Abstract
Trichomonads closely related to the bovid parasite Tritrichomonas foetus were identified in the bronchoalveolar lavage sample from a patient with AIDS in association with Pneumocystis pneumonia. This human case of T. foetus-like infection emphasizes the zoonotic potential of trichomonads, although the existence of a human-host-adapted T. foetus strain cannot be excluded.
CASE REPORT
A 54-year-old woman was admitted to the intensive care unit of Mantes-la-Jolie hospital, presenting with fever, severe fatigue, and dyspnea with polypnea that had been increasing over the previous month. The patient was known to be seropositive for human immunodeficiency virus, which was revealed 3 years previously following pneumocystosis. At that time, an antiretroviral therapy was proposed but the patient denied the diagnosis. Since the patient had no history of blood transfusion or injection of illicit drugs, transmission by unprotected sex remained the presumed mode of human immunodeficiency virus contamination. In addition, the patient was overweight and had type 2 diabetes. Hemodynamic parameters were within normal limits. Blood gases were 52 mm Hg O2 and 36 mm Hg CO2. A chest X-ray showed bilateral interstitial syndrome and several alveolar opacities. Bronchoalveolar lavage (BAL) was performed by fiber optic bronchoscopy and confirmed pneumocystosis. Interestingly, microscopic examination of a May-Grünwald-Giemsa-stained cytospin slide revealed numerous cells assumed to be trichomonad organisms, mixed with Pneumocystis parasites. The BAL sample was therefore immediately frozen for further analysis. Nasal oxygenation, intravenous injection of trimethoprim-sulfamethoxazole and methylprednisolone hemisuccinate, and subcutaneous injection of insulin were started, and recovery was rapid. On day 4, the patient was transferred to the Department of Infectious Diseases of the same institution where the investigations were completed. The CD4 cell count was 60 per mm3, with a CD4/CD8 ratio of 0.06. Plasma viral load was 5.34 log10 copies/ml. Hypercalcemia with hyperparathyroidism was also noticed. The patient returned home 13 days after her hospitalization with trimethoprim-sulfamethoxazole per os. Antiretroviral therapy and exploration of hyperparathyroidism were scheduled.
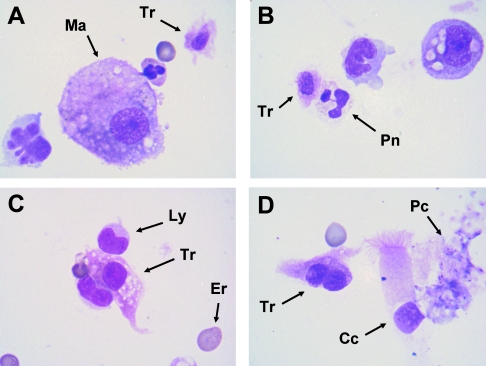
One part of the BAL sample obtained by fiber optic bronchoscopy was centrifuged, and cytospin slides were obtained for subsequent standard microscopic examination. The BAL specimen examined contained numerous parasites provisionally identified as trichomonads, exhibiting an amoeboid shape and mixed with alveolar macrophages, lymphocytes, erythrocytes, and aggregates of Pneumocystis organisms (Fig. 1). Parasitic cells showed one or two elliptically shaped nuclei and a poorly defined cytoplasm. This appearance had already been described for trichomonads from cytological specimens stained with May-Grünwald-Giemsa or Papanicolaou techniques (1-5). In parallel, DNA was extracted from the remaining part of the BAL sample by use of a QIAamp DNA minikit (QIAGEN, Hilden, Germany), with some modifications to the manufacturer's recommendations. In order to identify the trichomonad species found in the BAL sample, a nested-PCR strategy was developed, allowing the DNA amplification of the internal transcribed spacer 1 (ITS1)-5.8S rRNA-ITS2 region by use of trichomonad-specific primers. The first PCR was performed using the sense primer TRICHO-F (5′-CGGTAGGTGAACCTGCCGTT-3′) and the antisense primer TRICHO-R (5′-TGCTTCAGTTCAGCGGGTCT-3′) as described previously (8). PCR was carried out for 40 cycles (GeneAmp PCR system 9700 apparatus; Applied Biosystems) according to standard conditions for Platinum Taq high-fidelity DNA polymerase (Invitrogen, Groningen, The Netherlands). Negative (BAL sample without trichomonads) and positive (trichomonad DNAs extracted from axenic cultures) controls were included in the series. The second amplification was performed using the sense primer TRICHO-FBIS (5′-GGTGAACCTGCCGTTGGATC-3′) and the antisense primer TRICHO-RBIS (5′-TCAgTTCAGCGGGTCTTCCT-3′). The second PCR product was separated by agarose gel electrophoresis, and the band of the expected size (323 bp excluding the amplification primers) was purified using a QIAEX II gel extraction kit (QIAGEN). The purified PCR product was cloned in the T vector pCR 2.1-TOPO (Invitrogen) and amplified in Escherichia coli TOP10 competent cells. Ten clones were arbitrarily isolated, and the resulting minipreparations of plasmid DNAs were done using a QIAprep spin miniprep kit (QIAGEN). Selected clones were sequenced on both strands by use of a Big Dye Terminator cycle sequencing kit (Applied Biosystems) and an automated PRISM 377 DNA sequencer (Applied Biosystems).
FIG. 1.
Cytological appearance of trichomonad cells in the BAL sample (May-Grünwald-Giemsa staining). (A) A trichomonad cell (Tr) with an oval nucleus is easily recognizable in the vicinity of a macrophage cell (Ma). (B) A trichomonad cell is seen in contact with a neutrophile polymorphonuclear (Pn). (C) An amoeboid trichomonad with a round nucleus is seen between two lymphocytes (Ly); an erythrocyte (Er) gives the scale (diameter, 7 μm). (D) An amoeboid trichomonad exhibiting two nuclei is seen in the vicinity of a bronchial ciliated cell (Cc) and an aggregate of Pneumocystis organisms (Pc). Magnification, ×1,000.
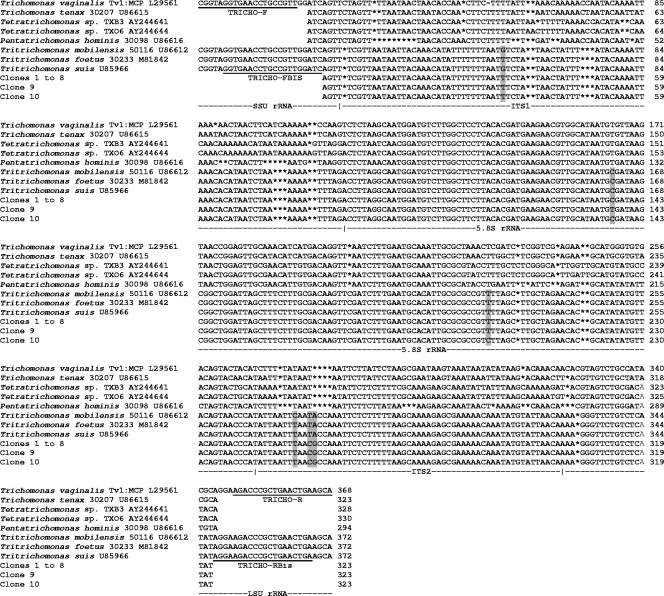
Among the 10 sequenced clones, 8 of them (clones 1 to 8) exhibited 100% identity, whereas clones 9 and 10 differed from clones 1 to 8 at only one position (C→T and T→C in positions 138 and 203, respectively; numbering as for the 323-bp PCR product). These differences are likely to be due to normal variation within the multiple copies of the RNA genes in any given genome. These sequences were aligned with all of the trichomonad ITS1-5.8S-ITS2 sequences available in databases by use of the BioEdit v7.0.1 package (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). A part of this alignment, including only trichomonad species of interest in our study, is shown in Fig. 2. In the common part of our alignment (339 positions including gaps), the sequences of clones 1 to 10 showed a lower degree of similarity (57.9 to 64.3%) to sequences of other trichomonad species found in humans. These included Trichomonas vaginalis, Trichomonas tenax, Pentatrichomonas hominis, and the recently identified Tetratrichomonas sp. strains TXB3 and TXO6, isolated from the oral cavity and bronchi of patients, respectively (9). Strikingly, clones 1 to 10 exhibited 98.6 to 99.2% identity (3 to 5 nucleotide differences) to homologous sequences from Tritrichomonas foetus, Tritrichomonas suis, and Tritrichomonas mobilensis, three probably synonymous trichomonad species isolated from cattle, pigs, and squirrel monkeys, respectively (6, 9, 12, 19). These results showed that the trichomonad species found in the lungs of this patient belonged to the Tritrichomonas genus and indicated that these organisms were closely related to the parasites of bovids, pigs, and nonhuman primates.
FIG. 2.
Alignment of sequences of the ITS1-5.8S rRNA-ITS2 region of clones 1 to 10 obtained in this study and those of trichomonad species of interest found in humans and animals. Differences between the clones obtained in this study and differences between these clones and the homologous sequences of Tritrichomonas foetus, Tritrichomonas suis, and Tritrichomonas mobilensis are shaded. Sequences of the primers used in this study are underlined. Gaps are represented by asterisks. SSU, small subunit; LSU, large subunit.
Trichomonads are flagellated protozoa often found in the digestive or reproductive systems of both invertebrates and vertebrates, including mammals. Four trichomonad species are currently found in humans (7): Trichomonas tenax in the oral cavity, Pentatrichomonas hominis and Dientamoeba fragilis in the intestinal tract, and Trichomonas vaginalis in the genitourinary tract. Only T. vaginalis is considered pathogenic and is the causative agent of human trichomoniasis, which has emerged as the most prevalent nonviral sexually transmitted disease worldwide in recent years (17). Until recently, trichomonad parasites of humans were thought to be site specific. However, trichomonads identified as T. tenax have been found in numerous cases in the upper or lower respiratory tract of humans (11, 13, 14). This organism is a commensal of the human oral cavity and is believed to enter the respiratory tract by aspiration from the contaminated oropharynx. Interestingly, other trichomonad species have also been identified in human lungs by molecular means. These include T. vaginalis (4, 15, 18), P. hominis (8), and a new Tetratrichomonas species that had not previously been reported as a human parasite (9). Thus, the presence of these protozoa in the human respiratory tract is not unusual, and we have recently shown that trichomonads are frequently found in lungs of immunocompromised patients in association with Pneumocystis organisms (5). Pulmonary trichomoniases have been reported almost exclusively with patients with underlying respiratory diseases and/or immunodepression, and trichomonads could not persist, apparently, without associated bacterial or fungal infections. These observations suggested that these protozoa were unable to cause the pulmonary disease on their own. However, their presence should not be considered a benign finding until the pathogenic potential of these parasites has been resolved. In addition, their occurrence in the respiratory tract of humans is likely overlooked, due mainly to observers' lack of awareness in unusual sites and amoeboid transformation of these microorganisms (4, 5).
Recently, the amplification of the ITS1-5.8S rRNA-ITS2 region by PCR followed by sequencing has become a reliable tool for detection and identification of trichomonads at the species level. In the case reported here, we have unexpectedly identified T. foetus-like organisms in the BAL sample from an adult patient with AIDS, which reinforces the increasing interest in trichomonad parasites. To our knowledge, this is the second human case of T. foetus-like infection, the first one being represented by a case of T. foetus meningoencephalitis in a recipient of allogeneic peripheral blood stem cell transplantation (16). However, in the latter case, these microorganisms were identified by scanning electron microscopy examination and not by using molecular tools. Trichomonads were thought to have strict host specificity, and T. foetus is associated in nature only with bovine diseases such as vaginitis. However, it has been shown recently that this parasite is also the etiological agent of feline trichomonal diarrhea (10). In addition, molecular data have confirmed previous morphological observations suggesting that T. foetus, Tritrichomonas suis from pigs, and probably Tritrichomonas mobilensis isolated from nonhuman primates should be considered strains of the same species (6, 9, 12, 19). To prevent confusion, it has been proposed to maintain the name T. foetus at least for both bovid and porcine Tritrichomonas (19). The suggestion that the same T. foetus species could be able to colonize several hosts, including bovids, pigs, felids, nonhuman primates, and humans (this study), raises the question of the as-yet-unknown zoonotic potential of trichomonads. These data also suggest the existence of a large potential reservoir in animals for T. foetus-like infections in humans. Kutisova et al. (9) have identified Tetratrichomonas strains isolated from the respiratory tract of humans which exhibited a very close genetic relationship with a common avian species, Tetratrichomonas gallinarum, reinforcing the hypothesis of zoonotic human infections. However, the same authors failed to transmit Tetratrichomonas of human origin to birds, which could be explained by a biological separation of the human-host-adapted T. gallinarum-like trichomonads. Thus, the existence of a human-host-adapted T. foetus-like strain cannot be excluded and this hypothesis has to be tested in further experimental infections. In parallel, additional studies are required to clarify how frequent human infections by T. foetus-like organisms are and to determine the pathogenic potential of these parasites. From a more general point of view, the present report highlights the widening pathological spectrum of trichomonads in humans, especially in the context of immunodepression-related emerging infections.
Nucleotide sequence accession numbers.
The nucleotide sequences obtained in this study have been deposited in GenBank under accession numbers DQ243910 to DQ243912.
Acknowledgments
The study was funded by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Institut Pasteur of Lille, and the Ministry of Research (contract no. EA3609) and was developed in the framework of IFR-17 (Institut Pasteur of Lille) and of the “Eurocarinii” network (FP-5, QLK2, CT-2000, 01369).
REFERENCES
- 1.Demirezen, S., Z. Safi, and S. Beksac. 2000. The interaction of Trichomonas vaginalis with epithelial cells, polymorphonuclear leucocytes and erythrocytes on vaginal smears: light microscopic observation. Cytopathology 11:326-332. [DOI] [PubMed] [Google Scholar]
- 2.Duboucher, C., M. Mogenet, and G. Périé. 1995. Salivary trichomoniasis: a case report of infestation of a submaxillary gland by Trichomonas tenax. Arch. Pathol. Lab. Med. 119:277-279. [PubMed] [Google Scholar]
- 3.Duboucher, C., F. Farto-Bensasson, M. Chéron, J.-Y. Peltier, F. Beaufils, and G. Périé. 2000. Lymph node infection by Trichomonas tenax: report of a case with coinfection by Mycobacterium tuberculosis. Hum. Pathol. 31:1317-1321. [DOI] [PubMed] [Google Scholar]
- 4.Duboucher, C., C. Noël, I. Durand-Joly, D. Gerbod, P. Delgado-Viscogliosi, S. Jouveshomme, C. Leclerc, G.-L. Cartolano, E. Dei-Cas, M. Capron, and E. Viscogliosi. 2003. Pulmonary co-infection by Trichomonas vaginalis and Pneumocystis sp. as a novel manifestation of AIDS. Hum. Pathol. 34:508-511. [DOI] [PubMed] [Google Scholar]
- 5.Duboucher, C., D. Gerbod, C. Noël, I. Durand-Joly, P. Delgado-Viscogliosi, C. Leclerc, S. Pham, M. Capron, E. Dei-Cas, and E. Viscogliosi. 2005. Frequency of trichomonads as coinfecting agents in Pneumocystis pneumonia. Acta Cytol. 49:273-277. [DOI] [PubMed] [Google Scholar]
- 6.Felleisen, R. S. J. 1997. Comparative sequences analysis of the 5.8 S rRNA genes and internal transcribed spacer (ITS) regions of trichomonad protozoa. Parasitology 115:111-119. [DOI] [PubMed] [Google Scholar]
- 7.Honigberg, B. M. 1990. Trichomonads found outside the urogenital tract of humans, p. 342-393. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, N.Y.
- 8.Jongwutiwes, S., U. Silachamroon, and C. Putaporntip. 2000. Pentatrichomonas hominis in empyema thoracis. Trans. R. Soc. Trop. Med. Hyg. 94:185-186. [DOI] [PubMed] [Google Scholar]
- 9.Kutisova, K., J. Kulda, I. Cepicka, J. Flegr, B. Koudela, J. Teras, and J. Tachezy. 2005. Tetratrichomonads from the oral cavity and respiratory tract of humans. Parasitology 131:1-11. [DOI] [PubMed] [Google Scholar]
- 10.Levy, M. G., J. L. Gookin, M. Poore, A. J. Birkenheuer, M. J. Dykstra, and R. W. Litaker. 2003. Tritrichomonas foetus and not Pentatrichomonas hominis is the etiologic agent of feline trichomonal diarrhea. J. Parasitol. 89:99-104. [DOI] [PubMed] [Google Scholar]
- 11.Lewis, K. L., D. E. Doherty, J. Ribes, J. P. Seabolt, and E. S. Bensadoun. 2003. Empyema caused by Trichomonas. Chest 123:291-292. [DOI] [PubMed] [Google Scholar]
- 12.Lun, Z.-R., X.-G. Chen, X.-Q. Zhu, X.-R. Li, and M.-Q. Xie. 2005. Are Tritrichomonas foetus and Tritrichomonas suis synonyms? Trends Parasitol. 21:122-125. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud, M. S. E., and G. A. Rahman. 2004. Pulmonary trichomoniasis: improved diagnosis by using polymerase chain reaction targeting Trichomonas tenax 18S rRNA gene in sputum specimens. J. Egypt. Soc. Parasitol. 34:197-211. [PubMed] [Google Scholar]
- 14.Mallat, H., I. Podglajen, V. Lavarde, J.-L. Mainardi, J. Frappier, and M. Cornet. 2004. Molecular characterization of Trichomonas tenax causing pulmonary infection. J. Clin. Microbiol. 42:3886-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaren, L. C., L. E. Davis, G. R. Haely, and C. G. James. 1983. Isolation of Trichomonas vaginalis from the respiratory tract of infants with respiratory diseases. Pediatrics 71:888-890. [PubMed] [Google Scholar]
- 16.Okamoto, S., M. Wakui, H. Kobayashi, N. Sato, A. Ishida, M. Tanabe, T. Takeuchi, S. Fukushima, T. Yamada, and Y. Ikeda. 1998. Trichomonas foetus meningoencephalitis after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 21:89-91. [DOI] [PubMed] [Google Scholar]
- 17.Soper, D. 2004. Trichomoniasis: under control or undercontrolled? Am. J. Obstet. Gynecol. 190:281-290. [DOI] [PubMed] [Google Scholar]
- 18.Szarka, K., P. Temesvari, A. Kerekes, A. Tege, and A. Repkeny. 2002. Neonatal pneumonia caused by Trichomonas vaginalis. Acta Microbiol. Immunol. Hung. 49:15-19. [DOI] [PubMed] [Google Scholar]
- 19.Tachezy, J., R. Tachezy, V. Hampl, M. Sedinova, S. Vanacova, M. Vrlik, M. Van Ranst, J. Flegr, and J. Kulda. 2002. Cattle pathogen Tritrichomonas foetus (Riedmuller, 1928) and pig commensal Tritrichomonas suis (Gruby & Delafond, 1843) belong to the same species. J. Eukaryot. Microbiol. 49:154-163. [DOI] [PubMed] [Google Scholar]