Abstract
The use of new flocked swabs, compared to kit swabs, enhanced the ability of three commercial nucleic acid amplification tests to detect low levels of Chlamydia trachomatis and Neisseria gonorrhoeae nucleic acids when the organisms were diluted in a universal transport medium as mocked specimens.
Chlamydia trachomatis and Neisseria gonorrhoeae infections are prevalent in sexually active men and women (10). Upper-genital-tract morbidity can be reduced by screening programs and treatment of lower tract infections (14). Nucleic acid amplification tests (NAAT) are available commercially and are used extensively (2). Most of these tests can be used on cervical, urethral, and vaginal swabs and on urine samples (2, 5, 11, 13, 16, 17). There is a debate on the practicality of confirming NAAT-positive specimens by retesting the specimen with an alternate NAAT (12). The analytical sensitivity of each test and susceptibility to amplification inhibitors may contribute to clinical sensitivity (2). The amount of target nucleic acid collected from infected cells or fluids, the ability of the specimen transport system to stabilize or preserve the target for amplification, and the efficiency of target extraction may also determine clinical sensitivity. We compared here a new flocked swab (FS) and room temperature universal transport medium (UTM-RT) from Copan Diagnostics, Inc., to the swabs and transport tubes from three diagnostic kits for C. trachomatis and N. gonorrhoeae: APTIMA Combo 2 (AC2) from Gen-Probe, Inc., San Diego, CA; Amplicor (AMP) from Roche Molecular Systems, Montreal, Quebec, Canada; and ProbeTec ET (PT) from Becton Dickinson Biosciences, Sparks, MD (Fig. 1). Laboratory strains of C. trachomatis (L2 434) and N. gonorrhoeae (ATCC 43069) were serially diluted in UTM-RT from 10−1 to 10−10, and 0.3 ml of each dilution for each organism was divided into aliquots into 30 tubes with FS and 10 for each kit swab (KS). The swabs were processed into kit dilution buffers as mocked swab samples (3). The instructions in the package inserts for cervical swabs for each kit were followed. No internal (amplification) controls were used. Dilutions of each organism with FS were also cultured by using McCoy cells and scored after 48 h by determining fluorescent inclusions forC.trachomatis and colony counts on chocolate agar for N. gonorrhoeae.
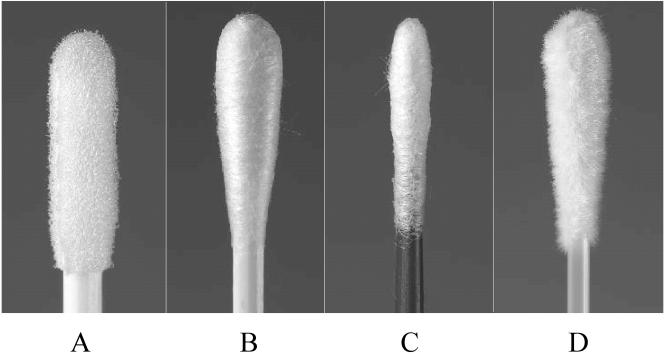
FIG. 1.
Swabs from NAAT kits for C. trachomatis and N. gonorrhoeae: Becton Dickinson ProbeTec ET (A), Roche Amplicor (B), GenProbe Aptima (C), and Copan flocked swab (D).
The titration endpoints for C. trachomatis are shown in Table 1. The AC2 test had the greatest analytical sensitivity (P < 0.001), and the 100% endpoint of detection for AC2 was equal for FS and the Gen-Probe KS at 10−7, but from 10−8 to 10−10 50% (15/30) of the replicates were positive in the FS series compared to 10% (3/30) for the Gen-Probe KS. In the AMP assay the FS increased the 100% endpoint to 10−7 compared to 10−6 for the AMP KS. Seventy percent (7/10) of the AMP KS were positive at the next dilution (10−7), and 1 of 10 FS results was positive at 10−8.
TABLE 1.
Detection of C. trachomatis diluted in UTM from FS and KS processed in AC2, AMP, and PT assaysa
| Dilutions of C. trachomatis in UTM (10 tubes/dilution) | No. of positive results (n = 40) obtained by NAAT:
|
Culture in McCoy shell vial cultures (fluorescent inclusions in duplicate/ total no.) | |||||
|---|---|---|---|---|---|---|---|
| AC2
|
AMP
|
PT
|
|||||
| KS | FS | KS | FS | KS | FS | ||
| 10−5 | 10 | 10 | 10 | 10 | 10 | 10 | 43/40 |
| 10−6 | 10 | 10 | 10 | 10 | 10 | 10 | 10/6 |
| 10−7 | 10 | 10 | 7 | 10 | 1 | 3 | 0 |
| 10−8 | 2 | 8 | 0 | 1 | 0 | 0 | 0 |
| 10−9 | 1 | 4 | 0 | 0 | 0 | 0 | 0 |
| 10−10 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
Each dilution was used to inoculate 10 replicates for each swab type, resulting in a maximum of 10 positive results for each dilution-swab combination in each assay. The numbers of positive results (%) per 40 samples that were below the 100% endpoint (below 10−6) were as follows: AC2 KS, 13 (32.5%); AC2 FS, 25 (62.5%); AMP KS, 7 (17.5%); AMP FS, 11 (27.5%); PT KS, 1 (2.5%); and PT FS, 3 (7.5%). P values for these results were as follows: AC2, P < 0.001; AMP, P = 0.13; PT, P = 1.0; AC2 versus AMP, P = 0.3; AC2 versus PT, P < 0.001.
The PT assay demonstrated the lowest 100% endpoint at 10−6 for both FS and PT KS, and over the next 40 samples (between 10−7 and 10−10) 7.5% (3/40) of the FS were positive (all at 10−7) compared to 2.5% (1/40) for PT KS at the same dilution (P = 1.0). The performances of the other two assays were compared beyond the 10−6 dilution. The FS detected 62.5% (25/40) positives compared to 32.5% (13/40) for the KS in the AC2 test (P < 0.001). In the AMP test the FS detected 27.5% (11/40) compared to 17.5% (7/40) for the AMP KS beyond the 10−6 dilution (P = 0.13). When we pooled the C. trachomatis data from all three tests, the FS was clearly superior to the KS (P < 0.001). Probit regression analysis (15) for AC2 estimated that KS required a 16-fold (P < 0.05)-greater concentration of C. trachomatis than FS (median detection concentrations of 5.1 × 10−9 for KS and 3.2 × 10−10 for FS).
The PT assay detected a few positives just beyond the culture positivity threshold, whereas the AC2 test combined with the FS detected 1,000-fold more positives. These observations may be attributed to the intrinsic differences in the amount of target available for each assay. The AC2 amplifies rRNA target which would be at an increased level compared to the DNA target amplified by AMP and PT (1).
Similar but less dramatic observations with the mocked N. gonorrhoeae samples were recorded for the three assays (Table 2). The AC2 test had the highest 100% endpoints at 10−6 for FS and KS compared to 10−5 for the other two assays. In each assay the FS detected a higher percentage of extra positives than KS beyond the lowest 100% endpoint of 10−5. In the AC2 N. gonorrhoeae test the FS samples detected 73.3% (22/30) positive compared to the AC2 KS, which detected 56.6% (17/30) between 10−6 and 10−8 (P = 0.06). In the PT and AMP tests the comparative values for FS and KS were 36.6% versus 23.3% (P = 0.13) and 26.6% versus 13.3% (P = 0.13), respectively. Each of the NAAT detected N. gonorrhoeae positives beyond the dilution of culture positivity, with a few more positives using the FS in the AC2 test.
TABLE 2.
Detection of N. gonorrhoeae diluted in UTM from FS and KS processed in AC2, AMP, and PT assaysa
| Dilutions of N. gonorrhoeae in UTM (10 tubes/dilution) | No. of positive results (n = 30) obtained by NAAT:
|
Culture in chocolate agar (no. of colonies/ 0.010 ml in duplicate) | |||||
|---|---|---|---|---|---|---|---|
| AC2
|
AMP
|
PT
|
|||||
| KS | FS | KS | FS | KS | FS | ||
| 10−4 | 10 | 10 | 10 | 10 | 10 | 10 | >1,000 |
| 10−5 | 10 | 10 | 10 | 10 | 10 | 10 | 48/42 |
| 10−6 | 10 | 10 | 4 | 6 | 6 | 8 | 7/9 |
| 10−7 | 7 | 9 | 0 | 2 | 1 | 3 | 0 |
| 10−8 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
Each dilution was used to inoculate 10 replicates for each swab type, resulting in a maximum of 10 positive results for each dilution-swab combination in each assay. The numbers of positive results (%) per 30 samples that were below the 100% endpoint (below 10−5) were as follows: AC2 KS, 17 (56.6%); AC2 FS, 22 (73.3%); AMP KS, 4 (13.3%); AMP FS, 8 (26.6%); PT KS, 7 (23.3%); and PT FS, 11 (36.6%). P values for these results were as follows: AC2, P = 0.06; AMP, P = 0.13; PT, P = 0.13; AC2 versus AMP, P < 0.001; AC2 versus PT, P = 0.002.
The presence of an adequate number of cells has been shown to affect the sensitivity of cultures (7), antigen detection (6), and PCR (4, 8, 9, 18). The presence of cellular DNA reflected by β-globin gene amplification can be a measure of specimen adequacy for C. trachomatis detection by PCR (4). Several studies support our findings that samples can test positive in the absence of cells or cellular DNA due to extracellular bacteria (4, 8, 18). Our data show that there are significant differences in analytical sensitivity among NAAT; this is probably influenced by the arbitrary setting of the positivity level of each test and the amount of assay target in the specimens.
Compared to the traditional method of winding long strands of material on the end of an applicator, the flocking process, inside a flocking chamber, attaches short nylon fiber strands to the glued end of molded plastic applicators of a desired shape. The strands are electrostatically charged and are propelled at high velocity, so that their polar ends strike the adhesive to bond them at right angles to the surface, resulting in a hydrophilic layer of nylon pile with efficient collection and release of particulate matter. The FS enhanced the analytical sensitivity of each of the assays for both C. trachomatis and N. gonorrhoeae, presumably by trapping, stabilizing, and releasing more target for assay extraction and amplification.
UTM-RT, like M4 medium, was designed to enable the transportation of specimens for laboratory diagnosis in multiple methods such as culture, antigen detection, or nucleic acid testing. UTM-RT is a defined media of modified Hanks balanced salt solution supplemented with buffers, bovine serum albumin, gelatin, antibiotics, and other ingredients (the exact formulation is published on the Copan website). The product can be stored at room temperature for l year and has a claim for specimen transit to the laboratory at room temperature for up to 48 h. The UTM-RT and FS could allow confirmatory testing from one NAAT to another near the dilution of 100% positivity, but assays such as PT, with lower analytical sensitivity, would not be expected to confirm the extra positives detected by a more sensitive AC2 assay. This finding confirms similar observations seen with clinical specimens for C. trachomatis (12). In conclusion, the universality of the system tested and apparent enhancement for detection of dilute positives in mocked specimens may translate to better clinical sensitivity for NAAT performed on patient specimens.
REFERENCES
- 1.Boyadzhyan, B., T. Yashina, J. H. Yatabe, M. Patnaik, and C. S. Hill. 2004. Comparison of the APTIMA CT and GC assays with the APTIMA combo 2 assay, the Abbott LCx assay, and direct fluorescent-antibody and culture assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae J. Clin. Microbiol. 42:3089-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernesky, M. A. 2002. Chlamydia trachomatis diagnostics. Sex. Transm. Infect. 78:232-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong, S., D. Jang, X. Song, J. Mahony, A. Petrich, P. Barriga, and M. Chernesky. 2003. Specimen processing and concentration of Chlamydia trachomatis added can influence false-negative rates in the LCx assay but not in the APTIMA Combo 2 assay when testing for inhibitors. J. Clin. Microbiol. 41:778-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutlée, F., M. DeLadurantaye, C. Tremblay, J. Vincelette, L. Labrecque, and M. Roger. 2000. An important proportion of genital samples submitted for Chlamydia trachomatis detection by PCR contain small amounts of cellular DNA as measured by β-globin gene amplification. J. Clin. Microbiol. 38:2512-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaydos, C. A., M. Theodore, N. Dalesio, B. J. Wood, and T. C. Quinn. 2004. Comparison of three nucleic acid amplification tests for detection of Chlamydia trachomatis in urine specimens. J. Clin. Microbiol. 42:3041-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellogg, J. A., J. W. Seiple, C. L. Murray, and J. S. Levisky. 1990. Effect of endocervical specimen quality on detection of Chlamydia trachomatis and on the incidence of false-positive results with the Chlamydiazyme method. J. Clin. Microbiol. 28:1108-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellogg, J. A. 1995. Impact of variation in endocervical specimen collection and testing techniques on frequency of false-positive and false-negative Chlamydia detection results. Am. J. Clin. Pathol. 104:554-559. [DOI] [PubMed] [Google Scholar]
- 8.Kellogg, J. A., J. W. Seiple, J. L. Klinedinst, E. S. Stroll, and S. H. Cavanaugh. 1995. Improved PCR detection of Chlamydia trachomatis by using an altered method of specimen transport and high-quality endocervical specimens. J. Clin. Microbiol. 33:2765-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellogg, J. A., J. W. Seiple, J. L. Klinedinst, and E. Stroll. 1996. Diff-quick stain as a simplified alternative to Papanicolaou stain for determination of quality of endocervical specimens submitted for PCR detection of Chlamydia trachomatis. J. Clin. Microbiol. 34:2590-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller, W. C., C. A. Ford, M. Morris, M. S. Handcock, J. L. Schmitz, M. M. Hobbs, M. S. Cohen, K. M. Harris, and J. R. Udry. 2004. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291:2229-2236. [DOI] [PubMed] [Google Scholar]
- 11.Moncada, J., J. Schachter, E. W. Hook III, D. Ferrero, C. Gaydos, T. C. Quinn, D. Willis, A. Weissfeld, and D. H. Martin. 2004. The effect of urine testing in evaluations of the sensitivity of the Gen-Probe Aptima Combo 2 assay on endocervical swabs for Chlamydia trachomatis and Neisseria gonorrhoeae: the infected patient standard reduces sensitivity of single site evaluation. Sex. Transm. Dis. 31:273-277. [DOI] [PubMed] [Google Scholar]
- 12.Schachter, J., E. W. Hook, D. H. Martin, D. Willis, P. Fine, D. Fuller, J. Jordan, W. M. Janda, and M. Chernesky. 2005. Confirming positive results of nucleic acid amplification tests (NAATs) for Chlamydia trachomatis: all NAATs are not created equal. J. Clin. Microbiol. 43:1372-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schachter, J., W. M. McCormack, M. A. Chernesky, D. H. Martin, P. B. Van Der, P. A. Rice, E. W. Hook III, W. E. Stamm, T. C. Quinn, and J. M. Chow. 2003. Vaginal swabs are appropriate specimens for diagnosis of genital tract infection with Chlamydia trachomatis. J. Clin. Microbiol. 41:3784-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholes, D., A. Stergachis, F. E. Heidrich, H. Andrilla, K. K. Holmes, and W. E. Stamm. 1996. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N. Engl. J. Med. 334:1362-1366. [DOI] [PubMed] [Google Scholar]
- 15.Smieja, M., J. B. Mahony, C. H. Goldlsmith, S. Chong, A. Petrich, and M. Chernesky. 2001. Replicate PCR testing and probit analysis for detection and quantitation of Chlamydia pneumoniae in clinical specimens. J. Clin. Microbiol. 39:1796-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Pol, B., T. C. Quinn, C. A. Gaydos, K. Crotcheft, J. Schachter, J. Moncada, D. Jungkind, D. H. Martin, B. Turner, C. Peyton, and R. B. Jones. 2000. Multicenter evaluation of the AMPLICOR and automated COBAS AMPLICOR CT/NG tests for detection of Chlamydia trachomatis. J. Clin. Microbiol. 38:1105-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Pol, B., D. V. Ferrero, L. Buck-Barrington, E. Hook III, C. Lenderman, T. Quinn, C. A. Gaydos, J. Lovchik, J. Schachter, J. Moncada, G. Hall, M. J. Tuohy, and R. B. Jones. 2001. Multicenter evaluation of the BDProbeTec ET system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J. Clin. Microbiol. 39:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsh, L. E., T. C. Quinn, and C. Gaydos. 1997. Influence of endocervical specimen adequacy on PCR and direct fluorescent-antibody staining for detection of Chlamydia trachomatis infections. J. Clin. Microbiol. 35:3078-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]