Abstract
The first two cases of canine visceral leishmaniasis in French Guiana are described. One infected dog was most probably imported from France. A second dog was then infected with Leishmania infantum in French Guiana. These observations exemplify the intercontinental transportation theory for L. infantum.
The presence of visceral leishmaniasis in America was suspected in 1911 by the Brazilian Carlos Chagas, who reported a nonmalaric splenomegaly in children of the Amazon Basin (6). The parasite was then named Leishmania chagasi Cunha & Chagas, 1937, in his honor. However, the first documented case of visceral leishmaniasis in America was that of Migone in 1913 in Paraguay (13).
Today, canine visceral leishmaniases (CVL) are widespread in the New World and visceral leishmaniasis is endemic in many areas of Latin America (8). Although the principal foci of visceral leishmaniasis are located in the drier, poorly forested areas, a small number of human and canine infections were recorded in the densely forested Amazon region, such as in the State of Pará. In Roraima, a particularly large focus also extends into Venezuela and Guyana (8). In Venezuela, it occurs sporadically in almost every state of the country with a low endemicity (8). Till now, visceral leishmaniasis has been reported a few times in Suriname (8) but never in French Guiana (16). In the present paper, we describe the first two cases of CVL in French Guiana that have recently occurred in Cayenne, the main city of this French territory.
In February 2005, a female rottweiler (dog 1) was found positive for CVL by the seroimmunological veterinary commercial test Leishmania Witness (Synbiotics Corp.) in a veterinary clinic of Cayenne (French Guiana). For about 6 months, dog 1 had been presenting clinical symptoms of CVL (exhaustion, weight loss, desquamation, ulcerated cutaneous lesions, fever and hepatosplenomegaly). To confirm this result, four different diagnostic methods were employed, as follows.
(i) Blood sample, bone marrow aspirate, and biopsy samples from cutaneous lesions, liver, and spleen were Giemsa stained and examined by direct microscopy. Parasites were observed in cutaneous ulcer scrapings and bone marrow smears.
(ii) All the samples were cultured for 2 months in Novy-Nicolle-MacNeal medium, RPMI 1640 supplemented with 20% fetal calf serum, 2 mM l-glutamine, 25 mM HEPES (pH 7.4), 1% nonessential and essential amino acids in minimal essential medium, and 50 UI/ml penicillin-streptomycin, Schneider's medium, and liver infusion tryptose supplemented with 20% fetal calf serum. None of these cultures was positive.
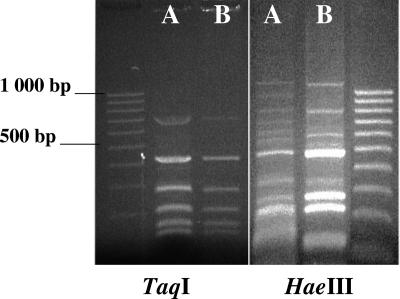
(iii) All the samples were analyzed by PCR-restriction fragment length polymorphism (RFLP) in duplicate. DNAs were extracted from blood, bone marrow, cutaneous lesions, liver, and spleen with the DNeasy tissue kit (QIAGEN) according to the manufacturer's recommendations. DNAs were then amplified by a Leishmania-specific PCR, routinely used for diagnosis in our laboratory of human cutaneous punch biopsy samples. The internal transcribed spacer 1 sequences, located between the end of the small subunit and the 5.8S region of the nuclear rRNA genes, were amplified with the primers SSU-12103-D (5′-GGGAATATCCTCAGCACGT-3′) and 5.8S-13333-R (5′-CGACACTGAGAATATGGCATG-3′) as described elsewhere (17). Leishmanial DNA was detected in ulcerated cutaneous lesions, bone marrow, and spleen biopsy samples. After digestion of these three samples by the discriminating enzymes TaqI, RsaI, and HaeIII (17), fingerprints were found identical to those obtained with the L. infantum zymodeme MON-1 World Health Organization reference strain MHOM/FR/78/LEM75 (Fig. 1).
FIG. 1.
PCR-RFLP profiles of (A) cultured L. infantum zymodeme MON-1 World Health Organization reference strain (MHOM/FR/78/LEM75) and (B) a dermal lesion biopsy from dog 1. Following a 2-h digestion with TaqI and HaeIII, samples were loaded in a 2% agarose electrophoresis gel with a 100-bp molecular size ladder.
(iv) Sequencing of the end of the RNA polymerase II large subunit gene (5) on DNA extracted from the spleen confirmed this identification. Following this diagnosis but notwithstanding our recommendation for treatment, dog 1 was killed upon its owners' request.
Subsequently, several determinant points appeared from the owners' interview. In July 2002, they first acquired a male rottweiler (dog 2) presenting little desquamation. This dog was born in the south of France in September 1998 and registered at the Spanish Royal Canine Society (Madrid, Spain) for contests before being sent to French Guiana in 2000. Dog 2 was found positive with Leishmania Witness by direct examination in May 2003, but no alert was given. Despite three intramuscular pentamidine injections, CVL symptoms increased until its death in May 2004. In early 2003, the owners decided to get a second rottweiler (dog 1). In total, these two dogs lived about 1.5 years together and had a puppy (dog 3). All three dogs were said never to have left their home in the Patawa district of Cayenne since 2002.
Several literature reports of “unexplainable” leishmaniasis in geographical areas where it is not known to be endemic are available (3). In addition, the asymptomatic period of dog 2 could appear unusually long. However, it is unlikely that the transmission of L. infantum to dogs has occurred in French Guiana between 2000 and 2003 as (i) neither known nor suspected vector species for this particular parasite have been recorded in French Guiana and (ii) neither human nor animal cases have been observed before, despite an important epidemiological surveillance of leishmaniases during the last 20 years (16). Unfortunately, parasites were not isolated from dog 2.
To our knowledge, L. guyanensis/L. panamensis has rarely been isolated from dogs in the New World except in Ecuador (2, 7). Dog 2 presented no dermal lesion and L. braziliensis has never been reported in dogs in the Amazon region before. Moreover, dog 2 was found strongly positive with the Leishmania Witness serological test, based on L. infantum antigen detection, whereas circulating antibodies are known to be low in cutaneous forms. In total, dog 2 seemed to have most probably been infected by L. infantum in the area of the Old World where it is endemic, that is, the Mediterranean basin, rather than in French Guiana.
In parallel, a preventive screening of three neighboring dogs of the district was managed. No clinical symptom was observed in the 1-year-old dog (dog/3), and it was found negative by an immunoserological rapid test, as was another puppy living in the district. Two other dogs that were found slightly positive with Leishmania Witness were finally proved to be negative by cultures, direct examinations, and PCR-RFLP of blood, bone marrow, and spleen. These false-positives were probably due to cross-reactions of the rapid test with Trypanosoma.
Five trapping nights with seven Centers for Disease Control and Prevention (CDC) light-traps were also conducted to investigate the presence of potential Leishmania vectors in March and April 2005 in the Patawa district of Cayenne. CDC light-traps were distributed in the neighboring gardens, up to 400 m around the infected dogs' home, and placed close to henhouses, kennels, mango trees, old walls, etc. Sandfly populations were very low (only 0.5 individuals/trap/night) and the dominance of the Pressatia subgenus was marked with 66.7% of Lutzomyia choti. None of the species caught has been reported previously or suspected elsewhere for the transmission of L. infantum (B. Rotureau et al., submitted). Finally, the owners of the infected rottweilers were all found negative by two serological tests (Leishmania Spot IF, BioMérieux, and Cellognost Leishmaniasis, Behring).
When combined, the results of these investigations revealed that (i) the index case (dog 2) with suspected CVL due to L. infantum was imported from France or Spain to French Guiana in 2000; (ii) a second dog (dog 1), born in French Guiana, was infected with L. infantum in French Guiana between January 2003 and May 2004; (iii) as no potential vector and no other dog or person was found to be infected, transmission between dog 1 and dog 2 could possibly have occurred sexually, as previously observed (4, 9, 14), or by fighting (12); and (iv) as their puppy (dog 3) was found negative for CVL, no vertical transmission seemed to have occurred, as previously reported (1). Nevertheless, as the transmission mode was not clearly identified, further surveys of dog and sandfly populations in this particular district should be conducted. The potential transmission risk that could have existed between 2000 and 2002 with dog 2 remains unclear.
Overall, this is the first report of L. infantum in French Guiana and, to our knowledge, the first documented example of probable importation of this particular Leishmania species in a dog from the Old World to South America, although it has been well documented for North America. Most cases of visceral leishmaniasis in North America have been diagnosed in canine or human patients that had previously resided in or traveled to countries where the infection is known to be endemic (reviewed in reference 15). L. infantum zymodeme MON-1 was incriminated in most of these cases and the common origin of the infective agent was suggested to be the Mediterranean region.
Some authors have suggested that L. infantum could have been imported first from Europe, carried by dogs or rats on board caravels sailed by conquistadors (10). From various experiments, L. chagasi was then proved to be indistinguishable from L. infantum (11). However, this hypothesis is far from other arguments that support the autochthonous origin of this species, possibly maintained in neotropical canids since the separation of the American and African continents (11). The origin of this clinically important Leishmania species is still under debate. However, our observations tend to support the intercontinental transportation theory for L. infantum (10). On the other hand, these CVL cases emphasize on the importance of keeping a close eye on the global ecoepidemiology of Leishmania parasites. In the actual global context, such cases are unlikely to be isolated and could have important public health consequences.
Acknowledgments
Thanks to O. Louguet, B. Bonnemains and D. Blanchet for their help in the sampling of the dogs. Thanks to P. Gaborit for his help in the field.
This work was supported by the University of the French West Indies and French Guiana (Cayenne, French Guiana), by the Contrat Plan Etat-Region 2365, by the Institut National de la Sante et de la Recherche Medicale (INSERM, Paris, France), and by the Centre National de la Recherche Scientifique (CNRS, Paris, France).
REFERENCES
- 1.Andrade, H. M., P. de Toledo, M. J. Marques, J. C. Franca Silva, W. L. Tafuri, W. Mayrink, and O. Genaro. 2002. Leishmania (Leishmania) chagasi is not vertically transmitted in dogs. Vet. Parasitol. 103:71-81. [DOI] [PubMed] [Google Scholar]
- 2.Banuls, A. L., R. Jonquieres, F. Guerrini, F. Le Pont, C. Barrera, I. Espinel,R. Guderian, R. Echeverria, and M. Tibayrenc. 1999. Genetic analysis of Leishmania parasites in Ecuador: are Leishmania (Viannia) panamensis and Leishmania (V.) guyanensis distinct taxa? Am. J. Trop. Med. Hyg. 61:838-845. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, C., G. Schonian, A. L. Banuls, M. Hide, F. Pratlong, E. Lorenz, M. Rollinghoff, and R. Mertens. 2001. Visceral leishmaniasis in a German child who had never entered a known endemic area: case report and review of the literature. Clin. Infect. Dis. 32:302-306. [DOI] [PubMed] [Google Scholar]
- 4.Catone, G., G. Marino, G. Poglayen, M. Gramiccia, A. Ludovisi, and A. Zanghi. 2003. Canine transmissible venereal tumour parasitized by Leishmania infantum. Vet. Res. Commun. 27:549-553. [DOI] [PubMed] [Google Scholar]
- 5.Croan, D. G., D. A. Morrison, and J. T. Ellis. 1997. Evolution of the genus Leishmania revealed by comparison of DNA and RNA polymerase gene sequences. Mol. Biochem. Parasitol. 89:149-159. [DOI] [PubMed] [Google Scholar]
- 6.Cunha, A. M., and E. Chagas. 1937. Nova especie de protozoariodo genero Leishmania patogenica para o homen, Leishmania chagasi n. sp. Hospital 11:148-152.
- 7.Dereure, J., I. Espinel, C. Barrera, F. Guerrini, A. Martini, R. Echeverria, R. H. Guderian, and F. Le Pont. 1994. Leishmaniasis in Ecuador. 4. Natural infestation of the dog by Leishmania panamensis. Ann. Soc. Belg. Med. Trop. 74:29-33. (In French.) [PubMed] [Google Scholar]
- 8.Desjeux, P. 1991. Information sur l'épidémiologie des leishmanioses et la lutte contre ces maladies par pays ou territoire, vol. 30. World Health Organization, Geneva, Switzerland.
- 9.Diniz, S. A., M. S. Melo, A. M. Borges, R. Bueno, B. P. Reis, W. L. Tafuri, E. F. Nascimento, and R. L. Santos. 2005. Genital lesions associated with visceral leishmaniasis and shedding of Leishmania sp. in the semen of naturally infected dogs. Vet. Pathol. 42:650-658. [DOI] [PubMed] [Google Scholar]
- 10.Killick-Kendrick, R., D. H. Molyneux, J. A. Rioux, G. Lanotte, and A. J. Leaney. 1980. Possible origins of Leishmania chagasi. Ann. Trop. Med. Parasitol. 74:563-565. [DOI] [PubMed] [Google Scholar]
- 11.Mauricio, I. L., J. R. Stothard, and M. A. Miles. 2000. The strange case of Leishmania chagasi. Parasitol. Today 16:188-189. [DOI] [PubMed] [Google Scholar]
- 12.Melby, P. C. 1991. Experimental leishmaniasis in humans: review. Rev. Infect. Dis. 13:1009-1017. [DOI] [PubMed] [Google Scholar]
- 13.Migone, L. E. 1913. Un caso de Kalazar en Assunción (Paraguay). Bull. Soc. Pathol. Exot. 6:118-120. [Google Scholar]
- 14.Nuwayri-Salti, N., and H. Fallah Khansa. 1985. Direct non-insect-vector transmission of Leishmania parasites in mice. Int. J. Parasitol. 15:497-500. [DOI] [PubMed] [Google Scholar]
- 15.Rosypal, A. C., A. M. Zajac, and D. S. Lindsay. 2003. Canine visceral leishmaniasis and its emergence in the United States. Vet. Clin. N. Am. Small Anim. Pract. 33:921-937. [DOI] [PubMed] [Google Scholar]
- 16.Rotureau, B. 2006. Ecology of the Leishmania species in the Guianan Ecoregion Complex. Am. J. Trop. Med. Hyg. 74:81-96. [PubMed] [Google Scholar]
- 17.Rotureau, B., C. Ravel, P. Couppié, F. Pratlong, M. Nacher, J.-P. Dedet, and B. Carme. 2006.. Use of PCR-restriction fragment length polymorphism analysis to identify the main New World Leishmania species and analyze their taxonomic properties and polymorphism by application of the assay to clinical samples. J. Clin. Microbiol. 44:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]