Abstract
This report of an accidental exposure to Mycobacterium tuberculosis in a microbiological laboratory illustrates the value of gamma interferon enzyme-linked immunospot assay using peptides of ESAT-6, CFP-10, TB37.6, and TB7.7 for the diagnosis of latent infection. In particular, positive responses to peptides 2 to 6 of TB37.6 were observed exclusively in recently infected persons.
CASE REPORT
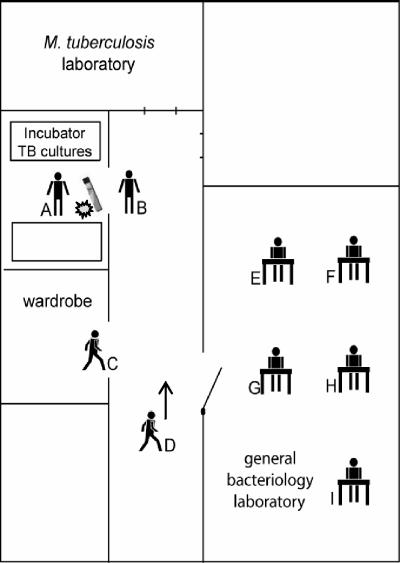
In a microbiological laboratory in The Netherlands, a technician (referred to as A) accidentally dropped a glass tube, containing a culture of Mycobacterium tuberculosis on solid Löwenstein-Jensen medium, on the floor. The sputum sample had been incubated for 3 to 4 weeks and many M. tuberculosis colonies were visible. The tube broke and the culture medium with M. tuberculosis colonies was spilled on the floor of the hall (Fig. 1). Technician A threw a paper cloth soaked in chlorine solution (1,000 ppm) on top of the spills. Subsequently, he cleaned up everything with a colleague (B). They wore gloves but did not wear protective masks. At the time of the accident, seven other colleagues were at work in surrounding rooms (Fig. 1, C through I). As all doors and windows were open, they could also have been exposed to mycobacteria.
FIG. 1.
Schematic drawing of the microbiological laboratory where the accident with the TB culture took place. The position of each technician (A through I), at the time of the accident, is indicated in the floor plan, as well as the place where the M. tuberculosis culture on solid Löwenstein-Jensen medium was dropped ( ).
).
Three months after the accident, a contact investigation was carried out among all nine potentially exposed technicians, of whom five were Mycobacterium bovis BCG vaccinated. Technician B, a non-BCG-vaccinated Dutch person, had a tuberculin skin test (TST) conversion, as the TST was 25 mm, while previous yearly routine TST results had been negative (0 mm) (see Table 2, subject code B). Thus, B had most likely become infected with M. tuberculosis during the accident. Technician A had a TST of 20 mm (see Table 2, subject code A). No previous TST had been done, as he was BCG vaccinated (>20 years ago) and was born in a country where tuberculosis (TB) is endemic. Chest radiographs of technician B and all BCG-vaccinated persons were normal, excluding active pulmonary TB. However, a recently acquired latent M. tuberculosis infection due to the accidental exposure could not be excluded by radiography.
TABLE 2.
| Codec | BCG | TST (mm) | TB | Treatment | PPDe
|
ESAT-6e
|
CFP-10e
|
TB37.6e
|
TB7.7e
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yr 0 | Yr 1 | Yr 0 | Yr 1 | Yr 0 | Yr 1 | Yr 0 | Yr 1 | Yr 0 | Yr 1 | |||||
| A | Yes | 20 | No | Yes | 275 | 76 | 71 | 78 | 469 | 190 | 52 | < | 7 | < |
| B | No | 25 | No | Yes | 146 | 160 | 370 | 275 | 104 | 148 | 53 | 8 | 5 | < |
| C | Yes | 3 | No | No | 5 | 98 | < | 9 | < | < | < | < | 0 | < |
| D | No | 0 | No | No | 12 | 16 | 6 | 3 | 4 | 1 | 1 | 1 | 0 | 8 |
| E | Yes | ND | No | No | 27 | 23 | 7 | < | 7 | 4 | 6 | 0 | 6 | < |
| F | Yes | ND | No | No | * | * | * | * | * | * | * | * | * | * |
| G | Yes | >10 | No | No | < | 5 | 5 | 11 | < | |||||
| H | No | 0 | No | No | 5 | 42 | < | 4 | < | < | < | < | < | < |
| I | No | 0 | No | No | 46 | < | < | 1 | 2 | |||||
| Lab 1 | No | 0 | No | No | < | < | < | < | 2 | |||||
| Lab 2 | Yes | 0 | No | No | 15 | < | < | < | < | |||||
| Lab 3 | Yes | 0 | No | No | 2 | < | < | < | 3 | |||||
| Lab 4 | Yes | 22 | No | No | 14 | < | < | < | < | |||||
| Lab 5 | No | >10 | No | No | 0 | < | < | < | < | |||||
| Lab 6 | Unknown | >10 | No | No | 271 | 27 | 7 | < | 19 | |||||
| Lab 7 | No | >10 | No | Yes | 3 | < | < | < | < | |||||
| Lab 8 | No | 11 | No | No | 6 | < | < | < | < | |||||
| Lab 9 | Yes | ND | Cured TBd | Yes | 22 | < | < | 3 | < | |||||
| Lab 10 | Yes | 11 | Cured TBd | Yes | 17 | < | < | < | < | |||||
| TB 1 | No | ND | Cured TB | Yes | 144 | 18 | 19 | 5 | 12 | |||||
| TB 2 | Unknown | ND | Cured TB | Yes | 52 | 84 | 34 | < | 16 | |||||
| TB 3 | No | ND | Cured TB | Yes | 196 | 63 | 104 | < | 3 | |||||
| TST 1 | No | >10 | No | Yes | 325 | 35 | 275 | < | < | |||||
| TST 2 | No | >10 | No | Yes | 243 | 77 | 111 | 2 | 115 | |||||
| TST 3 | No | >10 | No | Yes | 181 | 324 | 240 | 7 | 16 | |||||
IFN-γ ELISPOT was done 4 months (year 0) and 16 months (year 1) after the accidental exposure. ELISPOT results are shown as mean numbers of spot-forming cells (SFC)/106 PBMC minus the mean of medium value.
Symbols: <, number of SFC less than in medium; ND, not done; *, not interpretable due to high medium values.
A to I, technicians present during the accident, by decreasing exposure level; Lab 1 to 10, technicians not present during the accident; TB 1 to 3, cured TB patients, treated 10, 3, and 2 years before; TST 1 to 3, tuberculin skin test converters after a known exposure to TB 5, 6, and 10 years before.
Limited pulmonary TB diagnosed during routine checkup before clinical symptoms became apparent; Lab 9, 33 years ago and Lab 10, 8 years ago.
A value that is >20 constitutes a positive response.
Therefore, we evaluated an M. tuberculosis-specific immunodiagnostic assay (2, 25). Ten colleagues who were not at work on the day of the accident (see Table 2, subject codes Lab 1 to Lab 10) were included as a control group with comparable levels of background exposure to M. tuberculosis. Furthermore, six well-defined M. tuberculosis-infected individuals (three latently infected and three cured tuberculosis patients), with known positive responses to ESAT-6 and/or CFP-10 in a 6-day lymphocyte stimulation assay (3), were included as a positive control group (see Table 2, subject codes TB 1 to 3 and TST 1 to 3).
From a venous blood sample peripheral blood mononuclear cells (PBMC) were isolated and an overnight gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay was performed as previously described (5). In the positive control group, available frozen PBMC were used. A positive test result was predefined as a response of at least 20 spot-forming cells (SFC) per million PBMC (after subtraction of the background value) and of at least twice the background value. As antigenic stimuli we used ESAT-6 and CFP-10, those having been used in most previous studies of M. tuberculosis-specific immunodiagnostic assays, as was recently reviewed (25). In addition, we included overlapping peptides of TB7.7 and TB37.6 that were the most promising novel diagnostic antigens in earlier studies (1, 7, 21) (Table 1). All four coding genes are situated on genomic regions of difference (RD) that are present in M. tuberculosis but absent from BCG and most environmental mycobacterial species (6). TB7.7 is encoded by a phage-inserted region (phiRv2) which is highly specific for M. tuberculosis (6, 8, 12). TB37.6 is a PPE protein (PPE68) which is predominantly found in the membrane and cell wall fraction (13, 24). Despite being encoded by RD1, TB37.6 was recognized by BCG-vaccinated individuals (7, 11, 21), due to cross-reactivity to conserved epitopes within the PPE family that are also present in BCG (24). We tested only peptides 2 to 6 of TB37.6, which were found to be highly specific for M. tuberculosis (7). Purified protein derivative (PPD) was used as a nonspecific mycobacterial antigenic stimulus.
TABLE 1.
Diagnostic antigens and peptides
| RDa region | Rv code | Antigen | Antigenic stimulus | Sequence | Concn |
|---|---|---|---|---|---|
| PPD | PPD (RT 49 SSI) | 5 μg/ml | |||
| RD1 | Rv3875 | ESAT-6 | Peptide pool (p1-p9) | Complete sequence | 10 μg/ml/peptide |
| Rv3874 | CFP-10 | Peptide pool (p1-p9) | Complete sequence | 10 μg/ml/peptide | |
| Rv3873 | TB37.6 | Peptide pool (p2-p6) | Amino acids 13-70 | 10 μg/ml/peptide | |
| RD11 | Rv2654 | TB7.7 | Peptide pool (p1-p6) | Complete sequence | 10 μg/ml/peptide |
RD, regions of difference that are present in M. tuberculosis but absent from BCG and most environmental mycobacterial species.
The individual results of the ELISPOT are shown in Table 2. High numbers of ESAT-6- and CFP-10-specific T cells were detected in the two laboratory technicians (A and B) who had been most closely involved in the accident and had a positive TST. None of the other seven potentially exposed technicians (C through I) were positive. From the 10 colleagues who had not been present during the accident, only 1 TST-positive individual (Lab 6) responded to PPD and ESAT-6 in the ELISPOT. Of note, specimens from four other TST-positive individuals and two technicians who had been treated for limited pulmonary TB 33 and 8 years earlier (Lab 9 and Lab 10, respectively) did not recognize any of the four M. tuberculosis-specific peptide pools in an overnight ELISPOT assay. All three well-documented TST converters and two of the three TB patients responded to ESAT-6 and CFP-10 in the overnight IFN-γ ELISPOT assay (Table 2, subject codes TST 1 to 3 and TB 2 to 3). For one of the cured TB patients (TB 1), who suffered from TB disease 10 years ago, ESAT-6- and CFP-10-specific T cells were just below the cutoff level for a positive response.
The peptide pool of TB7.7 was poorly recognized (Table 2). Stimulation with peptides of TB37.6 using PBMC from the recently exposed technicians A and B did result in a significant number of IFN-γ-producing T cells (Table 2). Interestingly, none of the samples from the other nine TST-positive individuals responded to TB37.6, even though samples from some of these persons with a TST conversion in the past did respond strongly to ESAT-6 and CFP-10. Also, none of the samples from the five individuals with a history of TB recognized the TB37.6 peptides.
A follow-up analysis of M. tuberculosis-specific T-cell responses was done 1 year later, including those of all laboratory technicians who had been present at the time of the accident with M. tuberculosis. The results showed that the number of IFN-γ-producing T cells in response to PPD and the peptide pools of ESAT-6, CFP-10, and TB7.7 had remained of the same order of magnitude as 1 year earlier (Table 2). In contrast, responses to the TB37.6 peptides had become undetectable in subjects A and B, those being the subjects who were most likely infected with M. tuberculosis during the laboratory accident, who had both been treated for 6 months with isoniazid (to which the isolate was sensitive).
This report illustrates the diagnostic potential of an ELISPOT assay using M. tuberculosis-specific peptide pools of ESAT-6, CFP-10, and TB37.6 for diagnosis of recent infection with M. tuberculosis. The TST has limited value for diagnosis of recent TB infection, especially when many exposed individuals are BCG vaccinated or are already TST positive due to previous exposure to mycobacteria, as was the case in our study setting, where personnel of a microbiological laboratory were accidentally exposed to M. tuberculosis. Based on ELISPOT, two laboratory technicians with the highest level of exposure during the accident were found to respond strongly to ESAT-6 and CFP-10. Interestingly, peptides 2 to 6 of TB37.6 were exclusively recognized by specimens from these two recently exposed individuals and not by any of the controls with a history of TST conversion or cured TB who did respond to ESAT-6 and CFP-10. Moreover, follow-up with ELISPOT 1 year after the accident revealed that responses to TB37.6 had become undetectable, while responses to ESAT-6 and CFP-10 remained unchanged. These observations suggest that the pattern of antigen-specific responses may reflect the phase of infection and that a positive ELISPOT response to TB37.6 peptides could be a marker for recent infection.
Previous studies demonstrated that the antigens used in this study are highly specific for M. tuberculosis (3, 7, 10, 20, 22, 23, 27). Therefore, positive responses can be regarded as a correlate of infection. In contacts of smear-positive pulmonary TB patients, results of ESAT-6/CFP-10 ELISPOT correlated better with the degree of exposure than did the TST and, moreover, seemed to be more sensitive (15, 17, 18). Thus, we think that the persons with negative ELISPOT results had not been infected during the accident. During a follow-up period of 3 years, none of them developed active TB disease.
A positive response to ESAT-6 was observed in only one of the laboratory controls who had been infected with M. tuberculosis in the past. This is in contrast with previous reports that ESAT-6 and CFP-10 are frequently recognized by samples from TST-positive individuals and cured TB patients (3, 26, 30). This apparent discrepancy may be explained by a waning of ELISPOT responses during effective treatment, as has been reported (9). Another explanation could be that an overnight ELISPOT detects mainly responses of circulating effector memory T cells (16), which could cause a negative test result in case of a remote infection. In accordance, in some of technicians who were infected with M. tuberculosis in the past, we observed positive responses to ESAT-6 and CFP-10 in a 6-day culture, while overnight ELISPOT results were negative (data not shown). Thus, ELISPOT could be the method of choice for diagnosis of recent infection with M. tuberculosis, while more prolonged cultures may be required when diagnosis of more remote infection is relevant as well, such as in immunocompromised persons or those eligible for immunosuppressive treatment (4, 19).
Our findings illustrate that ELISPOT responses to ESAT-6 and CFP-10 can remain positive for years after adequate treatment and thus cannot discriminate between recent and remote infection. The study setting of an accidental exposure to M. tuberculosis in a laboratory was particularly suitable to evaluate novel antigens for the diagnosis of recent infection and to study the kinetics of immune responses, because the exact moment of exposure was known. We evaluated two promising novel diagnostic antigens, TB37.6 and TB7.7, of which TB7.7 was recognized poorly. The M. tuberculosis-specific peptides 2 to 6 of TB37.6 were exclusively recognized by samples from recently infected individuals. In seeming contrast to our results, another study reported that samples from remote TST converters could recognize TB37.6. In that study, whole recombinant antigen was tested in a 5-day culture assay (24), while we tested only the M. tuberculosis-specific peptides and used a short culture assay, which could explain the difference in observation. Previously, the M. tuberculosis-specific peptide pool of TB37.6 was tested only in patients with active TB and a small group of recently and latently infected individuals, inducing IFN-γ responses in 38 to 46% of these persons (7). However, the number of subjects in our study was too small to conclude definitively that responses to TB37.6 are found exclusively in recently infected individuals, and the observation could be coincidental. Further studies are needed to validate our findings. In both accidentally infected persons, responses to TB37.6 had become undetectable after treatment was completed, but this does not necessarily imply a causal relationship between treatment and the kinetics of the immune response. Two other studies indicate that there might be a sequential appearance of antigen-specific responses after infection with M. tuberculosis (28, 29). It has been reported that ELISPOT responses to ESAT-6 and CFP-10 became negative during successful treatment of active TB, while remaining positive in those without clinical improvement (9). A prospective follow-up study of recently infected persons with or without treatment for latent TB infection may clarify the natural kinetics of T-cell responses to TB37.6 and assess the effect of treatment.
A diagnostic assay that could specifically diagnose recently acquired latent infection would allow more targeted treatment, because the risk of progression to active TB decreases significantly with time after infection in persons with a normal immune system. The present report provides a novel hypothesis in this regard, namely, that ELISPOT responses to TB37.6 indicate recent infection. Targeting treatment to recently infected individuals would benefit even more if it would be possible to identify persons specifically at risk of developing active TB disease. One study found that recently exposed individuals with high ESAT-6 responses were most likely to progress to active TB within 2 years (14).
In conclusion, ELISPOT using peptide pools of ESAT-6, CFP-10, and TB37.6 allowed specific diagnosis of recent M. tuberculosis infection after accidental exposure to M. tuberculosis, irrespective of BCG vaccination or TST results. In particular, responses to M. tuberculosis-specific peptides of TB37.6 were exclusively found in association with a recently acquired infection. Clearly, the ideal assay for diagnosis of latent M. tuberculosis infection is not yet available, but our findings may represent another step in the right direction.
Acknowledgments
We thank all laboratory technicians who participated in this study. Sophie Toumanian, municipal health services (GGD), Department of TB Control, Twente, The Netherlands, is gratefully acknowledged for performing the contact investigation with the TSTs.
This work was supported by the European Commission.
REFERENCES
- 1.Aagaard, C., I. Brock, A. Olsen, T. H. Ottenhoff, K. Weldingh, and P. Andersen. 2004. Mapping immune reactivity toward Rv2653 and Rv2654: two novel low-molecular-mass antigens found specifically in the Mycobacterium tuberculosis complex. J. Infect. Dis. 189:812-819. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 3.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 4.Arend, S. M., F. C. Breedveld, and J. T. van Dissel. 2003. TNF-alpha blockade and tuberculosis: better look before you leap. Neth. J. Med. 61:111-119. [PubMed] [Google Scholar]
- 5.Arend, S. M., K. E. van Meijgaarden, K. de Boer, E. C. de Palou, D. van Soolingen, T. H. Ottenhoff, and J. T. van Dissel. 2002. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J. Infect. Dis. 186:1797-1807. [DOI] [PubMed] [Google Scholar]
- 6.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 7.Brock, I., K. Weldingh, E. M. Leyten, S. M. Arend, P. Ravn, and P. Andersen. 2004. Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection. J. Clin. Microbiol. 42:2379-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 99:3684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrara, S., D. Vincenti, N. Petrosillo, M. Amicosante, E. Girardi, and D. Goletti. 2004. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin. Infect. Dis. 38:754-756. [DOI] [PubMed] [Google Scholar]
- 10.Chapman, A. L., M. Munkanta, K. A. Wilkinson, A. A. Pathan, K. Ewer, H. Ayles, W. H. Reece, A. Mwinga, P. Godfrey-Faussett, and A. Lalvani. 2002. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 16:2285-2293. [DOI] [PubMed] [Google Scholar]
- 11.Cockle, P. J., S. V. Gordon, A. Lalvani, B. M. Buddle, R. G. Hewinson, and H. M. Vordermeier. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 13.Demangel, C., P. Brodin, P. J. Cockle, R. Brosch, L. Majlessi, C. Leclerc, and S. T. Cole. 2004. Cell envelope protein PPE68 contributes to Mycobacterium tuberculosis RD1 immunogenicity independently of a 10-kilodalton culture filtrate protein and ESAT-6. Infect. Immun. 72:2170-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty, T. M., A. Demissie, J. Olobo, D. Wolday, S. Britton, T. Eguale, P. Ravn, and P. Andersen. 2002. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J. Clin. Microbiol. 40:704-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewer, K., J. Deeks, L. Alvarez, G. Bryant, S. Waller, P. Andersen, P. Monk, and A. Lalvani. 2003. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361:1168-1173. [DOI] [PubMed] [Google Scholar]
- 16.Godkin, A. J., H. C. Thomas, and P. J. Openshaw. 2002. Evolution of epitope-specific memory CD4(+) T cells after clearance of hepatitis C virus. J. Immunol. 169:2210-2214. [DOI] [PubMed] [Google Scholar]
- 17.Hill, P. C., R. H. Brookes, A. Fox, K. Fielding, D. J. Jeffries, D. Jackson-Sillah, M. D. Lugos, P. K. Owiafe, S. A. Donkor, A. S. Hammond, J. K. Otu, T. Corrah, R. A. Adegbola, and K. P. McAdam. 2004. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin. Infect. Dis. 38:966-973. [DOI] [PubMed] [Google Scholar]
- 18.Hill, P. C., A. Fox, D. J. Jeffries, D. Jackson-Sillah, M. D. Lugos, P. K. Owiafe, S. A. Donkor, A. S. Hammond, T. Corrah, R. A. Adegbola, K. P. McAdam, and R. H. Brookes. 2005. Quantitative T cell assay reflects infectious load of Mycobacterium tuberculosis in an endemic case contact model. Clin. Infect. Dis. 40:273-278. [DOI] [PubMed] [Google Scholar]
- 19.Keane, J., S. Gershon, R. P. Wise, E. Mirabile-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 20.Lalvani, A., A. A. Pathan, H. McShane, R. J. Wilkinson, M. Latif, C. P. Conlon, G. Pasvol, and A. V. Hill. 2001. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Respir. Crit. Care Med. 163:824-828. [DOI] [PubMed] [Google Scholar]
- 21.Liu, X. Q., D. Dosanjh, H. Varia, K. Ewer, P. Cockle, G. Pasvol, and A. Lalvani. 2004. Evaluation of T-cell responses to novel RD1- and RD2-encoded Mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection. Infect. Immun. 72:2574-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori, T., M. Sakatani, F. Yamagishi, T. Takashima, Y. Kawabe, K. Nagao, E. Shigeto, N. Harada, S. Mitarai, M. Okada, K. Suzuki, Y. Inoue, K. Tsuyuguchi, Y. Sasaki, G. H. Mazurek, and I. Tsuyuguchi. 2004. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am. J. Respir. Crit. Care Med. 170:59-64. [DOI] [PubMed] [Google Scholar]
- 23.Munk, M. E., S. M. Arend, I. Brock, T. H. Ottenhoff, and P. Andersen. 2001. Use of ESAT-6 and CFP-10 antigens for diagnosis of extrapulmonary tuberculosis. J. Infect. Dis. 183:175-176. [DOI] [PubMed] [Google Scholar]
- 24.Okkels, L. M., I. Brock, F. Follmann, E. M. Agger, S. M. Arend, T. H. Ottenhoff, F. Oftung, I. Rosenkrands, and P. Andersen. 2003. PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family. Infect. Immun. 71:6116-6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pai, M., L. W. Riley, and J. M. Colford, Jr. 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761-776. [DOI] [PubMed] [Google Scholar]
- 26.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 27.Scarpellini, P., S. Tasca, L. Galli, A. Beretta, A. Lazzarin, and C. Fortis. 2004. Selected pool of peptides from ESAT-6 and CFP-10 proteins for detection of Mycobacterium tuberculosis infection. J. Clin. Microbiol. 42:3469-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi, L., Y. J. Jung, S. Tyagi, M. L. Gennaro, and R. J. North. 2003. Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc. Natl. Acad. Sci. USA 100:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva, V. M., G. Kanaujia, M. L. Gennaro, and D. Menzies. 2003. Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int. J. Tuberc. Lung Dis. 7:478-484. [PubMed] [Google Scholar]
- 30.Wu-Hsieh, B. A., C. K. Chen, J. H. Chang, S. Y. Lai, C. H. Wu, W. C. Cheng, P. Andersen, and T. M. Doherty. 2001. Long-lived immune response to early secretory antigenic target 6 in individuals who had recovered from tuberculosis. Clin. Infect. Dis. 33:1336-1340. [DOI] [PubMed] [Google Scholar]