Abstract
We describe a case of bacteremia due to imipenem-susceptible Shewanella algae. Despite treatment with imipenem, the patient developed a spinal epidural abscess, from which imipenem-resistant S. algae was isolated. The development of resistance should be monitored when S. algae infection is treated with imipenem, even though the strain is initially susceptible to imipenem.
CASE REPORT
A 65-year-old man underwent distal pancreatectomy with cholecystectomy because of intraductal papillary mucinous tumor. On hospital day 12 (postoperative day 7), the patient complained of chills and fever. Abdominal sonography showed fluid collection in the abdomen, and percutaneous drainage was performed. Cultures of blood and percutaneous drainage fluid yielded Shewanella algae, which was susceptible to ceftazidime, cefepime, aztreonam, imipenem, and amikacin. It was resistant to piperacillin and gentamicin, as determined by disk diffusion testing. Based on this result, treatment with imipenem (500 mg intravenously [i.v.] every 6 h [q6h]) was instituted. Despite the treatment, the patient remained febrile. On hospital day 25, the patient became agitated and disoriented, and signs of meningismus were evident on his examination. His cerebrospinal fluid (CSF) contained 180 white blood cells/mm3. The CSF protein concentration was 307 mg/dl (reference range, 15 to 45 mg/dl), and the CSF sugar concentration was 41 mg/dl (reference range, 40 to 80 mg/dl). The CSF culture was negative. Imipenem treatment was changed to meropenem treatment. On hospital day 32, he complained of pain in his neck, and weakness of his left arm and left leg developed suddenly. Magnetic resonance imaging (MRI) revealed a spinal epidural abscess extending from the fourth to the sixth cervical vertebrae. The patient underwent hemilaminectomy and discectomy of C4 and C5 with drainage of the epidural abscess, which yielded S. algae on culture. The isolate had a susceptibility profile the same as that of the previous isolate, except that it was now resistant to imipenem. The initial blood isolate of S. algae was susceptible to imipenem, as determined by the broth dilution test (MIC, 2 μg/ml). The S. algae strain isolated from the epidural abscess, however, was resistant to imipenem (imipenem MIC, 16 μg/ml; meropenem MIC, 0.5 μg/ml). Following laminectomy, the patient responded to treatment, with resolution of his fever. Based upon the susceptibility test results, cefepime (2 g i.v. q12h) was substituted for meropenem (1,000 mg i.v. q8h). On postoperative day 54, the follow-up MRI showed no definitive evidence of the epidural abscess. The patient was discharged without neurological sequelae.
Shewanella algae is a gram-negative bacillus that is widely distributed in the environment, and its natural habitats are water and soil. The organism was formerly called Pseudomonas putrefaciens, Alteromonas putrefaciens, Achromobacter putrefaciens, and CDC group Ib; and it has now been placed in the genus Shewanella (10). S. algae and Shewanella putrefaciens have been associated with a broad range of human infections, including skin and soft tissue infections, biliary tract infections, ocular infections, otitis media, empyema, peritonitis, and sepsis (1, 3, 4, 8, 14). Khashe and Janda have reported that S. algae may be the predominant human pathogen within the genus (9). Shewanellae are generally susceptible to most antimicrobial agents in vitro (6). However, there is little clinical experience with the treatment of Shewanella infections. We have described here a case of bacteremia caused by an S. algae isolate that was initially susceptible to imipenem, but the bacterium later became resistant to imipenem during treatment with that drug. In addition, we investigated the propensity of S. algae to develop resistance to imipenem by using a serial passage technique.
The two clinical isolates of imipenem-susceptible and imipenem-resitant S. algae were identified with a VITEK II automated system (bioMérieux, Marcy l'Etoile, France) and standard microbiological techniques (15). We performed a nucleic acid-based confirmatory test by using 16S rRNA gene sequencing analysis, as described previously, using primers fD2 (5′-AGAGTTTGATCATGGCTCAG-3′) and rP2 (5′-ACG GCT ACC TTG TTA CGA CTT-3′) (5, 19). We compared the sequence to those available in the GenBank and EMBL databases by using the Clustal N program with the BLAST package (http://www.ncbi.nlm.nih.gov/BLAST/BLAST.cgi). Over 701 bp, the isolate gene sequence shared 99%, 94%, and 94% similarities with the sequences of Shewanella algae strain ATCC 51192 (GenBank accession number AB205581), Aeromonas veronii strain LMG13695 (GenBank accession number AF418209), and Shewanella putrefaciens strain KIN80 (GenBank accession number AY136079), respectively. The two clinical isolates were ultimately identified by 16S rRNA gene sequencing analysis as S. algae.
We also performed pulsed-field gel electrophoresis (PFGE) to confirm that the two Shewanella species isolated from the patient were the same strain of the bacterium. The two S. algae isolates were subjected to DNA restriction analysis with 10 U/μl of the SmaI enzyme in appropriate buffer. The DNA fragments were separated by pulsed-field gel electrophoresis through a 1.2% agarose gel as described previously (11). We could document the identical DNA banding patterns based on the typing results (Fig. 1).
FIG. 1.
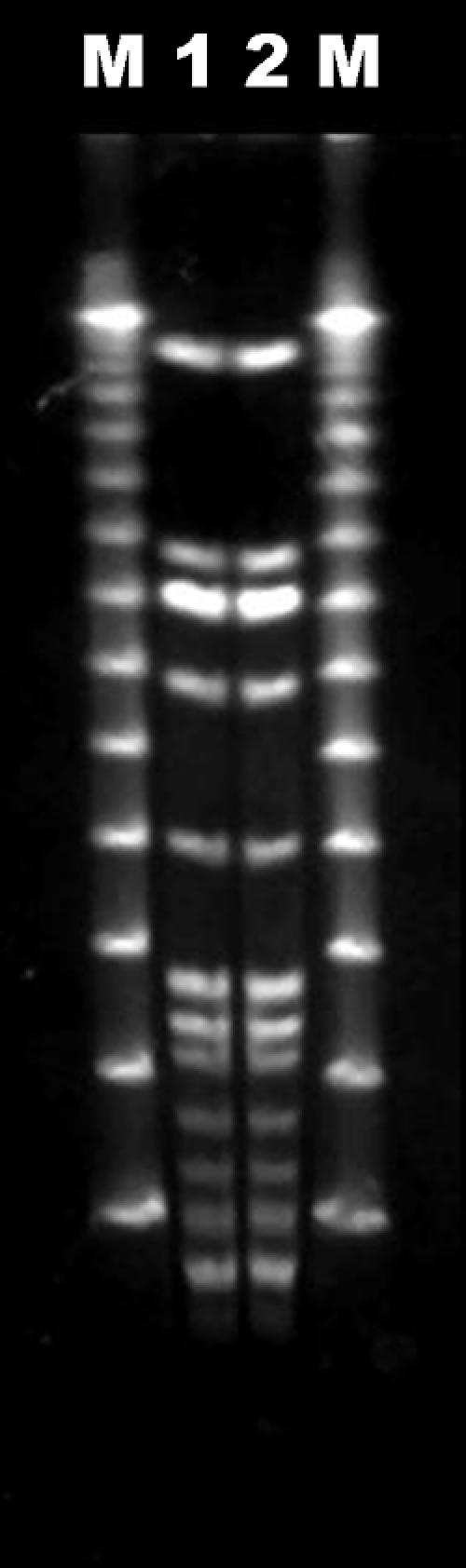
PFGE electrophoretic patterns of the isolated Shewanella species. Lane 1, blood isolate of imipenem-susceptible S. algae from patient; lane 2, epidural abscess isolate of imipenem-resistant S. algae from patient; lanes M, DNA ladders.
The bacterial strain used for the in vitro test for resistance induction was the imipenem-sensitive S. algae strain. Pseudomonas aeruginosa ATCC 27853 was used as a quality control strain. Single-step resistant variants were obtained from the imipenem-sensitive S. algae strain on Mueller-Hinton agar containing increasing amounts of imipenem (the MIC and two, four, and eight times the MIC). MIC interpretive standards for S. algae have not been established. For the purposes of this study, the MIC was interpreted as susceptible or resistant according to the guidelines of the Clinical and Laboratory Standards Institute MIC interpretive standards for P. aeruginosa, where applicable (12, 13). The frequency of single-step resistant variants was expressed as the ratio of the number of CFU grown in the presence of the antibiotic (at twice the MIC) to the number of CFU of the control grown without imipenem, as described previously (2). Along with these assays, the imipenem-sensitive S. algae strain was also subjected to a serial passage experiment with imipenem, as described by Tenney et al. (18). In brief, overnight growth of the S. algae strain on Mueller-Hinton agar was swabbed onto Mueller-Hinton agar plates containing one-half the MIC of imipenem. At 24 h, the surface growth was picked and placed onto agar containing twice the prior concentration of imipenem. This process was repeated serially.
Single-step resistant variants were selected from the imipenem-sensitive S. algae strain at up to four times the MIC, whereas the resistant variant from P. aeruginosa ATCC 27853 could be selected at up to two times the MIC. All the resistant variants of S. algae selected either by single-step or by sequential stepwise passage exhibited MICs of up to 8 to 16 μg/ml, whereas those of P. aeruginosa ATCC 27853 showed MICs of up to 16 μg/ml. The frequencies of resistant variants from the imipenem-sensitive S. algae strain at twice the MIC of imipenem ranged from 0.6 × 10−6 to 4 × 10−5, whereas those from P. aeruginosa ATCC 27853 at the same MIC ranged from 0.2 × 10−6 to 4 × 10−5.
It is well known that the rapid emergence of imipenem resistance during treatment of patients with pseudomonal infections is relatively common, and this may lead to treatment failure when this drug has been used alone and where dense inocula are present (16, 17). Until now, however, there have been no previous reports of the emergence of resistance during treatment of an S. algae infection. In the present study, we documented that imipenem-susceptible S. algae subsequently became resistant to imipenem during treatment. We also demonstrated in vitro that S. algae organisms have a propensity toward resistance to imipenem. The mechanism of resistance to imipenem in the organism may be related to a carbapenem-hydrolyzing Ambler class D β-lactamase, as described previously (7). PCR experiments were performed as described previously with the specific primers OXA-55/1 (5′-CATCTACCTTTAAAATTCCC-3′) and OXA-55/2 (5′-AGCTGTTCCTGCTTGAGCAC-3′) to amplify the blaOXA-55 gene from the imipenem-resistant S. algae isolate. We could detect a chromosome-encoded carbapenem-hydrolyzing Ambler class D β-lactamase from our S. algae isolates. This suggests that a carbapenem-hydrolyzing β-lactamase plays a central role in the emergence of resistance to imipenem in S. algae (7). When it is considered that the two isolates had the same PFGE pulsotype and that we had detected Ambler class D β-lactamase by performing PCR, it is likely that the emergence of resistance was due to a mutation that resulted in the derepression of Ambler class D β-lactamase synthesis. It would be of interest to know why the difference in antibiotic susceptibility between cefepime and carbapenem occurred. It might have been due to the difference in the rates of hydrolysis between cefepime and carbapenem. Further studies are required, however, to test the relevance of these possibilities.
This case is clinically significant in two aspects. First, this is the first case report of the emergence of resistance to imipenem in a patient with S. algae bacteremia treated with imipenem. Second, to our knowledge, this is the first report of a spinal epidural abscess caused by S. algae. Our case highlights the fact that clinicians should be aware of the potential for clinical failure when imipenem is used for the treatment of serious infections caused by S. algae.
Acknowledgments
We are grateful to Yeong Seon Lee and Jeong Ok Cha from the Department of Bacteriology, National Institute of Health, Korea Center for Disease Control & Prevention, for excellent technical assistance.
REFERENCES
- 1.Brink, A. J., A. van Straten, and A. J. van Rensburg. 1995. Shewanella (Pseudomonas) putrefaciens bacteremia. Clin. Infect. Dis. 20:1327-1332. [DOI] [PubMed] [Google Scholar]
- 2.Buscher, K. H., and W. Cullmann. 1987. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob. Agents Chemother. 31:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butt, A. A., J. Figueroa, and D. H. Martin. 1997. Ocular infection caused by three unusual marine organisms. Clin. Infect. Dis. 24:740. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez, H., B. Fonnesbech Vogel, L. Gram, S. Hoffmann, and S. Schaebel. 1996. Shewanella algae bacteremia in two patients with lower leg ulcers. Clin. Infect. Dis. 22:1036-1039. [DOI] [PubMed] [Google Scholar]
- 5.Drancourt, M., C. Bollet, A. Carlioz, R. Martelin, J. P. Gayral, and D. Raoult. 2000. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbiol. 38:3623-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fass, R. J., and J. Barnishan. 1980. In vitro susceptibility of nonfermentative gram-negative bacilli other than Pseudomonas aeruginosa to 32 antimicrobial agents. Rev. Infect. Dis. 2:841-853. [DOI] [PubMed] [Google Scholar]
- 7.Heritier, C., L. Poirel, and P. Nordmann. 2004. Genetic and biochemical characterization of a chromosome-encoded carbapenem-hydrolyzing ambler class D β-lactamase from Shewanella alga. Antimicrob. Agents Chemother. 48:1670-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwata, M., K. Tateda, T. Matsumoto, N. Furuya, S. Mizuiri, and K. Yamaguchi. 1999. Primary Shewanella alga septicemia in a patient on hemodialysis. J. Clin. Microbiol. 37:2104-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khashe, S., and J. M. Janda. 1998. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens. J. Clin. Microbiol. 36:783-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDonell, M. T., and R. R. Colwell. 1985. Phylogeny of the Vibrionaceae, and recommendation for two new genera, Listonella and Shewanella. Syst. Appl. Microbiol. 6:171-182. [Google Scholar]
- 11.Murchan, S., M. E. Kaufmann, A. Deplano. R. de Ryck, M. Struelens, C. E. Zinn, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, N. El Solh, C. Cuny, W. Witte, P. T. Tassios, N. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, I. Laconcha, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, G. Coombes, and B. Cookson. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests, 8th ed. M2-A8. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Pagani, L., A. Lang, C. Vedovelli, O. Moling, G. Rimenti, R. Pristera, and P. Mian. 2004. Soft tissue infection and bacteremia caused by Shewanella putrefaciens. J. Clin. Microbiol. 41:2240-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickett, M. J., D. G. Hollis, and E. J. Bottone. 1991. Miscellaneous gram-negative bacteria, p. 410-428. In A. Balows, W. J. Hausler, K. L. Herrmann, H. D. Isenberg, and H. J. Shadomy (ed.), Manual of clinical microbiology, 5th ed. American Society for Microbiology, Washington, D.C.
- 16.Quinn, J. P., and A. E. Studemeister. 1988. Resistance to imipenem in Pseudomonas aeruginosa: clinical experience and biochemical mechanisms. Rev. Infect. Dis. 10:892-898. [DOI] [PubMed] [Google Scholar]
- 17.Quinn, J. P., E. J. Dudek, C. A. Divincenzo, D. A. Lucks, and S. A. Lerner. 1986. Emergence of resistance of imipenem during therapy for Pseudomonas aeruginosa infections. J. Infect. Dis. 154:289-294. [DOI] [PubMed] [Google Scholar]
- 18.Tenney, J. H., R. W. Maack, and G. R. Chippenedale. 1983. Rapid selection of organisms with increasing resistance on subinhibitory concentrations of norfloxacin in agar. Antimicrob. Agents Chemother. 23:188-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisburg, W. G., S. M. Barns, D.A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]


