Abstract
We report a case of Ulocladium atrum keratitis in a 43-year-old man. No predisposing event was known. He received natamycin and fluconazole drops and the infection resolved. The isolate was identified by morphological and rRNA gene sequence analyses. U. atrum is a dematiaceous hyphomycete not hitherto reported to infect humans.
CASE REPORT
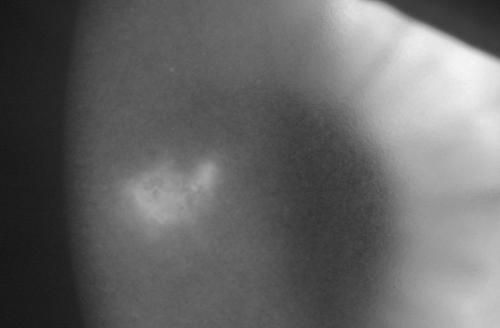
A 43-year-old truck driver from suburban Adelaide, Australia, woke one night with a suddenly painful, tearing right eye. His symptoms worsened through the day and he presented to a local health service the following day. A small corneal ulcer was seen, and he was given chloramphenicol ointment. However, the ulceration progressed and his vision deteriorated over the next 24 h, prompting a referral to Flinders Medical Centre. The patient was found to have photophobia, marked conjunctival injection, corneal edema, Descemet's membrane folding, and a central stromal infiltrate with a feathery edge (Fig. 1). The visual acuity (VA) in the right eye was hand motions only. No predisposing factors for a corneal infection were identified. The patient could not recall any ocular injury or irritation and did not wear contact lenses. He transported home appliances in his truck. The only medical history of note was tinea pedis and tinea cruris for which he was applying clotrimazole cream. Corneal scrapings were taken, and media were inoculated for aerobic and anaerobic bacterial culture, fungal culture, and Gram stain. A scraping was also taken for viral investigations: rapid culture (7) and PCR for herpes simplex virus and PCR for varicella-zoster virus. The PCRs were performed as a duplex based on the method of Read and Kurtz (17).
FIG. 1.
The clinical presentation showing corneal infiltration and edema (broad tangential illumination).
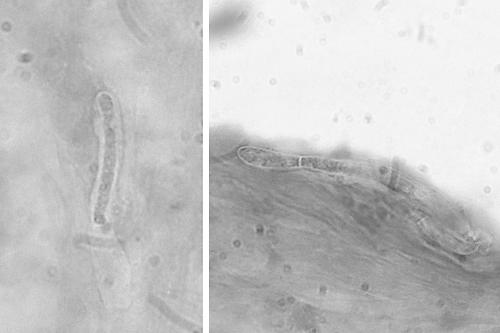
The Gram stain showed epithelial cells and dense patches of an acellular material, assumed to be anterior corneal stroma. Septate hyphae were embedded in the largest patch; no pus cells or bacteria were seen (Fig. 2). The chloramphenicol was discontinued and natamycin (5%) and fluconazole (0.2%; the neat intravenous preparation) were given as drops each hour. On review the next day, the patient was more comfortable and the infiltrate was smaller. By the next day, the VA had improved to count fingers, the infiltrate continued to resolve, and the anterior chamber could be examined and was found to be quiet. After incubation for 2 days, no growth was seen on the bacteriologic media and these were discarded. The viral assays were negative. The improvement in signs and symptoms continued, and treatment was tapered over the next 2 weeks. By 24 days after referral, the patient was comfortable and off all treatment. The eye was not inflamed, there was a small central corneal scar, and the VA was 20/20.
FIG. 2.
The Gram stain of the corneal scraping showing hyphae (magnification, ×960).
The primary fungal medium (Sabouraud's dextrose agar, no antibiotics) had been incubated at 28°C. A colony appeared at the inoculation site after 4 days. By day 8, the colony had a diameter of 2 cm and was greyish brown and powdery. It was sporulating by this time. Many of the conidia were dark brown and had transverse and longitudinal septa, suggestive of Alternaria species. However, some conidia were coarsely verrucose.
The isolate was sent, with clinical details only, to a fungal reference laboratory. It was identified as a Ulocladium species on morphological grounds. Antifungal susceptibility tests were performed using the National Committee for Clinical Laboratory Standards M38-A method for molds (11). MICs were determined for amphotericin B (4.0 μg/ml), flucytosine (64 μg/ml), fluconazole (64 μg/ml), itraconazole (0.125 μg/ml), ketoconazole (0.125 μg/ml), and voriconazole (0.25 μg/ml). Two other agents were tested using an agar disk method adapted from M38-A. Neo-Sensitabs tablets (Rosco Diagnostica, Taastrup, Denmark) containing either diffusible natamycin (50 μg) or terbinafine (30 μg) (19) were used on RPMI 1640 agar supplemented with glucose (0.2%) and buffered with MOPS (morpholinepropanesulfonic acid; 0.165 M). The inoculum was standardized to between 0.4 × 104 and 5 × 104 CFU/ml using a spectrophotometer. The plates were incubated at 35°C and examined at 48 and 72 h. The zone sizes indicated that the fungus was sensitive to natamycin and terbinafine (19).
The isolate was sent to a third laboratory for identification by rRNA gene sequencing. For DNA extraction, the isolate was cultured on potato dextrose agar at 30°C for 5 days. A suspension to a McFarland standard of 2.0 was prepared in saline (2 ml) and centrifuged. The pellet was resuspended in 200 μl of sorbitol buffer containing 200 U of lyticase (Sigma-Aldrich Corporation, St. Louis, Mo.) as described previously (25). The preparation was incubated at 37°C for 60 min and centrifuged (5,400 × g; 5 min). Spheroplasts were resuspended in 180 μl of lysis solution T and 20 μl of proteinase K (GenElute Mammalian Genomic DNA Miniprep kit; Sigma-Aldrich) and then incubated at 55°C for 60 min. DNA was extracted according to the manufacturer's instructions with a final elution volume of 200 μl. Samples were stored at −20°C until use.
Universal fungal primers were used to amplify the internal transcribed spacer (ITS) region of the rRNA gene complex, incorporating ITS 1, the 5.8S gene, and ITS 2 (24). Amplifications were performed in 25-μl volumes containing 1× GeneAmp PCR buffer (Applied Biosystems, Foster City, Calif.), 5% glycerol, 125 μM each deoxynucleoside triphosphate, 0.5 μM each primer (ITS 1, 5′-TCCGTAGGTGAACCTGCGG; and ITS 4, 5′-TCCTCCGCTTATTGATATGC), 1.25 U of Taq DNA polymerase (Applied Biosystems), and 10 μl of DNA. PCR conditions were 94°C for 2 min and 30 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s, with a final extension of 72°C for 6 min. The product was purified using a GFX PCR DNA and gel band purification kit (Amersham Biosciences, Piscataway, N.J.) and then sequenced using the ITS 1 primer and a BigDye Terminator v. 3.1 cycle sequencing kit in an ABI PRISM 3100-Avant genetic analyzer (Applied Biosystems). The result was edited using Chromas v. 2.23 software (Technelysium Pty. Ltd.). The 520-base sequence was deposited into GenBank and compared with other sequences (02/06) using FASTA (13).
Three sequences were identical to ours, four differed by one base, and three differed by two bases (Table 1). The sequences were assigned to five named species of Ulocladium. The table has one strain recognized as an epitype by de Hoog and Horré: Ulocladium atrum ATCC 18040 (4). The next two sequences differed from ours by six bases, one being assigned to the epitype for Ulocladium chartarum (4).
TABLE 1.
GenBank sequences most similar to AY943384
| Accession no. | Strain designation | No. of overlapping bases | Sequence similarity (%) | Refer- ence |
|---|---|---|---|---|
| AY372681 | U. botrytis UB32 | 519 | 100.00 | |
| AY625071 | U. chartarum UAMH 7842 | 514 | 100.00 | 10 |
| AY762941 | U. cucurbitae HSAUP_XF030282 | 510 | 100.00 | |
| AY278837 | U. consortiale | 514 | 99.8 | 14 |
| AY625072 | U. atrum UAMH 7840 | 514 | 99.8 | 10 |
| AF229487 | U. botrytis ATCC 18043 | 513 | 99.8 | 15 |
| AF229486 | U. atrum ATCC 18040 | 513 | 99.8 | 15 |
| AY372683 | U. atrum UA 36 | 519 | 99.6 | |
| AY208795 | Ulocladium sp. strain Po51 | 498 | 99.6 | |
| AY587137 | Ulocladium sp. strain Mos ID7 | 496 | 99.6 |
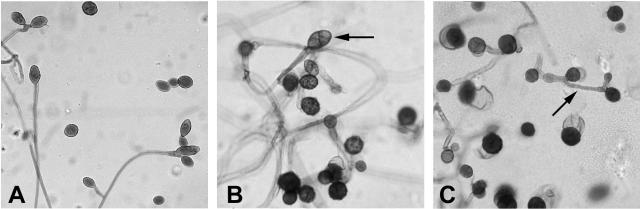
Further observations on the corneal isolate were made from subcultures on cornmeal agar incubated at 23 or 28°C (Fig. 3). The conidiophores varied from long, flexuous, and simple to short, geniculate, and branched. Conidia appeared within 48 h of culture. They were spherical to ellipsoidal and single but for the occasional chain of two. Dark brown, verrucose conidia were present by 72 h at either temperature. Increasing numbers of secondary conidiophores were seen. Many conidia were septate, and the septa often intersected at right angles. Of the species of Ulocladium described by Simmons in 1967, our isolate most closely resembles U. atrum (20). He later divided U. atrum-like strains into U. atrum, U. cucurbitae, U. dauci, or U. multiforme (21). Our isolate is most similar to the first of these species, although spherical and verrucose conidia appear quite early. We conclude that our patient had a corneal infection caused by U. atrum.
FIG. 3.
Micrographs of the isolate. A slide was prepared from a subculture (cornmeal agar; 28°C; 8 days) and stained with lactofuchsin. (A) Flexuous conidiophores with conidia (magnification, ×300). (B) Verrucose conidia. One septate conidium is indicated by an arrow (magnification, ×380). (C) Secondary conidiophore (arrow) and conidia (magnification, ×380).
Ulocladium is a genus of saprotrophic, darkly pigmented hyphomycetes. In medical microbiology laboratories, they appear most often in cultures for dermatophytes and are considered to be contaminants. The conclusion that a Ulocladium species was the causative agent in this case is based on the clinical appearance being consistent with a fungal infection, the discovery of hyphae in corneal tissue, the culture result and subsequent morphological and rRNA gene sequence analyses, the response to antifungal agents, and the failure to detect any other agent.
Ulocladium species closely resemble some of the saprotrophic Alternaria species, and in the past they have been classified as such. The morphological basis for separating the genera rests on observations such as whether developing conidia are ovoid or obovoid (20) and somewhat subtle differences in the pigmentation and verrucosity of mature conidia (3). Simmons has described as many as 12 Ulocladium species (20, 21), but recent ITS sequencing on a limited amount of material suggests this may be an overclassification. The sequencing supports the existence of U. atrum, U. botrytis, and U. chartarum as species within the cluster of Alternaria species (4, 15). de Hoog and Horré (4) concluded that about 14% of Alternaria and Ulocladium sequences in GenBank were misidentified; we suspect that the sequences listed in Table 1 all derive from the same organism.
This case is the first reported infection due to U. atrum. Human infections due to U. botrytis and U. chartarum, identified on morphological grounds, are known. U. botrytis was isolated from a case of onychomycosis (18). U. chartarum has been isolated from several cutaneous infections in immunocompromised individuals. One patient was on corticosteroids for Brill-Symmer's disease (1), one patient had Cushing's disease (23), and three others were on immunosuppressive therapy following heart (5) or kidney (2, 9) transplantation. In contrast, our patient was a healthy man, reflecting the vulnerability of the cornea to organisms of limited pathogenicity. Alternaria species have been reported from a variety of ocular infections, but a determined search of the literature failed to uncover any eye infections attributed to Ulocladium. Several large Indian series include corneal infections due to dematiaceous fungi that were not able to be identified (6, 8, 22), but it is likely that Ulocladium would have been recognized given its readiness to sporulate on simple fungal media.
A case of fungal keratitis without a recognized risk factor is unusual. Some degree of trauma, frequently associated with plant material, is a common predisposing event for keratomycosis. Anomalies of the ocular surface, structural alterations to the cornea, or poor compliance with contact lens care procedures can also predispose to fungal keratitis (12). How one or more spores breached the major barrier of the corneal epithelium in this case is not known.
The response of our patient to topical antifungals was rapid and impressive. The in vitro susceptibility data suggest that the response would have been due mainly to the natamycin. The findings were in general agreement with those of Pujol et al. (16), who determined the MICs of six antifungal agents against isolates of U. chartarum and U. botrytis by a broth microdilution method. Their results suggest that, of the drugs that can be used topically, amphotericin B, miconazole, and ketoconazole could be effective for Ulocladium keratitis. As the least number of isolates were resistant to miconazole and the mean MIC of this agent was 2.96 mg/liter, we suggest a combination of miconazole (1%; as the ophthalmic formulation) and natamycin for the initial treatment of Ulocladium keratitis. The susceptibility of the isolate to the newer azole, voriconazole, is also of note.
In summary, this case illustrates the vulnerability of the cornea to unusual organisms and those considered to have low pathogenicity. We believe that the successful outcome for our patient was due in part to a standard approach of taking corneal scrapings and thereby delivering appropriate treatment without delay.
Nucleotide sequence accession number.
The 520-base sequence of the Ulocladium isolate was deposited into GenBank under accession number AY943384.
REFERENCES
- 1.Altmeyer, P., and K. Schon. 1981. Schimmelpilzgranulome durch Ulocladium chartarum. Hautarzt 32:36-38. [PubMed] [Google Scholar]
- 2.Blanc, C., B. Lamey, and J. Lapalu. 1984. Alternariose cutanée chez un transplante rénal. Bull. Soc. Fr. Mycol. Med. 13:213-216. [Google Scholar]
- 3.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, p. 997-1001. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 4.de Hoog, G. S., and R. Horré. 2002. Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses 45:259-276. [DOI] [PubMed] [Google Scholar]
- 5.Duran, M. T., J. Del Pozo, M. T. Yebra, M. G. Crespo, M. J. Paniagua, M. A. Cabezon, and J. Guarro. 2003. Cutaneous infection caused by Ulocladium chartarum in a heart transplant recipient: case report and review. Acta Derm. Venereol. 83:218-221. [DOI] [PubMed] [Google Scholar]
- 6.Garg, P., U. Gopinathan, K. Choudhary, and G. N. Rao. 2000. Keratomycosis: clinical and microbiologic experience with dematiaceous fungi. Ophthalmology 107:574-580. [DOI] [PubMed] [Google Scholar]
- 7.Gleaves, C. A., D. J. Wilson, A. D. Wold, and T. F. Smith. 1985. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J. Clin. Microbiol. 21:29-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopinathan, U., P. Garg, M. Fernandes, S. Sharma, S. Athmanathan, and G. N. Rao. 2002. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in south India. Cornea 21:555-559. [DOI] [PubMed] [Google Scholar]
- 9.Magina, S., C. Lisboa, P. Santos, G. Oliveira, J. Lopes, M. Rocha, and J. Mesquita-Guimarães. 2000. Cutaneous alternariosis by Alternaria chartarum in a renal transplanted patient. Br. J. Dermatol. 142:1261-1262. [DOI] [PubMed] [Google Scholar]
- 10.Meklin, T., R. A. Haugland, T. Reponen, M. Varma, Z. Lummus, D. Bernstein, L. J. Wymer, and S. J. Vesper. 2004. Quantitative PCR analysis of house dust can reveal abnormal mold conditions. J. Environ. Monit. 6:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. NCCLS M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.O'Day, D. M. 1996. Fungal keratitis, p. 1048-1061. In J. S. Pepose, G. N. Holland, and K. R. Wilhelmus (ed.), Ocular infection and immunity. Mosby, St. Louis, Mo.
- 13.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pryor, B. M., and D. M. Bigelow. 2003. Molecular characterization of Embellisia and Nimbya species and their relationship to Alternaria, Ulocladium, and Stemphylium. Mycologia 95:1141-1154. [DOI] [PubMed] [Google Scholar]
- 15.Pryor, B. M., and R. L. Gilbertson. 2000. Molecular phylogenetic relationships amongst Alternaria species and related fungi based upon analysis of nuclear ITS and mtSSU rDNA sequences. Mycol. Res. 104:1312-1321. [Google Scholar]
- 16.Pujol, I., C. Aguilar, J. Gené, and J. Guarro. 2000. In vitro antifungal susceptibility of Alternaria spp. and Ulocladium spp. J. Antimicrob. Chemother. 46:337-338. [DOI] [PubMed] [Google Scholar]
- 17.Read, S. J., and J. B. Kurtz. 1999. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex PCR screening assay. J. Clin. Microbiol. 37:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano, C., E. Maritati, E. Paccagnini, and L. Massai. 2004. Onychomycosis due to Ulocladium botrytis. Mycoses 47:346-348. [DOI] [PubMed] [Google Scholar]
- 19.Rosco Diagnostica A/S. 2004. User's guide for Neo-Sensitabs susceptibility testing, 17th ed. Rosco Diagnostica A/S, Taastrup, Denmark. [Online.] http://www.rosco.dk/Default.asp?ID=198.
- 20.Simmons, E. G. 1967. Typification of Alternaria, Stemphylium, and Ulocladium. Mycologia 59:67-92. [PubMed] [Google Scholar]
- 21.Simmons, E. G. 1997. Multiplex conidium morphology in species of the Ulocladium atrum group. Can. J. Bot. 76:1533-1539. [Google Scholar]
- 22.Srinivasan, M., C. A. Gonzales, C. George, V. Cevallos, J. M. Mascarenhas, B. Asokan, J. Wilkins, G. Smolin, and J. P. Whitcher. 1997. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br. J. Ophthalmol. 81:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verret, J. L., F. Gaborieau, D. Chabasse, V. Rohmer, M. Avenel, and A. Smûlevici. 1982. Alternariose cutanée révélatrice d'une maladie de Cushing, un cas avec étude ultrastructurale. Ann. Dermatol. Venereol. 109:841-846. [PubMed] [Google Scholar]
- 24.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innes, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols. Academic Press, San Diego, Calif.
- 25.Williamson, E. C., J. P. Leeming, H. M. Palmer, C. G. Steward, D. Warnock, D. I. Marks, and M. R. Millar. 2000. Diagnosis of invasive aspergillosis in bone marrow transplant recipients by polymerase chain reaction. Br. J. Haematol. 108:132-139. [DOI] [PubMed] [Google Scholar]