Abstract
We report the first case of post-kala-azar dermal leishmaniasis due to Leishmania infantum in a human immunodeficiency virus type 1-infected patient in Australia. Molecular characterization of the isolate was performed using PCR restriction fragment length polymorphism targeting both repetitive sequences from Leishmania nuclear DNA and repetitive kinetoplast DNA minicircles for species differentiation.
CASE REPORT
A 45-year-old human immunodeficiency virus type 1 (HIV-1)-infected male was diagnosed with visceral leishmaniasis (VL) in 1996 after presenting with gastrointestinal symptoms. He probably acquired the disease as a child while living in Greece, and thus the presentation in 1996 represented reactivation of VL in the presence of HIV-related immunodeficiency. The Leishmania species was never formally identified at the first diagnosis; it was assumed to be L. infantum, having been acquired in the Mediterranean basin. Starting in 1996, he underwent several courses of induction and maintenance for VL with various agents, including pentavalent antimony, liposomal amphotericin B, and miltefosine, and was treated with highly active antiretroviral therapy continuously during this time. In 2003, he developed five nodular lesions on the head and neck and later in the pinna of one ear during induction and maintenance therapy for VL with liposomal amphotericin B. The development of these nodular skin lesions on two separate occasions correlated with clinical and immunological improvement, i.e., weight gain, defervescence, significant decrease in spleen size, and doubling of the CD4+ T-cell count from 176 to 374 cells/μl. The lesions subsequently resolved with continuation of maintenance therapy for VL and topical paromomycin for 1 month. These localized skin lesions on a background of improved visceral disease and rising CD4+ T cell levels are suggestive of para-/post-kala-azar dermal leishmaniasis (PKDL), and, in the setting of HIV infection, probably represent a form of immune restoration disease (5).
Skin biopsy specimens from the localized skin lesions on the neck and ear were sent to the Microbiology Department of St. Vincent's Hospital for staining, culture, and molecular analysis. Impression smears from the biopsy specium stained with a Leishman's stain showed numerous Leishmania amastigotes. The biopsy specium was also inoculated into Novy, Nicole, and MacNeal's (NNN) medium in duplicate. Both NNN culture tubes grew Leishmania promastigotes within 3 weeks.
DNA was extracted on a section of tissue by using the Roche High Pure PCR template preparation kit (Roche) according to manufacturer's instructions. Leishmania infection was confirmed by PCR using primers specific for the small-subunit rRNA gene of Leishmania as previously described (17). For determining the species of the Leishmania isolates, PCR restriction fragment length polymorphism (RFLP) targeting both repetitive sequences from Leishmania nuclear DNA and repetitive kinetoplast DNA minicircles were performed as described previously (11, 12). Both techniques gave banding patterns consistent with L. infantum.
Discussion.
Leishmania is an obligate intramacrophage parasite transmitted by phlebotomine female sand flies (Phlebotomus and Lutzomyia spp.), with more than 25 species capable of producing disease in humans and animal reservoirs (1). Leishmania infection can give rise to a spectrum of diseases depending on the causative Leishmania species, the virulence factors of the parasite, and the immune status of the host (7).
Leishmaniasis is distributed throughout at least 88 countries, 21 in the New World and 67 in the Old World (1). Leishmaniasis is a major parasitic disease with approximately 1.5 million new cases documented each year and over 350 million individuals living in areas of active parasite transmission (7).
In Mediterranean countries, leishmaniasis is hypoendemic, and the majority of cases of Leishmania-HIV coinfection are found in southern Europe. The entire clinical spectrum of leishmaniasis has been described in HIV-positive patients. The most frequent form is VL, in which the parasite targets the reticuloendothelial system. Cutaneous, mucocutaneous, and diffuse cutaneous leishmaniasis has also been described. In Mediterranean areas where L. infantum causes both cutaneous leishmaniasis and VL, cutaneous forms can appear occasionally, can precede a visceral form by some months, and can be concomitant with a visceral form or even appear after a previously treated visceral form (1).
Several studies have shown that even after effective treatment for leishmaniasis the parasite can remain quiescent in various organs, and after induction of immunosuppression in the host, the parasite can be reactivated (1, 5, 6, 7). This explains the recrudescence of infection in immunosuppressed patients. HIV-infected patients often have frequent recurrences of leishmaniasis, most likely due to reactivation (1).
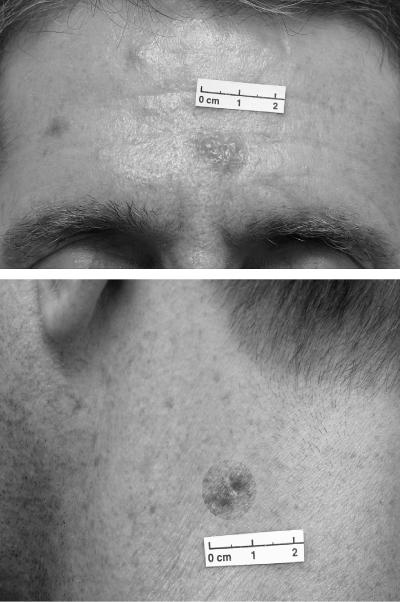
Post-kala-azar dermal leishmaniasis is a form of cutaneous leishmaniasis characterized by maculopapular or nodule lesions on the face, limbs, or trunk, which generally appear after apparently successful treatment of VL (Fig. 1). In spite of the fact that there are many reports about the association between VL and HIV infection, PKDL is rare in HIV-infected patients, with only a few cases reported in the literature (2, 6, 13, 14). PKDL is frequently associated with L. donovani, while the association of PKDL with L. infantum is controversial, with only two cases reported in the literature. Dereure et al. (4) were the first to identify an L. infatum strain from a PKDL case using starch gel electrophoresis and isoelectrofocusing. The only additional report is a case of PKDL caused by L. infantum that was confirmed by molecular testing in a woman with AIDS which occurred 13 months after a diagnosis of visceral leishmaniasis concomitantly with immunological recovery induced by highly active retroviral therapy (13).
FIG. 1.
Characteristic nodular lesions.
This patient presented with typical PKDL symptoms characterized by nodular lesions on the head, neck, and ear. Histology of tissue biopsies taken from the patient showed numerous amastigotes present in macrophages. Biopsy cultures in NNN medium grew Leishmania promastigotes. Various PCR-based technologies have been developed for species differentiation (11, 12, 17). The two molecular methods used to identify the Leishmania species using RFLP generated species-specific bands visualized in agarose gels, which allowed them to differentiate the isolate unequivocally. Both assays targeting different loci, repetitive kinetoplast DNA minicircles and repetitive genomic DNA (11, 12), gave concurrent results. With the advent of such molecular testing, Leishmania species causing PKDL can now be readily identified to the species level.
Australia and Oceania have long been considered free of endemic Leishmania species (7) and free of any suitable sandfly vectors, thus preventing locally acquired leishmaniasis. However imported cases of both cutaneous and visceral leishmaniasis have been reported in Australia in humans (3, 8, 9, 10, 16, 18). Recently, cutaneous leishmaniasis in kangaroos, where infection was acquired locally within Australia, was reported. Molecular analysis targeting the rRNA internal transcribed spacer 1, the DNA and RNA polymerase gene, and the miniexon gene locus confirmed the identity to the genus Leishmania but were not able to identify the species unequivocally (15). This suggests that the Leishmania parasite identified represents a novel species. What implication this has as a potential cause of human infection is unknown.
This case report is only the third time L. infantum, confirmed by molecular testing, has been implicated as a cause of PKDL and one of only a few PKDL cases in the setting of HIV infection.
REFERENCES
- 1.Alvar, J., C. Canavate, B. Guitierrez-Solar, M. Jimenez, F. Laguna, R. Lopez-Velez, R. Molina, and J. Moreno. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bittencourt, A., N. Silva, A. Straatmann, V. L. Nunes, I. Follador, and R. Badaro. 2003. Post-kala-azar dermal leishmaniasis associated with AIDS. Braz. J. Infect. Dis. 7:229-233. [DOI] [PubMed] [Google Scholar]
- 3.Charters, A. D., and P. A. Staer. 1970. Cutaneous leishmaniasis of long incubation period in an Italian immigrant in Western Australia. Med. J. Aust. 8:278-279. [PubMed] [Google Scholar]
- 4.Dereure, J., S. H. El-Safi, B. Bucheton, M. Boni, M. M. Kheir, B. Davoust, F. Pratlong, E. Feugier, M. Lambert, A. Dessein, and J. P. Dedet. 2003. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 5:1103-1108. [DOI] [PubMed] [Google Scholar]
- 5.French, M. A., P. Price, and S. F. Stone. 2004. Immune restoration disease after antiretroviral therapy. AIDS 18:1615-1627. [DOI] [PubMed] [Google Scholar]
- 6.Gilad, J., A. Borer, D. Hallel-Halevy, K. Reisenberg, M. Alkan, and F. Schlaeffer. 2001. Post-kala-azar dermal leishmaniasis manifesting after initiation of highly active anti-retroviral therapy in a patient with human immunodeficiency virus infection. Isr. Med. Assoc. J. 3:451-452. [PubMed] [Google Scholar]
- 7.Herwaldt, B. L. 1999. Leishmaniasis. Lancet 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 8.Jones, H. I. 1979. Cutaneous leishmaniasis in Western Australia. Med. J. Aust. 2:495-496. [PubMed] [Google Scholar]
- 9.Ju, O., D. I. Grove, W. J. Jaksic, and G. W. Dart. 2004. Visceral leishmaniasis: a trip to the Greek Islands is not always idyllic. Med. J. Aust. 181:446-447. [DOI] [PubMed] [Google Scholar]
- 10.Maguire, G. P., I. Bastian, S. Arianayagam, A. Bryceson, and B. J. Currie. 1998. New World cutaneous leishmaniasis imported into Australia. Pathology 30:73-76. [DOI] [PubMed] [Google Scholar]
- 11.Marfurt, J., I. Niederwieser, N. D. Makia, H. P. Beck, and I. Felger. 2003. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn. Microbiol. Infect. Dis. 46:115-124. [DOI] [PubMed] [Google Scholar]
- 12.Minodier, P., R. Piarroux, F. Gambarelli, C. Joblet, and H. Dumon. 1997. Rapid identification of causative species in patients with Old World leishmaniasis. J. Clin. Microbiol. 35:2551-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridolfo, A. L., C. Gervasoni, S. Antinori, M. Pizzuto, S. Santambrogio, D. Trabattoni, M. Clerici, and M. Galli. 2000. Post-kala-azar dermal leishmaniasis during highly active antiretroviral therapy in an AIDS patient infected with Leishmania infantum. J. Infect. 40:199-202. [DOI] [PubMed] [Google Scholar]
- 14.Rios-Buceta, L., G. F. Buezo, P. F. Penas, E. Dauden-Tello, M. Aragues-Montanes, J. Fraga-Fernandez, and A. Garcia-Diez. 1996. Post-kala-azar dermal leishmaniasis in an HIV-patient. Int. J. Dermatol. 35:303-304. [DOI] [PubMed] [Google Scholar]
- 15.Rose, K., J. Curtis, T. Baldwin, A. Mathis, B. Kumar, A. Sakthianandeswaren, T. Spruck, J. Low Choy, and E. Handman. 2004. Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms. Int. J. Parasitol. 34:655-664. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson, K. V. 1961. Cutaneous leishmaniasis in South Australia. Med. J. Aust. 48:193-195. [DOI] [PubMed] [Google Scholar]
- 17.Schonian, G., A. Nasereddin, N. Dinse, C. Schweynoch, H. D. Schallig, W. Presber, and C. L. Jaffe. 2003. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn. Microbiol. Infect. Dis. 47:349-358. [DOI] [PubMed] [Google Scholar]
- 18.Storer, E., and J. Wayte. 2005. Cutaneous leishmaniasis in Afghani refugees. Australas. J. Dermatol. 46:80-83. [DOI] [PubMed] [Google Scholar]