of the Department of Anatomic Pathology at Mayo Clinic for assistance with Fig. 4.
There was no financial support for this study. No conflict of interest exists for any of the authors.
REFERENCES
- 1.Blair, J. E., L. J. Fredrikson, B. A. Pockaj, and C. S. Lucaire. 2002. Locally invasive cutaneous Apophysomyces elegans infection acquired from snapdragon patch test. Mayo Clin. Proc. 77:717-720. [DOI] [PubMed] [Google Scholar]
- 2.Brown, S. R., I. A. Shah, and M. Grinstead. 1998. Rhinocerebral mucormycosis caused by Apophysomyces elegans. Am. J. Rhinol. 12:289-292. [DOI] [PubMed] [Google Scholar]
- 3.Burrell, S. R., D. J. Ostlie, M. Saubolle, M. Dimler, and S. D. Barbour. 1998. Apophysomyces elegans infection associated with cactus spine injury in an immunocompetent pediatric patient. Pediatr. Infect. Dis. J. 17:663-664. [DOI] [PubMed] [Google Scholar]
- 4.Caceres, A. M., C. Sardinas, C. Marcano, R. Guevara, J. Barros, G. Bianchi, V. Rosario, R. Balza, M. Silva, M. C. Redondo, and M. Nunez. 1997. Apophysomyces elegans limb infection with a favorable outcome: case report and review. Clin. Infect. Dis. 25:331-332. [DOI] [PubMed] [Google Scholar]
- 5.Carter, J. E., and O. Ulusarac. 2003. Widespread cutaneous involvement by invasive Apophysomyces elegans in a gravid patient following trauma. Cutis 72:221-224, 227-228. [PubMed] [Google Scholar]
- 6.Chakrabarti, A., A. Das, A. Sharma, N. Panda, S. Das, K. L. Gupta, and V. Sakhuja. 2001. Ten years' experience in zygomycosis at a tertiary care centre in India. J. Infect. 42:261-266. [DOI] [PubMed] [Google Scholar]
- 7.Chakrabarti, A., A. Ghosh, G. S. Prasad, J. K. David, S. Gupta, A. Das, V. Sakhuja, N. K. Panda, S. K. Singh, S. Das, and T. Chakrabarti. 2003. Apophysomyces elegans: an emerging zygomycete in India. J. Clin. Microbiol. 41:783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti, A., P. Kumar, A. A. Padhye, L. Chatha, S. K. Singh, A. Das, J. D. Wig, and R. N. Kataria. 1997. Primary cutaneous zygomycosis due to Saksenaea vasiformis and Apophysomyces elegans. Clin. Infect. Dis. 24:580-583. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti A., N. Panda, S. C. Varma, K. Singh, A. Das, S. C. Sharma, and A. A. Padhye. 1997. Craniofacial zygomycosis caused by Apophysomyces elegans. Mycoses 40:419-421. [DOI] [PubMed] [Google Scholar]
- 10.Cooter, R. D., I. S. Lim, D. H. Ellis, and I. O. Leitch. 1990. Burn wound zygomycosis caused by Apophysomyces elegans. J. Clin. Microbiol. 28:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dannaoui, E., J. F. Meis, D. Loebenberg, and P. E. Verweij. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dannaoui, E., J. W. Mouton, J. F. G. M. Meis, P. E. Verweij, and the Eurofung Network. 2002. Efficacy of antifungal therapy in a nonneutropenic murine model of zygomycosis. Antimicrob. Agents Chemother. 46:1953-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton, M. E., A. A. Padhye, D. A. Schwartz, and J. P. Steinberg. 1994. Osteomyelitis of the sternum caused by Apophysomyces elegans. J. Clin. Microbiol. 32:2827-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis, D. H. 2005. Systemic zygomycosis, p. 659-686. In W. G. Merz and R. J. Hay (ed.), Topley & Wilson's microbiology and microbial infections, medical mycology, 10th ed. Hodder Arnold, London, United Kingdom.
- 15.Fairley, C., T. J. Sullivan, P. Bartley, T. Allworth, and R. Lewandowski. 2000. Survival after rhino-orbital-cerebral mucormycosis in an immunocompetent patient. Ophthalmology 107:555-558. [DOI] [PubMed] [Google Scholar]
- 16.Fung-Tomc, J. C., E. Huczko, B. Minassian, and D. P. Bonner. 1998. In vitro activity of a new oral triazole, BMS-207147 (ER-30346). Antimicrob. Agents Chemother. 42:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Covarrubias, L., R. Bartlett, D. M. Barratt, and R. J. Wassermann. 2001. Rhino-orbitocerebral mucormycosis attributable to Apophysomyces elegans in an immunocompetent individual: case report and review of the literature. J. Trauma 50:353-357. [DOI] [PubMed] [Google Scholar]
- 18.Herbrecht, R. 2004. Posaconazole: a potent, extended-spectrum triazole anti-fungal for the treatment of serious fungal infections. Int. J. Clin. Pract. 58:612-624. [DOI] [PubMed] [Google Scholar]
- 19.Huffnagle, K. E., P. M. Southern, Jr., L. T. Byrd, and R. M. Gander. 1992. Apophysomyces elegans as an agent of zygomycosis in a patient following trauma. J. Med. Vet. Mycol. 30:83-86. [DOI] [PubMed] [Google Scholar]
- 20.Kimura, M., M. B. Smith, and M. R. McGinnis. 1999. Zygomycosis due to Apophysomyces elegans: report of 2 cases and review of the literature. Arch. Pathol. Lab. Med. 123:386-390. [DOI] [PubMed] [Google Scholar]
- 21.Lakshmi, V., T. S. Rani, S. Sharma, V. S. Mohan, C. Sundaram, R. R. Rao, and G. Satyanarayana. 1993. Zygomycotic necrotizing fasciitis caused by Apophysomyces elegans. J. Clin. Microbiol. 31:1368-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence, R. M., W. T. Snodgrass, G. W. Reichel, A. A. Padhye, L. Ajello, and F. W. Chandler. 1986. Systemic zygomycosis caused by Apophysomyces elegans. J. Med. Vet. Mycol. 24:57-65. [PubMed] [Google Scholar]
- 23.Lesueur, B. W., K. Warschaw, and L. Fredrikson. 2002. Necrotizing cellulitis caused by Apophysomyces elegans at a patch test site. Am. J. Contact Dermatitis. 13:140-142. [PubMed] [Google Scholar]
- 24.Mathews, M. S., A. Raman, and A. Nair. 1997. Nosocomial zygomycotic post-surgical necrotizing fasciitis in a healthy adult caused by Apophysomyces elegans in south India. J. Med. Vet. Mycol. 35:61-63. [DOI] [PubMed] [Google Scholar]
- 25.McGinnis, M. R., J. Midez, L. Pasarell, and A. Haque. 1993. Necrotizing fasciitis caused by Apophysomyces elegans. J. Mycol. Med. 3:175-179. [Google Scholar]
- 26.Meis, J. F., B. J. Kullberg, M. Pruszczynski, and R. P. Veth. 1994. Severe osteomyelitis due to the zygomycete Apophysomyces elegans. J. Clin. Microbiol. 32:3078-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naguib, M. T., M. M. Huycke, J. A. Pederson, L. R. Pennington, M. E. Burton, and R. A. Greenfield. 1995. Apophysomyces elegans infection in a renal transplant recipient. Am. J. Kidney Dis. 26:381-384. [DOI] [PubMed] [Google Scholar]
- 28.Okhuysen, P. C., J. H. Rex, M. Kapusta, and C. Fife. 1994. Successful treatment of extensive posttraumatic soft-tissue and renal infections due to Apophysomyces elegans. Clin. Infect. Dis. 19:329-331. [DOI] [PubMed] [Google Scholar]
- 29.Page, R., D. J. Gardam, and C. H. Heath. 2001. Severe cutaneous mucormycosis (zgomycosis) due to Apophysomyces elegans. ANZ J. Surg. 71:184-186. [DOI] [PubMed] [Google Scholar]
- 30.Pitisuttithum, P., R. Negroni, J. R. Graybill, B. Bustamante, P. Pappas, S. Chapman, R. S. Hare, and C. J. Hardalo. 2005. Activity of posaconazole in the treatment of central nervous system fungal infections. J. Antimicrob. Chemother. 56:745-755. [DOI] [PubMed] [Google Scholar]
- 31.Radner, A. B., M. D. Witt, and J. E. Edwards, Jr. 1995. Acute invasive rhinocerebral zygomycosis in an otherwise healthy patient: case report and review. Clin. Infect. Dis. 20:163-166. [DOI] [PubMed] [Google Scholar]
- 32.Sugar, A. M. 2005. Agents of mucormycosis and related species, p. 2973-2984. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's Principles and practice of infectious diseases, 6th ed., vol. 2. Elsevier Churchill Livingstone, Philadelphia, Pa. [Google Scholar]
- 33.Sun, Q. N., L. K. Najvar, R. Bocanegra, D. Loebenberg, and J. R. Graybill. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob. Agents Chemother. 46:2310-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobon, A. M., M. Arango, D. Fernandez, and A. Restrepo. 2003. Mucormycosis (Zygomycosis) in a heart-kidney transplant recipient: recovery after posaconazole therapy. Clin. Infect. Dis. 36:1488-1491. [DOI] [PubMed] [Google Scholar]
- 35.Walsh, T. J., J. W. Hiemenz, N. L. Seibel, J. R. Perfect, G. Horwith, L. Lee, J. L. Silber, M. J. DiNubile, A. Reboli, E. Bow, J. Lister, and E. J. Anaissie. 1998. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 26:1383-1396. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg, W. G., B. H. Wade, G. Cierny III, D. Stacy, and M. G. Rinaldi. 1993. Invasive infection due to Apophysomyces elegans in immunocompetent hosts. Clin. Infect. Dis. 17:881-884. [DOI] [PubMed] [Google Scholar]
- 37.Wieden, M. A., K. K. Steinbronn, A. A. Padhye, L. Ajello, and F. W. Chandler. 1985. Zygomycosis caused by Apophysomyces elegans. J. Clin. Microbiol. 22:522-526. [DOI] [PMC free article] [PubMed] [Google Scholar]