Abstract
Background
The rate of death from prostate cancer has recently declined in many areas of the world. Over the past 15 years prostate-specific antigen (PSA) screening has increased in popularity, which has resulted in increases in the incidence of prostate cancer. Over the same period there have been changes in the management of the disease and, in particular, the use of androgen ablation. We set out to examine the relation between changes in prostate cancer incidence (a surrogate for PSA screening) and subsequent changes in mortality in regions using common treatment recommendations.
Methods
We used data from prostate cancer cases and deaths reported to the British Columbia Cancer Registry during 1985–1999 to examine trends in incidence and mortality in 88 small health areas (SHAs) among men aged 50–74 years. We conducted 2 analyses. In the first we classified the SHAs by intensity of PSA screening (low, medium or high) according to their ranked age-standardized incidence rate of prostate cancer in 1990–1994 and examined subsequent trends in prostate cancer mortality. In the second analysis we examined the SHA-specific relative change in prostate cancer incidence between 1985–1989 and 1990–1994 and correlated it with the relative change in mortality for cases diagnosed after 1990.
Results
Between 1985–1989 and 1990–1994 the incidence of prostate cancer increased by 53.2% and 14.6% among men aged 50–74 and those 75 and over respectively. Between 1985–1989 and 1995–1999 prostate cancer mortality declined by 17.6% and 7.9% in the 2 age groups respectively. Among men aged 50–74 years SHAs with low, middle and high levels of screening had respective increases in prostate cancer incidence of 5.4%, 53.6% and 70.5% between 1985–1989 and 1990–1994. Corresponding decreases in mortality between 1985–1989 and 1995–1999 were 28.9%, 18.0% and 13.5%. Mortality declines were greatest in SHAs with low screening levels (p = 0.032). Before 1990 prostate cancer mortality was similar in the 3 screening groups (p = 0.72). Regions with the smallest increases in incidence had the largest declines in mortality.
Interpretation
We found no association between the intensity of PSA screening and subsequent decreases in prostate cancer mortality.
Screening for prostate cancer using prostate-specific antigen (PSA) remains controversial.1 Persuasive evidence exists that the PSA test can detect disease many years before symptoms will occur2 and that the rate of advanced disease has decreased among screened men.3 Autopsy studies show that occult prostate cancer is common,4 so the potential exists for diagnosis of clinically unimportant disease. Because complications associated with the treatment of prostate cancer by either radiotherapy5 or radical prostatectomy6 can be significant, the detection and treatment of clinically unimportant disease will cause harm. To date, the results of only 1 clinical trial of PSA screening have been reported, and they indicated a reduction in prostate cancer mortality.7 However, the analysis was not based on randomized assignment, and re-analysis according to group assignment showed no effect on mortality.8 In the absence of compelling evidence, differing guidelines on PSA screening have developed: some favour screening,9 some recommend providing information to patients so that they can make the decision,10 and some recommend against screening.11
Considerable evidence exists that PSA screening is being adopted, albeit unevenly, across Canada. A study in Ontario12 found that 63% of PSA tests in men without diagnosed prostate cancer were for screening. Analysis of prostate cancer incidence in Canada shows that the rate increased by about 60% between 1980–1985 and 1990–199513 and that this increase was, in part, due to PSA screening.14 Regions of the United States have displayed similar secular trends earlier, with increases exceeding 100%, and the pattern observed is compatible with a screening-related harvesting effect.15 Similar trends have been noted in other jurisdictions, and speculation has occurred about the impact of PSA screening on the decline in mortality.16 Studies of prostate cancer trends in different jurisdictions have provided data supporting17 and questioning18,19,20,21 a causal link.
The objective of our study was to examine what relation, if any, exists between regional declines in prostate cancer mortality in British Columbia and prior increases in prostate cancer incidence within those regions. Change in incidence was considered a surrogate for PSA screening. We considered incidence and mortality during 1985–1999 and focused on men aged 50–74 years (the group most frequently considered for screening).
Methods
We obtained data from the British Columbia Cancer Registry on all notifications of prostate cancer (International Classification of Diseases for Oncology, first edition22 [ICD-O-1] code C61 excluding sarcomas, leukemias, lymphomas and melanomas) during 1985–1999. Information abstracted included patient identification number, date of birth, date of diagnosis, histology code, postal code of residence at diagnosis, vital status and, if appropriate, date of death, cause of death and postal code at death. Demographic information was obtained from the Health Data Warehouse, British Columbia Ministry of Health, which provides inter-census estimates of population sizes and age and sex distribution for British Columbia and its regions. We gathered this information for small health areas (SHAs), the 88 regions used for the planning and delivery of educational and health care services.
Age-standardized incidence and mortality were calculated using 5-year age intervals and the 1991 Canadian population as standard. Prostate cancer notifications were assigned to SHAs according to the patient's postal code at diagnosis or death, depending on the analysis. Cases with missing postal codes were not included in the SHA-based analysis but were included in the province-wide calculations.
In the first analysis we classified SHAs by intensity of PSA screening (low, medium or high) according to their ranked age-standardized incidence rate of prostate cancer during 1990–1994 among men aged 50–74 years. We made statistical comparisons of rates by assuming the observed number of events followed a Poisson distribution23 with common relative risk for each of the 5-year age intervals. Tests of significance and estimation of parameters were performed using the method of maximum likelihood.
In the second analysis we examined prostate cancer mortality during 1995–1999 among men aged 50–74 in 1990–1994 by SHA of residence at diagnosis. SHA-specific incidence and mortality in 1985–1989 were included in the analysis to permit adjustment for pre-existing regional differences in these rates. We assumed fixed effects for the following covariates: SHA (separately for incidence and mortality), period (separately for incidence and mortality), age (separately for incidence and mortality by 5-year age groups) and an interaction term between SHA and period that was assumed to take the same value for both incidence and mortality components except for a multiplier, d. The d multiplier measures the strength of the relation between the relative change in incidence in an SHA (from 1985–1989 to 1990–1994) and the relative change in mortality (from 1985–1989 to 1995–1999) after controlling for overall age, and temporal and pre-existing regional differences in incidence and mortality. A negative value for d would indicate that PSA screening in 1990–1994 resulted in mortality declines in 1995–1999. A positive value for d would indicate that regions with the smallest increases in incidence (lowest PSA screening level) had the greatest declines in mortality and thus no beneficial effect of screening.
Results
Table 1 provides a breakdown of the data available for analysis as well as summary information on the data analyzed and the distribution of SHAs. In total, there were 32 745 cases of prostate cancer and 6592 deaths from the disease. We excluded 261 cases and 19 deaths involving men younger than 50. Of the remainder, 439 cases (1.4%) and 62 deaths (0.9%) had missing postal codes and thus were not included in the SHA-based analysis.
Table 1
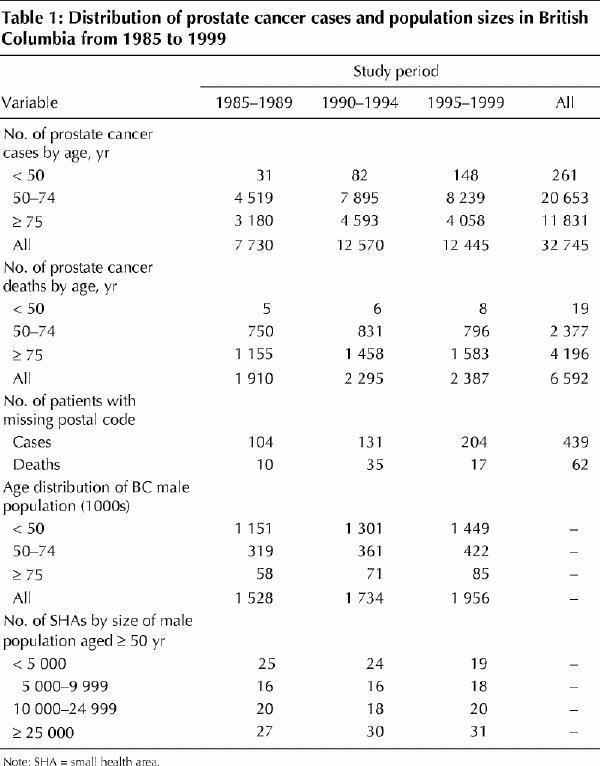
The relative values of age-standardized incidence and mortality from 1985 to 1999 (with 1985 = 1.0) are presented in Fig. 1 for the age groups 50–74 and 75 and over. During 1990–1994 the incidence of prostate cancer in the 50–74 age group increased more rapidly than during 1985–1989 (peaking in 1993) and then declined, but still remained at levels exceeding those of 1985. In the group aged 75 and over, the incidence in 1995–1999 was slightly lower than in 1985. Between 1985–1989 and 1990–1994 the incidence rate increased by 53.2% among men aged 50–74 and by 14.6% among those aged 75 and over. Conversely, mortality was quite stable throughout 1985–1989 and 1990–1994 for both age groups, but then declined in 1995–1999. Between 1985–1989 and 1995–1999 the mortality rate declined by 17.6% and 7.9% among men aged 50–74 and those aged 75 and over respectively.
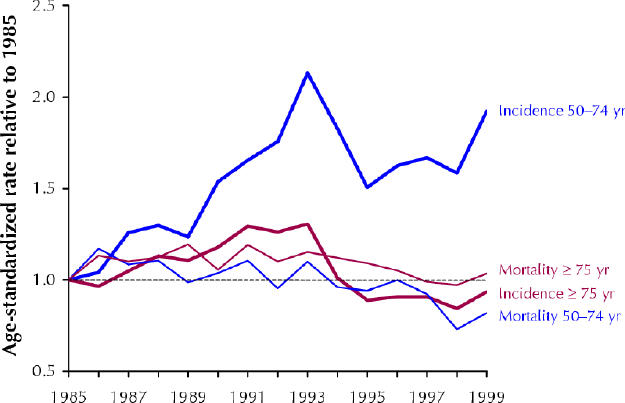
Fig. 1: Relative change (1985 = 1.0) in age-standardized incidence and mortality of prostate cancer in British Columbia from 1985 to 1999 among men aged 50–74 and among those 75 and older.
Changes in the incidence and mortality of prostate cancer for the 50–74 age group varied regionally (Fig. 2). The age-standardized incidence rates for the SHAs according to intensity of PSA screening are presented in Table 2 for the 3 study periods. The differences in incidence rates between the low-, medium- and high-level screening groups in each period were statistically significant (p < 0.001 for each period). The change in incidence rates between 1985–1989 and 1990–1994 for each screening group had the following p values: p = 0.14 (low screening level), p < 0.001 (medium) and p < 0.001 (high). Table 2 also provides the age-standardized mortality for the 3 screening groups in the same study periods, where location was determined by the postal code on the death certificates. The overall decline in mortality between 1985–1989 and 1995–1999 was statistically significant (p < 0.001). The differences in mortality between the screening groups in each period had the following p values: p = 0.72 (1985–1989), p = 0.50 (1990–1994) and p = 0.04 (1995–1999). The change in mortality between 1985–1989 and 1995–1999 for each screening group had the following p values: p < 0.001 (low screening level), p = 0.011 (medium) and p = 0.07 (high).
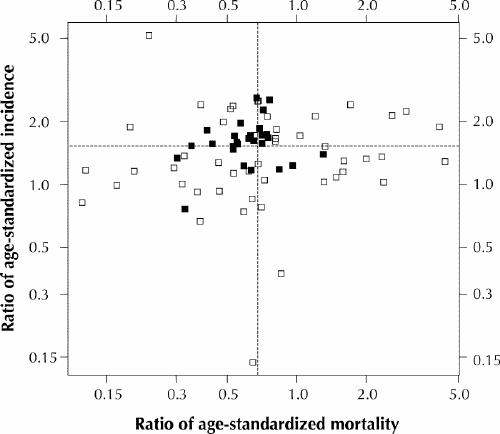
Fig. 2: Ratio of age-standardized incidence (rate in 1990–1994 divided by rate in 1985–1989) plotted against the ratio of age-standardized mortality (rate in 1995–1999 divided by rate in 1985–1989) among men aged 50–74 years in the small health regions (SHAs) in British Columbia. (Values are shown for the 75 SHAs whose values could be plotted on the logarithmic scales.) SHAs with 10 or more deaths from prostate cancer in 1985–1989 are represented by black sqaures; all other SHAs are represented by white squares. The dotted lines indicate provincial averages.
Table 2
The second analysis, in which we used Poisson regression to examine the relation between relative change in incidence and relative change in mortality, resulted in an estimated d value of 0.68 (95% confidence interval [CI] 0.15 to 1.21). This finding indicates a positive relation between increased screening use and smaller-than-average reductions in mortality. Restricting the same analysis to SHAs in which there were 10 or more deaths during 1985–1989 altered the estimated value of d to 0.48 (95% CI –0.06 to 1.02).
Interpretation
We found that the incidence of prostate cancer in British Columbia changed over the study period in line with changes seen elsewhere in North America. We also found that these changes were not uniform, with some SHAs showing large increases in incidence and others showing little change. The differences were statistically significant, which indicated that the observed changes were more than could be attributed to chance given the overall trends seen in the population. These findings are compatible with the detection of preclinical prostate cancer through PSA screening. We also saw a decline in mortality in the last period of our study, again in line with changes observed in other jurisdictions. We found no evidence that the increase in incidence was related to a decline in mortality, as would be expected if screening was a major cause of both effects. This study confirms the finding of other population-based studies that there is no evidence of a relation between community PSA screening and the observed decline in prostate cancer mortality.18,19,20,21 In fact, we found a significant link between relative increases in incidence and lesser declines in mortality (since d was greater than 0), as has been reported elsewhere.21 In non-screening situations, one would expect increases in incidence to be accompanied by subsequent increases in mortality, so such a relation is to be expected in the absence of a beneficial screening effect.
There are several possible reasons why we found no beneficial effect of PSA screening on prostate cancer mortality. First, we had no data on utilization of PSA screening and instead used change in prostate cancer incidence as a surrogate measure. Historical trends in prostate cancer among men aged 50–74 in British Columbia indicate that incidence rates would be expected to increase by about 18% over 5 years; however, a 53% increase was observed between 1985–1989 and 1990–1994. Screening with modalities whose objective is the detection of occult cancer, such as the PSA test, do bring about changes in cancer incidence and these are proportional to the amount of screening.24 Given the magnitude of changes in prostate cancer incidence, the consistency with observations in other jurisdictions and their relation to PSA use, and the anecdotal evidence of awareness of PSA testing in the public and the medical profession in British Columbia, it seems reasonable to conclude that observed changes in incidence in the province are largely attributable to PSA use.
Second, although the differences in incidence among SHAs were statistically significant in 1990–1994, the magnitude of these differences may have been small compared with random variations among regions because of their small size. However, when we repeated the Poisson regression analysis using only larger regions (SHAs with ≥ 10 deaths) we found that the parameter d did not change significantly and remained incompatible with a beneficial screening effect. If differences in observed rates among SHAs were purely random and not due to any systematic differences in screening intensity, then we would expect the incidence in 1990–1994 to be 29% higher in the high-level screening group than in the low-level screening group. However, the observed rate was 79% higher, which indicated that about 50% of the observed difference was likely due to PSA screening. For comparison, data from a trial of colorectal cancer screening by fecal occult blood tests25 showed that after 5 years the incidence rate was about 30% higher in the screened group (annual compliance rate of 50%–70%) than in the control group. Although it is difficult to compare different screening modalities and diseases because of different sojourn times (the time in the screen-detectable preclinical disease state), it does appear that there were real differences in PSA screening activity between the SHAs. Comparing groups in 1995–1999, we found that the mortality in the high-level screening group was 31% greater than the rate in the low-level screening group (Table 2), a difference similar in magnitude to the 29% difference expected from the manner in which the groups were selected.
Third, death from prostate cancer frequently occurs many years after diagnosis, and consequently the magnitude of the death rate depends on the number of cases diagnosed many years previously. When analyzing the effect of PSA screening, which became prevalent in the early 1990s, it is important to exclude deaths from cancer diagnosed before this time. Of the 2387 deaths from prostate cancer in 1995–1999, 558 (23%) involved men whose cancer was diagnosed before 1990. However, exclusion of these deaths in the regression analysis did not result in any qualitative difference in findings from those using overall incidence and mortality (i.e., no evidence of a beneficial relation).
Fourth, treatment may be confounded with screening, and it is possible that patients with screen-detected disease are not treated as adequately as those who present with symptoms. The British Columbia Cancer Agency has published guidelines for the management of cancer for over 25 years. Previous studies have shown that there is a higher consistency in the use of systemic and radiotherapeutic approaches to cancer management in British Columbia than in some other parts of Canada.26 This consistency is attributable both to the existence of these guidelines and to the centralized nature of much cancer management in the province. Therefore, we believe it unlikely that variation in patient treatment confounded our findings.
Finally, the elapsed time between the changes in prostate cancer incidence (and PSA screening) may be insufficient to result in measurable changes in prostate cancer mortality. Changes in mortality in screening trials do not occur until several years after the initiation of screening. The magnitude of the time lag between the initiation of screening and the creation of a mortality effect depends on the way in which the screening modality works and the distribution of the extent of disease in the population. Screening methods that rapidly reduce a large burden of metastatic disease will have the most immediate effects on mortality.
In conclusion, we believe that, given the evidence presented here and general considerations about the way cancer screening works, it is unlikely that the reductions seen in prostate cancer mortality in British Columbia in the late 1990s were due to PSA screening.
Acknowledgments
We thank Drs. Nhu Le, John Spinelli and Richard Gallagher for their comments on an earlier draft of the manuscript.
Footnotes
This article has been peer reviewed.
Contributors: All authors contributed to the design of the study and to the development of the analytical plan. Mr. Phillips extracted the data and performed the statistical analyses. All authors contributed to the preparation of the manuscript and approved the final version.
Competing interests: None declared for Dr. Coldman and Mr. Phillips. Dr. Pickles received speaker fees from Abbott Laboratories Ltd., Eli Lilly Canada Inc. and AstraZeneca Canada Inc. for lectures on the management of prostate cancer.
Correspondence to: Dr. Andrew J. Coldman, British Columbia Cancer Agency, 800–686 W Broadway, Vancouver BC V5Z 1G1; fax 604 660-3645; acoldman@bccancer.bc.ca
References
- 1.Iscoe NA. Prostate cancer screening: waiting for Godot. CMAJ 1998;159 (11): 1375-7. [PMC free article] [PubMed]
- 2.Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995; 273: 289-94. [PubMed]
- 3.Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA 1993;270:948-54. [PubMed]
- 4.Yatani K, Chigusa I, Akazaki K, Stemmermann GN, Welsh RH, Correa P. Geographic pathology of latent prostatic carcinoma. Int J Cancer 1982;29:611-6. [DOI] [PubMed]
- 5.Wasson JH, Cushman CC, Buskewitz RC, Littenberg B, Mulley AG, Wennberg JE. A structured literature review of treatment for localized prostate cancer. Prostate Disease Patient Outcome Research Team. Arch Fam Med 1993;2:487-93. [DOI] [PubMed]
- 6.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Leach GE, et al. Quality of life outcomes in men treated for localized prostate cancer. JAMA 1995; 273: 129-35. [DOI] [PubMed]
- 7.Labrie F, Candas B, Dupont A, Cusan L, Gomez JL, Suburu RE, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized control trial. Prostate 1999;38:83-91. [DOI] [PubMed]
- 8.Boer R, Schroeder FH. Quebec randomized trial on prostate cancer screening shows no evidence for mortality reduction. Prostate 1999;40:130-1. [DOI] [PubMed]
- 9.Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal and endometrial cancers. CA Cancer J Clin 2001;51:38-75. [DOI] [PubMed]
- 10.Donovan JL, Frankel SJ, Neal DE, Hamdy FC. Screening for prostate cancer in the UK. BMJ 2001;323:763-4. [DOI] [PMC free article] [PubMed]
- 11.Green CJ, Hadorn D, Bassett K, Kazanjian A. Prostate specific antigen in the early detection of prostate cancer. Vancouver: Centre for Health Services and Policy Research; 1993.
- 12.Bunting PS, Goel V, Williams JI, Iscoe NA. Prostate specific antigen testing in Ontario: reasons for testing patients without diagnosed prostate cancer. CMAJ 1999;160(1):70-5. [PMC free article] [PubMed]
- 13.National Cancer Institute of Canada. Canadian cancer statistics 2001. Toronto: The Institute; 2001.
- 14.Levy IG, Iscoe NA, Klotz LH. Prostate cancer: 1. The descriptive epidemiology in Canada. CMAJ 1998;159(5):509-13. [PMC free article] [PubMed]
- 15.Stephenson RA, Smart CR, Mineau GP, James BC, Janerich DT, Dibble RL. The fall in incidence of prostate carcinoma. Cancer 1995;77:1342-8. [DOI] [PubMed]
- 16.Mettlin CJ, Murphy GP. Why is prostate cancer death rate declining in the United States? Cancer 1998;82:249-51. [DOI] [PubMed]
- 17.Bartsch G, Horninger W, Klocker H, Reissigl A, Oberaigner W, Schonitzer D, et al. Prostate cancer mortality after introduction of prostate specific antigen mass screening in the federal state of Tyrol, Austria. Urology 2001;58(3):417-24. [DOI] [PubMed]
- 18.Crocetti E, Ciatto S, Zappa M. Prostate cancer: different incidence but not mortality trends within two areas of Tuscany, Italy. J Natl Cancer Inst 2001; 93: 876-7. [DOI] [PubMed]
- 19.Etzioni R, Legler JM, Feuer EJ, Merrill RM, Cronin KA, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer — part III: quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J Natl Cancer Inst 1999;91:1033-9. [DOI] [PubMed]
- 20.Oliver SE, Gunell D, Donovan JL. Comparison of trends in prostate cancer mortality in England and Wales and the USA. Lancet 2000;355:1788-9. [DOI] [PubMed]
- 21.Perron L, Moore L, Bairati I, Bernard PM, Meyer F. PSA screening and prostate cancer mortality. CMAJ 2002;166(5):586-91. [PMC free article] [PubMed]
- 22.International classification of diseases for oncology. 1st ed. Geneva: World Health Organization; 1976.
- 23.McCullagh P, Nelder JA. Generalized linear models. 2nd ed. London: Chapman and Hall; 1989. p. 194.
- 24.Fueur EJ, Wun LM. How much of the recent rise in breast cancer incidence can be explained by increases in mammography utilization? A dynamic population model approach. Am J Epidemiol 1992;136:1423-36. [DOI] [PubMed]
- 25.Mandel JS, Bond JH, Church TR, Shaver DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993;328:1365-71. [DOI] [PubMed]
- 26.Sawka C, Olivotto I, Coldman A, Goel V, Holowaty E, Hislop TG. The association between population-based treatment guidelines and adjuvant therapy for node-negative breast cancer. British Columbia/Ontario Working Group. Br J Cancer 1997;75(10):1534-42. [DOI] [PMC free article] [PubMed]



