Abstract
Recently, a third novel feline hemotropic Mycoplasma sp. (aka hemoplasma), “Candidatus Mycoplasma turicensis,” in a cat with hemolytic anemia has been described. This is the first study to investigate the prevalence, clinical manifestations, and risk factors for all three feline hemoplasma infections in a sample of 713 healthy and ill Swiss cats using newly designed quantitative real-time PCR assays. “Candidatus Mycoplasma haemominutum” infection was detected in 7.0% and 8.7% and Mycoplasma haemofelis was detected in 2.3% and 0.2% of healthy and ill cats, respectively. “Candidatus Mycoplasma turicensis” was only detected in six ill cats (1.1%); three of them were coinfected with “Candidatus Mycoplasma haemominutum.” The 16S rRNA gene sequence of 12 Swiss hemoplasma isolates revealed >98% similarity with previously published sequences. Hemoplasma infection was associated with male gender, outdoor access, and old age but not with retrovirus infection and was more frequent in certain areas of Switzerland. “Candidatus Mycoplasma haemominutum”-infected ill cats were more frequently diagnosed with renal insufficiency and exhibited higher renal blood parameters than uninfected ill cats. No correlation between hemoplasma load and packed cell volume was found, although several hemoplasma-infected cats, some coinfected with feline immunodeficiency virus or feline leukemia virus, showed hemolytic anemia. High M. haemofelis loads (>9 × 105 copies/ml blood) seem to lead to anemia in acutely infected cats but not in recovered long-term carriers. A repeated evaluation of 17 cats documented that the infection was acquired in one case by blood transfusion and that there were important differences among species regarding whether or not antibiotic administration led to the resolution of bacteremia.
Hemobartonella felis, the causative agent of feline infectious anemia, has recently been reclassified as a hemotropic mycoplasma (hemoplasma), and two different species, named Mycoplasma haemofelis and “Candidatus Mycoplasma haemominutum,” have been recognized worldwide (6, 8, 11, 12, 16, 20, 22, 23, 27, 29, 31). In experimental infection studies, M. haemofelis seemed more pathogenic than “Candidatus Mycoplasma haemominutum” (11, 32), and an influence of retroviral status on the clinical manifestation of the latter is suspected (4, 13, 14). Recently, we identified a third novel and unique feline hemoplasma isolate, herein designated “Candidatus Mycoplasma turicensis,” in a Swiss cat with a history of severe intravascular hemolysis (33). After experimental transmission to two specific-pathogen-free (SPF) cats, “Candidatus Mycoplasma turicensis” induced mild to severe anemia. Phylogenetic analyses based on the 16S rRNA gene sequence data revealed that “Candidatus Mycoplasma turicensis” was closely related to Mycoplasma coccoides and Mycoplasma haemomuris, both rodent hemoplasma species (7, 15, 30).
This is the first study to investigate the prevalence, clinical manifestations, and risk factors for infection for all three feline hemoplasma species. Diagnosis, quantification, and follow-up of hemoplasma infection were performed using three newly designed sensitive real-time PCR assays.
MATERIALS AND METHODS
Sample collection.
A total of 996 feline EDTA-anticoagulated blood samples were available. They comprised initial samples from 713 Swiss cats and 283 follow-up samples that were sought specifically to monitor the course of hemoplasma infections. The initial samples were derived from 86 healthy and 627 ill cats. Ninety-eight cats were sampled because a hemoplasma infection was suspected (designated “preselected samples”).
Hematology, biochemistry, and urine analysis.
For the ill cats, all the parameters requested by the attending veterinarian were analyzed. For most of the healthy cats, only packed cell volume (PCV) values were determined. Hemograms were performed using a Cell-Dyn 3500 system (Abbott, Baar, Switzerland). Blood smears were Giemsa stained using an AMES Hema Tek slide stainer (Bayer, Zürich, Switzerland) and visually evaluated for white blood cell differential counts, red blood cell morphological characteristics, and the presence of hemoplasmas. Aggregate reticulocytes were visually counted after brilliant cresyl blue staining. Cats with a PCV of <33% (5% quantile [see below]) were considered anemic. Regeneration was defined by a reticulocyte count of >60,000 reticulocytes/μl. Serum biochemistry was performed using a Cobas Integra 700 system (Roche Diagnostics, Rotkreuz, Switzerland) and included bilirubin, glucose, blood urea nitrogen (BUN), creatinine, protein, albumin, cholesterol, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, lipase, sodium, chloride, potassium, and phosphorus. Urine analysis comprised gross appearance, specific gravity, urine pH (Neutralit; Merck AG, Dietikon, Switzerland), urine nitrite, protein, glucose, ketones, urobilinogen, bilirubin, blood (Combur-Test; Roche), and the urine protein-to-creatinine ratio (Cobas Integra 700). Reference ranges are stated as 5% and 95% quantiles.
DNA extraction.
DNA was purified from 200 μl blood using the MagNaPure LC DNA Isolation Kit I (Roche). To monitor for cross-contamination, negative controls consisting of 200 μl phosphate-buffered saline were concurrently prepared with each batch of 11 to 15 samples.
Development of quantitative real-time PCR assays and standards.
Species-specific oligonucleotides were designed as follows: for “Candidatus Mycoplasma haemominutum,” forward primer 5′-GAA AGT CTG ATG GAG CAA TAC CAT-3′, reverse primer 5′-CTG GCA CAT AGT TWG CTG TCA CTT A-3′, and probe 6-carboxyfluorescein-AAG GCT TAA TCA TTT CCT-minor groove binder were used; for M. haemofelis, forward primer 5′-GAA AGT CTG ATG GAG CAA TAC CAT-3′, reverse primer 5′-CTG GCA CAT AGT TWG CTG TCA CTT A-3′, and probe VIC-AGT ACT ATC ATA ATT ATC CCT CG-minor groove binder were used. For “Candidatus Mycoplasma turicensis,” a previously published real-time PCR assay was used (33). The PCR mixtures were comprised of 12.5 μl 2× qPCR Mastermix (Eurogentec, Seraing, Belgium), 880 nM of each primer, 200 nM probe, and 5 μl template DNA made up to 25 μl with water. The amplification buffer contained dUTP for use with uracil-N-glycosylase to prevent carryover of PCR amplicons. Assays were performed using an ABI PRISM 7700 sequence detection system (Applied Biosystems, Rotkreuz, Switzerland). The first 500 samples were subjected to conventional (16) and real-time PCR. The conventional PCR, although able to amplify all three feline hemoplasma isolates, cannot differentiate between M. haemofelis and “Candidatus Mycoplasma turicensis.” DNA samples from uninfected SPF cats and water were used as negative controls. DNA from the following bacteria was used to determine the specificity of the real-time PCR assays: Chlamydophila felis, Pasteurella multocida, Bartonella henselae, Mycoplasma penetrans, Mycoplasma equigenitalium, Mycoplasma argini, Mycoplasma agalactiae, Mycoplasma suis, Mycoplasma wenyonii, Mycoplasma haemocanis, and “Candidatus Mycoplasma haematoparvum.” For absolute quantification, plasmids containing the cloned 16S rRNA gene of “Candidatus Mycoplasma haemominutum,” M. haemofelis, or “Candidatus Mycoplasma turicensis” were generated as described below. Purified DNA was linearized, quantified, and serially 10-fold diluted in a solution of 30 μg/ml of salmon sperm DNA (Invitrogen, Basel, Switzerland).
Sequencing of 16S rRNA genes.
The near-complete 16S rRNA genes from 12 Swiss hemoplasma isolates (five “Candidatus Mycoplasma haemominutum,” four M. haemofelis, and three “Candidatus Mycoplasma turicensis” isolates) were sequenced. For “Candidatus Mycoplasma turicensis,” the sequencing method has been described previously (33). For “Candidatus Mycoplasma haemominutum” and M. haemofelis, species-specific primers (for “Candidatus Mycoplasma haemominutum,” forward primer 5′-AAG TCG AAC GAA GAG GGT TTA CTC-3′ and reverse primer 5′-TTW AAT ACG GTT TCA ACT AGT ACT TTC TCC-3′ were used; for M. haemofelis, forward primer 5′-TCG AAC GGA YYT TGG TTT CG-3′ and reverse primer 5′-CAA ATG AAT GTA TTT TTA AAT GCC CAC-3′ were used) that amplify 1,354 bp and 1,309 bp of the gene, respectively, were designed. The reaction mixture contained 5 μl of 5× HF PCR buffer (Finnzymes; BioConcept, Allschwil, Switzerland), 500 nM each primer, 200 μM each deoxynucleoside triphosphate (Sigma-Aldrich, Buchs, Switzerland), 1 U Phusion High-Fidelity DNA polymerase (Finnzymes), and 5 μl template DNA made up to 25 μl with water. The program was as follows: 98°C for 3 min; 35 cycles of 98°C for 10 s, 63 to 68°C for 30 s, and 72°C for 1 min; and a final elongation step at 72°C for 10 min. PCR products were gel purified with a MinElute gel extraction kit (QIAGEN, Hombrechtikon, Switzerland) and cloned using the Zero Blunt TOPO PCR cloning kit (Invitrogen), and plasmid DNA was purified using a QIAprep Spin Miniprep kit (QIAGEN). Sequencing was performed with M13 forward and reverse primers and an internal primer (5′-AGC AAT ACC ATG TGA ACG ATG AA-3′) using the BigDye Terminator cycle sequencing kit (Applied Biosystems) and standard cycling conditions. Products were purified with DyeEx Spin columns (QIAGEN) and analyzed with an ABI PRISM 310 genetic analyzer (Applied Biosystems). For sequence comparison, the GCG Wisconsin Package (Accelrys GmbH, Munich, Germany) was used.
Serological assays and FeLV PCR.
To diagnose feline leukemia virus (FeLV) infection, p27 antigenemia was detected by enzyme-linked immunosorbent assay (19); positive results were confirmed by real-time PCR (24). Feline immunodeficiency virus (FIV) antibodies were measured by enzyme-linked immunosorbent assay (5); positive results were confirmed by Western blot analysis (18).
Statistical evaluation.
For observed prevalences, 95% confidence intervals (CIs) were calculated. Up to 104 different parameters per cat were compiled and analyzed with Excel (Microsoft, Wallisellen, Switzerland), Analyze-it Clinical Laboratory (Analyze-it Software, Leeds, United Kingdom), and Prism (GraphPad, San Diego, CA) software. The data included date of blood collection; anamnesis (n = 19 [breed, age, gender, place of domicile, outdoor access, number of cats in the household, foreign travel, vaccination status, deworming, reason for clinical presentation, appetite, drinking, weight loss, constitution, defecation, urination, lameness, previous disease, and previous treatment]); clinical examination findings (n = 27); hematological (n = 16), biochemical (n = 16), and urine (n = 11) parameters; serology (n = 3 [FIV, FeLV, and feline coronavirus]); PCR analyses (n = 7); and diagnosis (n = 5). Correlation between PCV and hemoplasma load in blood (copy numbers of DNA template per milliliter of blood) was assessed by the Spearman rank correlation coefficient (rs). Blood loads were tested for statistical differences among cats infected with different hemoplasmas by the Kruskal-Wallis one-way analysis of variance by ranks and the Dunn's posttest for multiple comparisons. For spatial association, the results were analyzed based on the zip codes of the place of domicile of the sampled cats; the frequency of each hemoplasma infection was compared between cats living in the West (zip codes 1000 to <3000), in the South (zip codes 6500 to <7000), and in the North-East (the rest of the zip codes) of Switzerland. Risk factors for hemoplasma infections were analyzed with the Mann-Whitney U test for continuous variables (age, PCV, BUN, creatinine, protein, and lipase) and with Fisher's exact test (cell frequencies of ≤5) or chi-square test (cell frequencies of >5) for categorical variables (gender, breed, hemoplasma coinfection, FIV and FeLV infection, outdoor access, place of domicile, and renal insufficiency). Differences were considered significant if the P value was <0.05.
Nucleotide sequence accession numbers.
The 16S rRNA gene nucleotide sequences generated from “Candidatus Mycoplasma haemominutum,” “Candidatus Mycoplasma turicensis,” and M. haemofelis isolates have been submitted to GenBank under accession numbers DQ157141 to DQ157149, DQ157150 to DQ157154, and DQ157155 to DQ157160, respectively.
RESULTS
Hemoplasma-specific real-time PCR assays.
Amplification of the newly developed real-time PCR assays specific for “Candidatus Mycoplasma haemominutum,” M. haemofelis, and “Candidatus Mycoplasma turicensis” using 10-fold serial dilutions of plasmid standards was linear over 9 orders of magnitude. All cats found to be hemoplasma positive by conventional PCR yielded positive results in the corresponding specific real-time PCR. The real-time PCR assays showed no cross-reactivity with other feline hemoplasmas in that each yielded negative results when 104 copies/reaction of standard plasmid DNA of nonmatching hemoplasma species were used as a template. As expected from the sequence comparison, M. haemocanis-derived DNA tested positive in the real-time PCR assay for M. haemofelis, and “Candidatus Mycoplasma haematoparvum”-derived DNA was amplified by the real-time PCR assay for “Candidatus Mycoplasma haemominutum.” All other template DNA derived from various bacterial pathogens did not result in product amplification. All negative controls were negative. The PCR assays were able to specifically detect 1 copy/reaction of “Candidatus Mycoplasma haemominutum” (10/10 reactions positive), M. haemofelis (9/10 reactions positive), and “Candidatus Mycoplasma turicensis” (10/10 reactions positive), respectively.
Characteristics of cases.
Case characteristics are shown in Tables 1 and 2. Healthy cats were significantly younger, had a higher PCV, and more frequently had outdoor access than ill cats. Gender, breed, and frequency of FIV and FeLV infections were not significantly different between healthy and ill cats.
TABLE 1.
Gender, breed, FIV and FeLV infection status, and outdoor access of healthy and ill cats
| Variable | No. of healthy cats (%) | No. of ill cats (%) | Odds ratio | 95% CI | P |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 32 (37.2) | 263 (44.4) | 1.0 | ||
| Male | 54 (62.8) | 330 (55.6) | 0.74 | 0.47-1.2 | 0.26 |
| Breed | |||||
| Nonpedigree | 74 (86.0) | 529 (84.4) | 1.0 | ||
| Pedigree | 12 (14.0) | 98 (15.6) | 1.1 | 0.60-2.2 | 0.81 |
| FIV | |||||
| Negative | 82 (95.3) | 408 (96.0) | 1.0 | ||
| Positive | 4 (4.7) | 17 (4.0) | 0.85 | 0.28-2.6 | 0.97 |
| FeLV | |||||
| Negative | 83 (96.5) | 437 (96.9) | 1.0 | ||
| Positive | 3 (3.5) | 14 (3.1) | 0.89 | 0.25-3.2 | 1.0 |
| Outdoor access | |||||
| No | 11 (14.5) | 187 (38.1) | 1.0 | ||
| Yes | 65 (85.5) | 304 (61.9) | 0.28 | 0.14-0.54 | 0.0001 |
TABLE 2.
Median age and PCV in healthy and ill cats
| Variable and cat group | No. of cats | Median | 95% CI | P |
|---|---|---|---|---|
| Age (yr) | ||||
| Healthy cats | 82 | 1.8 | 1.0-2.0 | <0.0001 |
| Ill cats | 560 | 8.0 | 7.0-9.0 | |
| PCV (%) | ||||
| Healthy cats | 86 | 36 | 35-38 | <0.0001 |
| Ill cats | 587 | 34 | 33-34 |
Prevalence.
Of the 615 nonpreselected samples, 52 (8.5%; 95% CI, 6.3% to 10.7%) tested PCR positive for “Candidatus Mycoplasma haemominutum,” 3 (0.5%; 95% CI, 0% to 1.1%) tested positive for M. haemofelis, and 6 (1%; 95% CI, 0.2% to 1.8%) tested positive for “Candidatus Mycoplasma turicensis.” Three of the six “Candidatus Mycoplasma turicensis”-infected cats were coinfected with “Candidatus Mycoplasma haemominutum” (50%; P = 0.022). When the preselected samples were included (total, n = 713), 71 (10.0%; 95% CI, 7.8% to 12.2%) tested PCR positive for “Candidatus Mycoplasma haemominutum,” 11 (1.5%; 95% CI, 0.6% to 2.4%) tested positive for M. haemofelis, and 9 (1.3%; 95% CI, 0.5% to 2.1%) tested positive for “Candidatus Mycoplasma turicensis.” Six of the nine “Candidatus Mycoplasma turicensis”-infected cats were coinfected with “Candidatus Mycoplasma haemominutum” (67%; P = 0.0001), whereas 2 of the 71 “Candidatus Mycoplasma haemominutum”-infected animals tested positive for M. haemofelis.
Antibiotic treatment with enrofloxacin or doxycycline has been shown to be effective against hemoplasma infection (10, 28) and could therefore influence the detectability of these agents by PCR. Concurrent antibiotic treatment was reported for 73 cats: 16 cats were treated with enrofloxacin, 4 cats were treated with doxycycline, and 53 cats were treated with other antibiotics. One of 11 (9%) samples from doxycycline-treated cats, 2 of 43 (4.7%) samples from enrofloxacin-treated cats, and 7 of 105 (6.7%) samples derived from cats treated with other antibiotics gave positive PCR results.
Sequencing of 16S rRNA gene.
To confirm the PCR results and to evaluate sequence variations among hemoplasma isolates, the 16S rRNA genes from all three feline hemoplasma species were sequenced. These data revealed more than 98% similarity with previously published hemoplasma sequences (GenBank accession numbers AF271154, AY150977, and AY831867) (data not shown).
Characteristics of hemoplasma PCR-positive cats.
Among nonpreselected cats, the prevalence of the three hemoplasma species was not significantly different between healthy and ill cats, and hemoplasma PCR-positive status was not associated with anemia. Hemoplasma-infected cats were significantly older than hemoplasma-uninfected cats (Table 3), and infection was associated with male gender, outdoor access (Table 4), and living in the South and West of Switzerland (Table 5). No significant difference was found for any hematology or biochemistry parameter between hemoplasma-infected and hemoplasma-uninfected ill cats (data not shown).
TABLE 3.
Median age of hemoplasma- or “Candidatus Mycoplasma haemominutum”-negative and -positive cats
| Organism and cat group | PCR negative
|
PCR positive
|
P | ||||
|---|---|---|---|---|---|---|---|
| No. of cats | Median age (yr) | 95% CI (yr) | No. of cats | Median age (yr) | 95% CI (yr) | ||
| Hemoplasma | |||||||
| Healthy cats | 74 | 1.0 | 1.0-2.0 | 8 | 9.5 | 1.0-13 | 0.0029 |
| Ill cats | 497 | 7.0 | 6.0-8.0 | 63 | 10 | 10-12 | 0.0005 |
| “Candidatus Mycoplasma haemominutum” | |||||||
| Healthy cats | 76 | 1.0 | 1.0-2.0 | 6 | 12 | 4.0-13 | 0.0006 |
| Ill cats | 504 | 7.0 | 6.0-8.0 | 56 | 11 | 10-12 | 0.0002 |
TABLE 4.
Gender and outdoor access of hemoplasma-, “Candidatus Mycoplasma haemominutum”-, and M. haemofelis-positive cats
| Organism and variable | No. of PCR
|
Odds ratio | 95% CI | P | |
|---|---|---|---|---|---|
| Negative (%) | Positive (%) | ||||
| Hemoplasma | |||||
| Gender | |||||
| Female | 276 (45.8) | 19 (24.7) | 1.0 | ||
| Male | 326 (54.2) | 58 (75.3) | 2.6 | 1.5-4.4 | 0.0007 |
| Outdoor access | |||||
| No | 188 (36.9) | 11 (19.0) | 1.0 | ||
| Yes | 322 (63.1) | 47 (81.0) | 2.5 | 1.3-4.9 | 0.010 |
| “Candidatus Mycoplasma haemominutum” | |||||
| Gender | |||||
| Female | 277 (45.3) | 18 (26.9) | 1.0 | ||
| Male | 335 (54.7) | 49 (73.1) | 2.3 | 1.3-4.0 | 0.0059 |
| Outdoor access | |||||
| No | 190 (36.8) | 9 (17.6) | 1.0 | ||
| Yes | 327 (63.2) | 42 (82.4) | 2.7 | 1.3-5.7 | 0.010 |
| M. haemofelis | |||||
| Gender | |||||
| Female | 295 (44.0) | 0 (0) | 1.0 | ||
| Male | 375 (56.0) | 9 (100) | 15 | 0-57 | 0.011 |
TABLE 5.
Place of domicile of hemoplasma-, “Candidatus Mycoplasma haemominutum”-, and M. haemofelis-negative and -positive cats
| Organism and place of domicile | No. (%) of cats with PCR result was:
|
Odds ratio | 95% CI | P | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Hemoplasma | |||||
| South/West of Switzerland | 23 (4.0) | 8 (19.0) | 5.6 | 2.3-13 | <0.0001 |
| Rest of Switzerland | 548 (96.0) | 34 (81.0) | 1.0 | ||
| “Candidatus Mycoplasma haemominutum” | |||||
| South/West of Switzerland | 26 (4.5) | 5 (15.6) | 4.0 | 1.4-11 | 0.017 |
| Rest of Switzerland | 555 (95.5) | 27 (84.4) | 1.0 | ||
| M. haemofelis | |||||
| South/West of Switzerland | 27 (4.5) | 4 (44.4) | 17 | 4.3-67 | 0.0011 |
| Rest of Switzerland | 577 (95.5) | 5 (55.6) | 1.0 | ||
Characteristics of “Candidatus Mycoplasma haemominutum” PCR-positive cats.
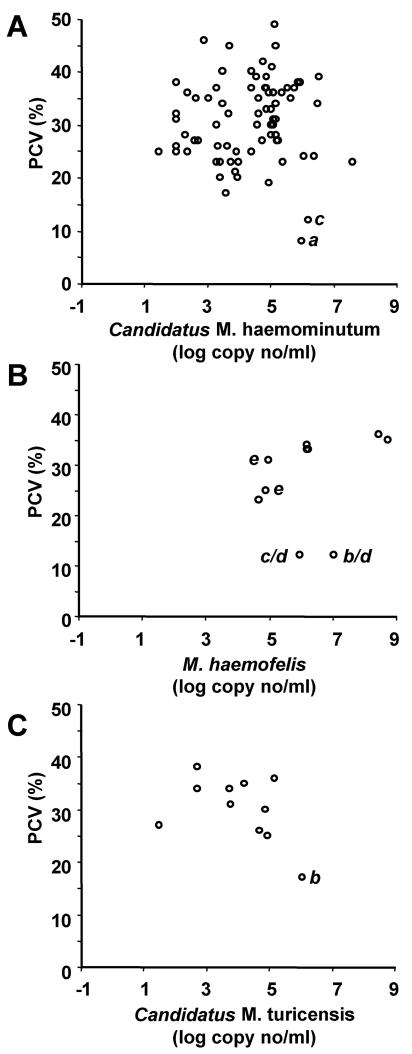
Including the preselected samples, 71 of 713 cats tested “Candidatus Mycoplasma haemominutum” positive; infected cats were significantly older than uninfected cats (Table 3), and PCR-positive status was associated with male gender, outdoor access (Table 4), and living in the South and West of Switzerland (Table 5). In the ill cat population, “Candidatus Mycoplasma haemominutum”-infected cats had significantly higher BUN, creatinine, protein, and lipase levels (Table 6) and were more frequently diagnosed with renal insufficiency (P = 0.021) than cats not infected with “Candidatus Mycoplasma haemominutum.” In addition, infection with “Candidatus Mycoplasma haemominutum” was significantly associated with “Candidatus Mycoplasma turicensis” coinfection (P = 0.0001) but not with retrovirus infections. Hematological parameters were not different between “Candidatus Mycoplasma haemominutum”-infected and “Candidatus Mycoplasma haemominutum”-uninfected cats, and infection was not associated with anemia. When 75 PCR-positive samples (from 71 cats) were analyzed, no correlation between PCV and “Candidatus Mycoplasma haemominutum” loads in the blood could be found (Fig. 1A) (rs = 0.11; CI, −0.12 to 0.33; P = 0.35). Two of the “Candidatus Mycoplasma haemominutum”-infected cats exhibited PCVs of <15% with signs of acute intravascular hemolysis; one cat was infected with FeLV and was diagnosed with lymphatic leukemia, while the second cat was coinfected with M. haemofelis.
TABLE 6.
Selected biochemical blood parameters of “Candidatus Mycoplasma haemominutum” PCR-negative and -positive ill cats
| Variable | PCR negative
|
PCR positive
|
Reference range | P | ||||
|---|---|---|---|---|---|---|---|---|
| No. of cats | Median | 95% CI | No. of cats | Median | 95% CI | |||
| BUN (mg/dl) | 438 | 23.6 | 11.5-86.2 | 40 | 29.2 | 12.6-78.3 | 20.8-35.4 | 0.014 |
| Creatinine (μmol/liter) | 438 | 116 | 61.4-392 | 39 | 145 | 82.9-472 | 98.0-163 | 0.0003 |
| Protein (g/liter) | 436 | 68.0 | 49.0-82.3 | 37 | 72.0 | 57.4-87.8 | 64.0-80.0 | 0.0009 |
| Lipase (U/liter) | 407 | 17.0 | 8.0-55.8 | 35 | 20.0 | 12.4-117 | 8.0-26.0 | 0.019 |
FIG. 1.
Correlation between PCV (percent) (y axis) and hemoplasma load (log DNA copy number per milliliter of blood) (x axis) in cats infected with (A) “Candidatus Mycoplasma haemominutum,” (B) M. haemofelis, and (C) “Candidatus Mycoplasma turicensis.” All PCR-positive samples were included. Specific cats are indicated as follows: a, FeLV-positive cats; b, FIV-positive cats; c, cats coinfected with “Candidatus Mycoplasma haemominutum” and M. haemofelis; d, cats reported as having acute hemolysis at the time of blood collection; e, cats reported as having acute hemolysis within the last 2 months before blood collection.
Characteristics of M. haemofelis PCR-positive cats.
Including the preselected samples, 11 of 713 cats tested M. haemofelis positive. Infection was significantly associated with male gender (Table 4) and living in the South and West of Switzerland (Table 5). All M. haemofelis-positive cats were nonpedigree cats ranging in age from 1 to 10 years. The retroviral status of five cats was known: one tested FeLV positive, and another cat tested FIV positive. Nine out of 11 samples were from ill cats including two cats with acute hemolysis at the time of sampling and three cats that had shown signs of hemolysis within 2 months prior to sampling but that had recovered after doxycycline-prednisolone treatment. No association between PCV and M. haemofelis loads in the blood was found (Fig. 1B) (n = 10; rs = 0.57; CI, −0.09 to 0.88; P = 0.084). Three healthy cats with PCV values within the reference range had M. haemofelis loads of 1.6 × 106 to 2.8 × 108 copies/ml blood. Conversely, two cats with moderate loads of 9.2 × 105 and 1.1 × 107 copies/ml blood had low PCV values of 12% (Fig. 1B). Both animals were reported to have acute hemolysis at the time of blood collection. One of these cats tested FIV positive and was euthanized for humane reasons due to deteriorating health.
Characteristics of “Candidatus Mycoplasma turicensis” PCR-positive cats.
Including the preselected samples, 9 of 713 cats tested “Candidatus Mycoplasma turicensis” positive. These nine cats were all ill, nonpedigree cats from 3 to 16 years of age. Seven cats were castrated males, and two cats were female (one intact, one neutered). The retroviral status of four cats was known, and one cat was FIV positive. Three of seven “Candidatus Mycoplasma turicensis”-infected cats were anemic. All five “Candidatus Mycoplasma turicensis”-infected cats for which the modus vivendi was reported had outdoor access. No correlation between “Candidatus Mycoplasma turicensis” loads and PCV was found (Fig. 1C) (n = 11; rs = −0.38; CI, −0.80 to 0.28; P = 0.25). One cat with a relatively high load and low PCV was coinfected with FIV. “Candidatus Mycoplasma turicensis”-infected cats exhibited significantly lower hemoplasma loads in the blood (median copy number, 5.0 × 102 copies/ml) than “Candidatus Mycoplasma haemominutum”-infected cats (median copy number, 7.8 × 104 copies/ml; P = 0.0099; Dunn's posttest, P < 0.05) and M. haemofelis-infected cats (median copy number, 5.1 × 105 copies/ml; P = 0.0099; Dunn's posttest, P < 0.01). The diagnoses of seven of the nine “Candidatus Mycoplasma turicensis”-infected cats were known: three cats had neoplasia (malignant lymphoma, hepatocellular carcinoma, or fibrosarcoma), one cat had lower urinary tract disease, one cat had a mandibular fracture, one cat had lacerations, and one cat had immune-mediated hemolytic anemia. Transmission of heparinized blood from the latter cat to two SPF cats induced anemia in the recipients (33).
Course of “Candidatus Mycoplasma haemominutum” infection.
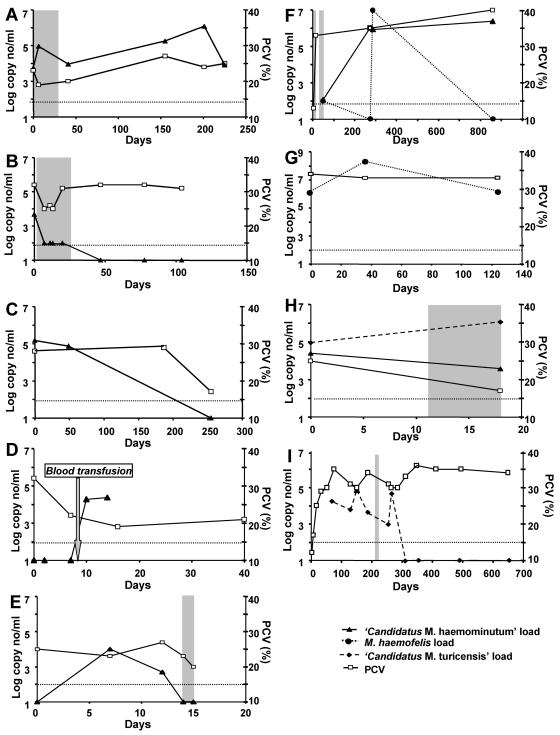
Follow-up blood samples were available for 15 “Candidatus Mycoplasma haemominutum”-infected cats (Table 7 and Fig. 2). Time periods of follow-up reached up to 8.5 months. For 11 cats, all blood samples collected within a period of several days to >7 months (two to six samples per cat) tested PCR positive. Six of the 15 “Candidatus Mycoplasma haemominutum”-infected cats were treated with antibiotics during follow-up (cats 06, 51, 71, 74, 98, and 880) (Table 7). For four of these cats (cats 71, 74, 98, and 880), each sample that was collected tested PCR positive (up to 810 days of follow-up), independent of antibiotic treatment. However, “Candidatus Mycoplasma haemominutum” loads decreased during treatment, with a 6-fold reduction with doxycycline or spiramycin-metronidazole treatment and a 10-fold reduction with enrofloxacin-amoxicillin treatment. Two of these cats were retrovirus positive: cat 71 was FeLV positive, and cat 98 was FIV positive. In cat 71, an increase in hemoplasma load to >106 copies/ml blood occurred several months after antibiotic treatment, yet this was not associated with a drop in PCV (Fig. 2). Three of the 15 “Candidatus M. haemominutum”-positive cats became PCR negative during the follow-up period (cats 06, 51, and 78) (Fig. 2); after enrofloxacin-amoxicillin treatment, cat 06 stayed PCR negative for the remaining follow-up time (three samples, 1 month apart). This cat exhibited already low “Candidatus Mycoplasma haemominutum” loads before treatment (100 to 4,700 copies/ml). Cat 78 yielded a negative PCR result on the last blood sample taken, yet no antibiotic treatment was reported. Cat 86 became infected with “Candidatus Mycoplasma haemominutum” via a blood transfusion. This cat had nasal mycosis with acute epistaxis and had received 56 ml of whole blood from an unrelated blood donor cat. After transfusion, cat 86 became PCR positive, with loads of 1.9 × 104 to 2.3 × 104 copies/ml blood. No blood sample from the donor cat was available for PCR analysis.
TABLE 7.
Course of infection in “Candidatus Mycoplasma haemominutum,” “Candidatus Mycoplasma turicensis,” and M. haemofelis PCR-positive cats
| Cat | Observation period (days) | No. of PCR-positive samples/total samples analyzed | Hemoplasma species | Copies/ml blood (range) | Antibiotic treatment |
|---|---|---|---|---|---|
| 03 | 6 | 2/2 | “Candidatus Mycoplasma haemominutum” | 1.1 × 105-1.2 × 105 | Not reported |
| 06 | 104 | 5/8 | “Candidatus Mycoplasma haemominutum” | 0-4.7 × 103 | Enrofloxacin, amoxicillin |
| 22 | 2 | 2/2 | “Candidatus Mycoplasma haemominutum” | 4.1 × 102-4.3 × 102 | Not reported |
| 41 | 1 | 2/2 | “Candidatus Mycoplasma haemominutum” | 2.5 × 104-1.1 × 105 | Not reported |
| 51 | 15 | 5/2 | “Candidatus Mycoplasma haemominutum” | 0-1.0 × 104 | Amoxicillin |
| 20 | 41 | 2/2 | “Candidatus Mycoplasma haemominutum” | 1.2 × 105-1.7 × 106 | Not reported |
| 61 | 100 | 2/2 | “Candidatus Mycoplasma haemominutum” | 1.0 × 105-1.2 × 105 | Not reported |
| 71 | 225 | 6/6 | “Candidatus Mycoplasma haemominutum” | 5.7 × 103-1.2 × 106 | Enrofloxacin, amoxicillin |
| 74 | 25 | 2/2 | “Candidatus Mycoplasma haemominutum” | 3.8 × 104-2.3 × 105 | Doxycycline |
| 78 | 255 | 2/3 | “Candidatus Mycoplasma haemominutum” | 0-1.5 × 105 | Not reported |
| 81 | 14 | 2/2 | “Candidatus Mycoplasma haemominutum” | 1.1 × 105-3.5 × 105 | Not reported |
| 86 | 14 | 2/5 | “Candidatus Mycoplasma haemominutum” | 0-2.3 × 104 | Not reported |
| 97 | 49 | 3/3 | “Candidatus Mycoplasma haemominutum” | 1.9 × 103-2.5 × 104 | Not reported |
| 880 | 854 | 4/4 | “Candidatus Mycoplasma haemominutum” | 1.2 × 102-2.4 × 106 | Tetracycline |
| 2/4 | M. haemofelis | 1 × 102-9.7 × 106 | |||
| 98 | 18 | 2/2 | “Candidatus Mycoplasma haemominutum” | 3.8 × 103-2.5 × 104 | Spiramycin, metronidazole |
| 2/2 | “Candidatus Mycoplasma turicensis” | 9.1 × 104-1.2 × 106 | |||
| 15 | 124 | 3/3 | M. haemofelis | 1.6 × 106-2.0 × 108 | Not reported |
| 946 | 646 | 6/10 | “Candidatus Mycoplasma turicensis” | 0-5.2 × 104 | Doxycycline |
FIG. 2.
Course of “Candidatus Mycoplasma haemominutum” (A to F and H), M. haemofelis (F to G), and “Candidatus Mycoplasma turicensis” (H and I) infections. The loads are presented as log DNA copy number per milliliter of blood (left y axis); the PCV is given as a percentage (right y axis). (A) Cat 71; (B) cat 06; (C) cat 78; (D) cat 86; (E) cat 51; (F) cat 880; (G) cat 15; (H) cat 98; (I) cat 946. Periods of antibiotic treatment are indicated by gray areas; treatments are listed in Table 7. The time point of blood transfusion for cat 86 is indicated by an arrow (D). The lower detection limit of the PCR assays (100 copies/ml blood) is indicated by a fine dotted line.
Course of M. haemofelis infection.
Follow-up blood samples for M. haemofelis infection were available for two cats (cats 15 and 880) (Table 7 and Fig. 2). Cat 15 was monitored for 124 days and was M. haemofelis PCR positive on all samples, with loads ranging from 1.6 × 106 to 2.0 × 108 copies/ml blood. On the second and third samples, the cat tested FeLV positive but subsequently overcame FeLV antigenemia. Despite the presence of FeLV infection and high hemoplasma loads, this cat remained healthy throughout and did not receive antibiotics at any time.
Four blood samples collected during a follow-up period of 28 months were available from one cat (cat 880) coinfected with M. haemofelis and “Candidatus Mycoplasma haemominutum” (Table 7 and Fig. 2). The cat had a history of acute hemolysis with pale mucous membranes, pyrexia, and severe anemia (PCV, 13%) 44 days before the first blood sample was analyzed. Following 4 weeks of treatment with tetracycline and prednisolone, the cat recovered, and no relapse was reported. The cat tested PCR positive for “Candidatus Mycoplasma haemominutum” for each sample analyzed, with loads ranging from 1.2 × 102 to 2.4 × 106 copies/ml blood. The M. haemofelis loads varied dramatically, and only two of four samples tested PCR positive. Within 12 days, a negative result and a positive result with a very high load of 9.7 × 106 copies/ml blood were found, yet antibiotic treatment was not given during this period.
Course of “Candidatus Mycoplasma turicensis” infection.
Follow-up samples were available for two cats (cats 946 and 98) infected with “Candidatus Mycoplasma turicensis.” Cat 946, which was diagnosed with immune-mediated hemolytic anemia (33), was monitored for 21 months (10 blood samples) (Table 7 and Fig. 2). Before antibiotic treatment, the cat tested “Candidatus Mycoplasma turicensis” positive for each of the six blood samples analyzed over a period of >6 months, with loads ranging from 9.6 × 102 to 7.4 × 104 copies/ml blood. After doxycycline treatment (10 mg/kg/day orally for 14 days), all four samples collected over a period of 1 year were PCR negative for “Candidatus Mycoplasma turicensis.”
Cat 98, which was coinfected with “Candidatus Mycoplasma haemominutum” and “Candidatus Mycoplasma turicensis,” was monitored for 18 days (Table 7 and Fig. 2). The cat was FIV positive and had gingival lymphoma. During the follow-up period, the cat was irradiated four times and received spiramycin-metronidazole treatment for 9 days. Antibiotic treatment was not associated with decreased “Candidatus Mycoplasma turicensis” loads, and, indeed, loads increased 6.6-fold. The cat was highly anemic during the entire observation period, although hemolysis or regeneration was not evident.
DISCUSSION
This is the first study to show that all three feline hemoplasma species are prevalent in pet cats. The study was performed with three newly designed real-time PCR assays that proved to be highly sensitive and specific and thus accurate for the diagnosis of feline hemoplasma infections.
In agreement with previously published studies (16, 25, 26), “Candidatus Mycoplasma haemominutum” was also the most prevalent hemoplasma in Swiss cats. The frequency of “Candidatus Mycoplasma turicensis” infection was found to be similarly low compared to that of M. haemofelis infection. The overall prevalence of hemoplasma infections in Swiss cats was lower than that reported in the three aforementioned studies, pointing to geographical variations in the distribution of feline hemoplasmal species. Sequencing of a number of 16S rRNA genes from isolates derived from Switzerland revealed almost-complete identity to American, British, Australian, South African, and Swiss isolates (16, 27, 29, 33).
There was no significant difference in hemoplasma prevalence between healthy and ill cats. This could imply that these agents have a low pathogenic potential. Alternatively, the prevalence in the healthy cats investigated in this study could have been overestimated, since they had significantly more outdoor access than the ill cats. This could also explain why retroviral infections were similarly prevalent in healthy and ill cats. In addition, some of the ill cats had been treated with doxycycline and enrofloxacin prior to blood sampling, which could have led to the resolution of bacteremia and thus to an underestimation of hemoplasma prevalence in ill cats.
Hemoplasma infection was not associated with anemia, and hemoplasma blood loads were not correlated with PCV. This appears especially remarkable for M. haemofelis; it is in contrast to data from a previous study (26), and the pathogenic potential of this agent has been demonstrated previously by different authors (11, 32). We found acute hemolysis in 5 of 11 M. haemofelis-infected cats, either at the time or within 2 months of blood collection. However, high loads (>106 copies/ml blood) were also observed in healthy animals. One of these cats had recently undergone hemolysis but had recovered completely. This led us to hypothesize that cats acutely infected with M. haemofelis indeed develop severe hemolysis in the presence of high hemoplasma loads in blood but that chronically infected animals that recover from acute illness can lack clinical signs, even in the presence of remarkably high loads in the blood.
Two “Candidatus Mycoplasma haemominutum”-infected cats had a PCV of <15% with signs of hemolysis. One of these cats was FeLV positive with lymphatic leukemia, while the other cat was coinfected with M. haemofelis. Our findings agree with previous observations that “Candidatus Mycoplasma haemominutum” alone does not usually lead to significant anemia and that cofactors are involved in the development of disease (11, 13). This also seems to be the case for “Candidatus Mycoplasma turicensis” infections. The highest “Candidatus Mycoplasma turicensis” load was observed in an anemic FIV-coinfected cat, which had developed lymphopenia and gingival lymphoma. Interestingly, we found that hemoplasma prevalence and loads in blood were similar in retrovirus-infected and uninfected cats. We therefore assume that a retrovirus-positive status alone is insufficient to induce hemoplasma disease and that retrovirus-induced immunosuppression or interaction with blood progenitor cells is required for the manifestation of hemoplasma-induced anemia.
Cats with outdoor access were more likely to be infected with hemoplasmas, supporting the hypothesis that these agents are indirectly transmitted by blood-sucking arthropods like ticks and fleas (17, 34). In line with this, we found geographical variations in the distribution of hemoplasma infections within Switzerland. The West and South, where infections were more prevalent than in the remainder of the country, are known for higher mean annual temperatures and for harboring additional kinds of arthropods, i.e., Rhipicephalus sanguineus and Dermacentor reticulatus (1-3). However, in agreement with previous studies (25, 26), the high prevalence of hemoplasma infections in male cats speaks for a direct transmission. Recently, it was demonstrated that a bold attitude makes outdoor male cats more vulnerable to pathogens that are directly transmitted, such as FIV (21). In addition, both “Candidatus Mycoplasma haemominutum” and “Candidatus Mycoplasma turicensis” have been detected by PCR in saliva of infected cats (9; B. Willi, unpublished data). Whichever transmission prevails, the finding that >50% of “Candidatus Mycoplasma turicensis”-infected cats were coinfected with “Candidatus Mycoplasma haemominutum” suggests that these two hemoplasma species exhibit a similar way of transmission.
Overall, “Candidatus Mycoplasma turicensis” loads in blood were found to be lower than those of M. haemofelis and “Candidatus Mycoplasma haemominutum.” While no explanation can be given for this observation, it might explain why “Candidatus Mycoplasma turicensis” could not affirmatively be identified visually by light microscopy on stained blood smears.
The present study observed for the first time an association between “Candidatus Mycoplasma haemominutum” PCR-positive status and elevated BUN, creatinine, protein, and lipase blood levels and the diagnosis of renal insufficiency. This association could be causal but could also be accounted for by, e.g., the older age of infected cats.
Interestingly, we observed different responses of the three feline hemoplasma species to antibiotic treatment. A cat infected with “Candidatus Mycoplasma turicensis” was able to clear bacteremia after doxycycline treatment. The cat remained PCR negative during the year-long follow-up period. In line with this, two SPF cats experimentally infected with “Candidatus Mycoplasma turicensis” eliminated the agent spontaneously from peripheral blood (33). One of these cats remained PCR negative in the 330-day follow-up period; immunosuppression with methylprednisolone did not reactivate “Candidatus Mycoplasma turicensis” infection in this cat (B. Willi, unpublished). Similarly, 3 of 15 “Candidatus Mycoplasma haemominutum”-infected cats became PCR negative either without antibiotic treatment or after antibiotic intervention with enrofloxacin-doxycycline or amoxicillin. In contrast, spontaneous or treatment-induced elimination of M. haemofelis was not observed, although in one cat, loads in the blood fluctuated markedly, and the animal transiently tested PCR negative for M. haemofelis.
It can be concluded from the present study that “Candidatus Mycoplasma turicensis” is common in the Swiss pet cat population. The pathogenic potential of this isolate seems to depend mainly on cofactors such as iatrogenic or retrovirus-induced immunosuppression or other as-yet-undefined factors. Our observations suggest that doxycycline is effective against this hemoplasma species. Treatment of infected cats should be considered even in the absence of anemia, as apparent clearance of the organism after antibiotic therapy was observed, and immunosuppression or stress in an infected cat may lead to severe acute hemolysis. Hemoplasma infection can be transmitted via blood transfusion. As the recipient animals are often in poor health, infection with M. haemofelis, but also with “Candidatus Mycoplasma haemominutum” or “Candidatus Mycoplasma turicensis,” could represent a life-threatening event. The screening of blood donor cats for infection with all three feline hemoplasma species using sensitive and specific PCR assays prior to transfusion is therefore highly recommended.
Acknowledgments
We thank the practicing veterinarians for their help in obtaining samples for the study. We thank M. M. Wittenbrink and L. Hoelzle (University of Zurich, Switzerland) as well as M. G. Doherr (University of Berne, Switzerland) and G. Regula (Swiss Federal Veterinary Office, Switzerland) for helpful contributions and excellent support. Special thanks go to B. Weibel, T. Meili Prodan, E. Gönczi, B. Pineroli, N. Tschopp, A. Pepin, R. Tandon, E. Rogg, E. Rhiner, Y. Bosshart, C. Brönnimann, U. Egger, E. Grässli, M. Huder, B. Lange, J. Setz, and J. Wälchli for excellent laboratory assistance. Laboratory work was performed using the logistics of the Center for Clinical Studies at the Vetsuisse Faculty of the University of Zurich.
This work was supported by a research grant (Forschungskredit 2002) of the University of Zurich and by Merial GmbH, Germany. R.H.-L. is the recipient of a professorship by the Swiss National Science Foundation (PP00B-102866). These studies were conducted by B.W. as partial fulfillment of the requirements for a Ph.D. degree at the Vetsuisse Faculty, University of Zurich.
REFERENCES
- 1.Aeschlimann, A., W. Büttiker, A. Elbl, and H. Hoogstraal. 1965. A propos des tiques de Suisse (Arachnoidea, Acarina, Ixodoidea). Rev. Suisse Zool. 72:577-583. [Google Scholar]
- 2.Aeschlimann, A., S. Schneeberger, K. Pfister, W. Burgdorfer, and A. Cotty. 1986. Données nouvelles sur les tiques ixodides du canton du Tessin (Suisse) et sur la présence d'agents rickettsiens dans leur hémolymphe. Annu. Soc. Helv. Sci. Nat. 1:58-68. [Google Scholar]
- 3.Bernasconi, M. V., C. Valsangiacomo, T. Balmelli, O. Péter, and J. C. Piffaretti. 1996. Zoonosi da zecche nel canton Ticino: aspetti faunistici ed epidemiologici. Boll. Soc. Tic. Sci. Nat. 84:15-24. [Google Scholar]
- 4.Bobade, P. A., A. S. Nash, and P. Rogerson. 1988. Feline haemobartonellosis: clinical, haematological and pathological studies in natural infections and the relationship to infection with feline leukaemia virus. Vet. Rec. 122:32-36. [DOI] [PubMed] [Google Scholar]
- 5.Calzolari, M., E. Young, D. Cox, D. Davis, and H. Lutz. 1995. Serological diagnosis of feline immunodeficiency virus infection using recombinant transmembrane glycoprotein. Vet. Immunol. Immunopathol. 46:83-92. [DOI] [PubMed] [Google Scholar]
- 6.Clark, P., S. F. Foster, and P. B. Spencer. 2002. Detection of Haemobartonella felis (Candidatus Mycoplasma haemofelis) in Australia that is similar to the ‘Ohio’ strain. Aust. Vet. J. 80:703-704. [DOI] [PubMed] [Google Scholar]
- 7.Cox, H. W., and G. Calaf-Iturri. 1976. Autoimmune factors associated with anaemia in acute Haemobartonella and Eperythrozoon infections of rodents. Ann. Trop. Med. Parasitol. 70:73-79. [DOI] [PubMed] [Google Scholar]
- 8.Criado-Fornelio, A., A. Martinez-Marcos, A. Buling-Sarana, and J. C. Barba-Carretero. 2003. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet. Microbiol. 93:307-317. [DOI] [PubMed] [Google Scholar]
- 9.Dean, R., C. R. Helps, T. J. Gruffydd-Jones, and S. Tasker. 2005. Use of real-time PCR to detect Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in the salivary glands of haemoplasma-infected cats. In BSAVA 2005 Proceedings. British Small Animal Veterinary Association, Gloucester, United Kingdom.
- 10.Dowers, K. L., C. Olver, S. V. Radecki, and M. R. Lappin. 2002. Use of enrofloxacin for treatment of large-form Haemobartonella felis in experimentally infected cats. J. Am. Vet. Med. Assoc. 221:250-253. [DOI] [PubMed] [Google Scholar]
- 11.Foley, J. E., S. Harrus, A. Poland, B. Chomel, and N. C. Pedersen. 1998. Molecular, clinical, and pathologic comparison of two distinct strains of Haemobartonella felis in domestic cats. Am. J. Vet. Res. 59:1581-1588. [PubMed] [Google Scholar]
- 12.Foley, J. E., and N. C. Pedersen. 2001. ‘Candidatus Mycoplasma haemominutum,’ a low-virulence epierythrocytic parasite of cats. Int. J. Syst. Evol. Microbiol. 51:815-817. [DOI] [PubMed] [Google Scholar]
- 13.George, J. W., B. A. Rideout, S. M. Griffey, and N. C. Pedersen. 2002. Effect of preexisting FeLV infection or FeLV and feline immunodeficiency virus coinfection on pathogenicity of the small variant of Haemobartonella felis in cats. Am. J. Vet. Res. 63:1172-1178. [DOI] [PubMed] [Google Scholar]
- 14.Harrus, S., E. Klement, I. Aroch, T. Stein, H. Bark, E. Lavy, M. Mazaki-Tovi, and G. Baneth. 2002. Retrospective study of 46 cases of feline haemobartonellosis in Israel and their relationships with FeLV and FIV infections. Vet. Rec. 151:82-85. [DOI] [PubMed] [Google Scholar]
- 15.Iralu, V., and K. D. Ganong. 1983. Agglutination of mouse erythrocytes by Eperythrozoon coccoides. Infect. Immun. 39:963-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen, W. A., M. R. Lappin, S. Kamkar, and W. J. Reagan. 2001. Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis in naturally infected cats. Am. J. Vet. Res. 62:604-608. [DOI] [PubMed] [Google Scholar]
- 17.Lappin, M. R., B. Griffin, J. Brunt, A. Riley, D. Burney, J. Hawley, M. M. Brewer, and W. A. Jensen. 2005. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J. Feline Med. Surg. [Online.] doi: 10.1016/j.jfms.2005.08.003. [DOI] [PMC free article] [PubMed]
- 18.Lutz, H., P. Arnold, U. Hubscher, H. Egberink, N. Pedersen, and M. C. Horzinek. 1988. Specificity assessment of feline T-lymphotropic lentivirus serology. Zentbl. Veterinarmed. B 35:773-778. [DOI] [PubMed] [Google Scholar]
- 19.Lutz, H., N. C. Pedersen, R. Durbin, and G. H. Theilen. 1983. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. J. Immunol. Methods 56:209-220. [DOI] [PubMed] [Google Scholar]
- 20.Messick, J. B., L. M. Berent, and S. K. Cooper. 1998. Development and evaluation of a PCR-based assay for detection of Haemobartonella felis in cats and differentiation of H. felis from related bacteria by restriction fragment length polymorphism analysis. J. Clin. Microbiol. 36:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natoli, E., L. Say, S. Cafazzo, R. Bonanni, M. Schmid, and D. Pontier. 2005. Bold attitude makes male urban feral domestic cats more vulnerable to feline immunodeficiency virus. Neurosci. Biobehav. Rev. 29:151-157. [DOI] [PubMed] [Google Scholar]
- 22.Neimark, H., K. E. Johansson, Y. Rikihisa, and J. G. Tully. 2001. Proposal to transfer some members of the genera Haemobartonella and Eperythrozoon to the genus Mycoplasma with descriptions of ‘Candidatus Mycoplasma haemofelis,’ ‘Candidatus Mycoplasma haemomuris,’ ‘Candidatus Mycoplasma haemosuis’ and ‘Candidatus Mycoplasma wenyonii.’ Int. J. Syst. Evol. Microbiol. 51:891-899. [DOI] [PubMed] [Google Scholar]
- 23.Rikihisa, Y., M. Kawahara, B. Wen, G. Kociba, P. Fuerst, F. Kawamori, C. Suto, S. Shibata, and M. Futohashi. 1997. Western immunoblot analysis of Haemobartonella muris and comparison of 16S rRNA gene sequences of H. muris, H. felis, and Eperythrozoon suis. J. Clin. Microbiol. 35:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tandon, R., V. Cattori, A. M. Gomes-Keller, M. L. Meli, M. C. Golder, H. Lutz, and R. Hofmann-Lehmann. 2005. Quantification of feline leukemia viral and proviral loads by TaqMan real-time polymerase chain reaction. J. Virol. Methods 130:124-132. [DOI] [PubMed] [Google Scholar]
- 25.Tasker, S., S. H. Binns, M. J. Day, T. J. Gruffydd-Jones, D. A. Harbour, C. R. Helps, W. A. Jensen, C. S. Olver, and M. R. Lappin. 2003. Use of a PCR assay to assess the prevalence and risk factors for Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in cats in the United Kingdom. Vet. Rec. 152:193-198. [DOI] [PubMed] [Google Scholar]
- 26.Tasker, S., J. A. Braddock, R. Baral, C. R. Helps, M. J. Day, T. J. Gruffydd-Jones, and R. Malik. 2004. Diagnosis of feline haemoplasma infection in Australian cats using a real-time PCR assay. J. Feline Med. Surg. 6:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tasker, S., C. R. Helps, C. J. Belford, R. J. Birtles, M. J. Day, A. H. Sparkes, T. J. Gruffydd-Jones, and D. A. Harbour. 2001. 16S rDNA comparison demonstrates near identity between an United Kingdom Haemobartonella felis strain and the American California strain. Vet. Microbiol. 81:73-78. [DOI] [PubMed] [Google Scholar]
- 28.Tasker, S., C. R. Helps, M. J. Day, D. A. Harbour, T. J. Gruffydd-Jones, and M. R. Lappin. 2004. Use of a Taqman PCR to determine the response of Mycoplasma haemofelis infection to antibiotic treatment. J. Microbiol. Methods 56:63-71. [DOI] [PubMed] [Google Scholar]
- 29.Tasker, S., C. R. Helps, M. J. Day, D. A. Harbour, S. E. Shaw, S. Harrus, G. Baneth, R. G. Lobetti, R. Malik, J. P. Beaufils, C. R. Belford, and T. J. Gruffydd-Jones. 2003. Phylogenetic analysis of hemoplasma species: an international study. J. Clin. Microbiol. 41:3877-3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurston, J. P. 1954. Anaemia in mice caused by Eperythrozoon coccoides (Schilling, 1928). Parasitology 44:81-98. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe, M., M. Hisasue, K. Hashizaki, M. Furuichi, M. Ogata, S. Hisamatsu, E. Ogi, M. Hasegawa, R. Tsuchiya, and T. Yamada. 2003. Molecular detection and characterization of Haemobartonella felis in domestic cats in Japan employing sequence-specific polymerase chain reaction (SS-PCR). J. Vet. Med. Sci. 65:1111-1114. [DOI] [PubMed] [Google Scholar]
- 32.Westfall, D. S., W. A. Jensen, W. J. Reagan, S. V. Radecki, and M. R. Lappin. 2001. Inoculation of two genotypes of Hemobartonella felis (California and Ohio variants) to induce infection in cats and the response to treatment with azithromycin. Am. J. Vet. Res. 62:687-691. [DOI] [PubMed] [Google Scholar]
- 33.Willi, B., F. S. Boretti, V. Cattori, S. Tasker, M. L. Meli, C. Reusch, H. Lutz, and R. Hofmann-Lehmann. 2005. Identification, molecular characterization, and experimental transmission of a new hemoplasma isolate from a cat with hemolytic anemia in Switzerland. J. Clin. Microbiol. 43:2581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods, J. E., M. M. Brewer, J. R. Hawley, N. Wisnewski, and M. R. Lappin. 2005. Evaluation of experimental transmission of Candidatus Mycoplasma haemominutum and Mycoplasma haemofelis by Ctenocephalides felis to cats. Am. J. Vet. Res. 66:1008-1012. [DOI] [PubMed] [Google Scholar]