Abstract
Several species of rhizobia were successfully transformed with broad-host-range plasmids of different replicons by using a modified freeze-thaw method. A generic binary vector (pPZP211) was maintained in Mesorhizobium loti without selection and stably inherited during nodulation. The method could extend the potential of rhizobia as a vehicle for plant transformation.
The symbiosis between legumes and the root nodule bacteria collectively known as rhizobia is of critical agronomic and environmental importance, accounting for a significant proportion of the nitrogen available to leguminous plants. Rhizobia are phylogenetically diverse; currently they are classified into five genera of α-proteobacteria (Rhizobium, Bradyrhizobium, Sinorhizobium, Azorhizobium, and Mesorhizobium) (26) and at least two genera of β-proteobacteria (Burkholderia and Ralstonia) (20). Although Agrobacterium is closely related to Rhizobium, the suggestion that Agrobacterium tumefaciens be reclassified as Rhizobium radiobacter has been disputed (28). However, it is not disputed that Agrobacterium, Bradyrhizobium, Sinorhizobium, Azorhizobium, and Mesorhizobium are phylogenetically distinct and differ in genomic organization (7, 27).
Until recently, Agrobacterium was widely considered to be the only bacterial genus capable of transferring genes into the genomes of plants. Broothaerts et al. (3) have shown that other plant-associated bacteria, including Sinorhizobium, Rhizobium, and Mesorhizobium, can be modified to mediate gene transfer into tobacco and Arabidopsis plants and, in the case of Sinorhizobium meliloti, into rice plants. These authors suggested that rhizobia could become a new resource for crop improvement and therefore an alternative to the patented Agrobacterium-mediated technology (3).
An important prerequisite for the use of rhizobia as a vehicle for gene transfer into plants is the availability of an efficient and easy transformation system for these bacteria. Early reports that Rhizobium meliloti could be transformed by using either conventional chemical transformation (25) or thermal shock (5) have not been adopted, and the introduction of foreign DNA into rhizobia is still conducted via conjugal mating with Escherichia coli (6) or by electroporation (8, 10, 12, 13). The conjugal transfer method, however, is time-consuming and confined to strains harboring plasmids that carry the mob gene, and while efficient transformation via electroporation is possible for a range of rhizobial species, it requires special equipment.
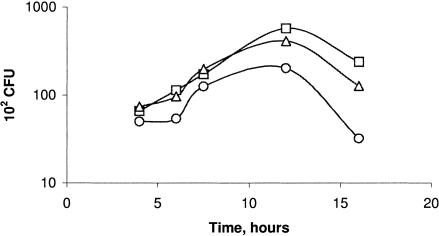
The close genetic relationship between Agrobacterium and rhizobia led us to test the widely used freeze-thaw method for Agrobacterium transformation (16) on rhizobia. Given the slower growth rate of rhizobia, a time course analysis was undertaken to identify the optimal incubation time for the preparation of competent Mesorhizobium loti cells. Efficiency of transformation with the binary vector pPZP211 (11) was monitored at intervals of 4, 6, 7.5, 12, and 16 h and at three plasmid concentrations (0.3, 0.9, and 1.5 μg/ml). The pPZP211 vector was chosen because it is a vector routinely used in our laboratory in Agrobacterium-mediated plant transformation experiments and has a replication origin (pVS1) similar to those of a wide range of other vectors currently used (Gateway vectors; Invitrogen). The results showed that the transformation efficiency reached a maximum after 12 h at all three plasmid concentrations. Figure 1 shows that sufficient numbers were obtained, even with the lowest concentration of 0.3 μg/ml after 6 h, although an increased plasmid concentration resulted in more transformants. Given this, the standard conditions adopted for preparing competent cells was a 6-h growth period and 1 μg of vector DNA for transformation. Since all cloning steps would be conducted with E. coli and the objective was to introduce plasmid DNA into the rhizobia, further optimization of the transformation protocol described here was considered unnecessary.
FIG. 1.
Efficiency of transformation of M. loti with pPZP211: effect of the DNA concentration and the length of the growth period on competent-cell preparation. Symbols: circles, M. loti cells transformed with 0.3-μg/ml DNA; squares, M. loti cells transformed with 0.9-μg/ml DNA; triangles, M. loti cells transformed with 1.5-μg/ml DNA. Note the logarithmic scale on the y axis.
The basic protocol for the preparation of competent rhizobial cells was as follows. Three milliliters of medium was inoculated with a rhizobial strain (the media and strains used in this study are listed in Table 1) and grown at 28°C with vigorous shaking until they reached the stationary growth phase. Two milliliters from the stationary-phase cultures was used to inoculate 50 ml of appropriate medium and incubated with shaking for 6 h at 28°C. Cells were harvested by centrifugation at 12,000 × g for 10 min at 4°C, and the pellet was resuspended in 2 ml of ice-cold 20 mM CaCl2 solution. The resulting cell suspension was placed into ice-cold 1.5-ml polypropylene microcentrifuge tubes in aliquots of 100 μl before snap-freezing in liquid N2. The prepared competent cells were kept at −80°C and were stable for at least 6 months.
TABLE 1.
Bacterial strains used in this study
To test the transformation efficiency of the competent rhizobial cells, approximately 1 μg of binary vector plasmid pPZP211 DNA (11) was made up to a volume of 5 μl with sterile distilled water. This was then added to a 100-μl aliquot of competent cells immediately after they were removed from −80°C. Subsequently, the mixture was kept at 37°C for 5 min without shaking. For the recovery phase, 1 ml of the appropriate medium was added to the transformed cells before they were transferred to 10-ml tubes and incubated at 28°C for 2 h with shaking. To determine the actual transformation efficiency, the cell suspension was diluted and plated on nonselective agar medium to count the cells. Cells without added DNA and the appropriately diluted transformation mixture were plated on selective medium to calculate the number of spontaneous resistant colonies and transformation efficiency, respectively.
A 6-h growth period for competent-cell preparation was sufficient to produce transformants of all fast-growing species of Mesorhizobium and Sinorhizobium studied. However, Bradyrhizobium lupini, a slow grower, needed at least a 24-h growth period (Table 2). Enough transformants were obtained under the conditions described above to demonstrate the method's general usefulness, even though the growth rates of the rhizobial species tested were widely different.
TABLE 2.
Transformation efficiencies of different rhizobial strains determined by using competent cells isolated after 6 h of growth
| Strains | Spectinomycin resistance (μg/ml) | OD600a after 6 h of cultivation | Transformation efficiency (103 CFU/μg of pPZP211 DNA) |
|---|---|---|---|
| M. loti 2037 | 200 | 0.269 | 8.4 |
| S. meliloti 1021 | 250 | 0.212 | 0.06 |
| S. meliloti 2011 | 250 | 0.569 | 11.2 |
| S. fredii 205 | 100 | 0.165 | 0.01 |
| B. lupini 2257 | 300 | 0.168b | 0.02b |
OD600, optical density at 600 nm.
B. lupini cells were grown for 24 h for competent-cell isolation.
The transformants of M. loti and S. meliloti 2011 were checked for the presence of the introduced pPZP211 plasmid. Six colonies from the different transformation experiments were selected and grown for 48 h in the presence of appropriate selection. Plasmid DNA was isolated from the M. loti and S. meliloti transformants by the alkaline extraction method (2). The isolated pPZP211 plasmid was the same size as the introduced pPZP211 DNA, but the DNA yield was too poor for restriction pattern analysis. Therefore, the plasmids isolated from the rhizobial strains were transformed into E. coli DH5α, whereupon the reisolated plasmid restriction pattern confirmed that the transformants indeed carried the pPZP211 plasmid (data not shown).
Having established the protocol, we assessed three binary vectors (pPZP211, pART27, and pSoup; Table 3) for their respective efficiencies of transformation of competent M. loti cells. These vectors were chosen as they represent the spectrum of replication origins commonly used in Agrobacterium-mediated plant transformation experiments. Transformants were obtained with all three vectors by using 1 μg of vector DNA (Table 3), and the presence of plasmids in M. loti was confirmed by the method described above. Transformation efficiency was high enough to obtain the desired M. loti derivatives, even with pSoup, which is a very low-copy-number plasmid (14).
TABLE 3.
Transformation efficiency of M. loti with different Agrobacterium vectors
Two M. loti colonies harboring pPZP211 were further tested for plasmid stability. The colonies were grown for 3 days in selective medium at 28°C with shaking. From this initial culture, 50 μl was inoculated into 5 ml of liquid YEB (1,000-fold dilution without selection); this was done in triplicate. Successive subcultures were established in the same way every 32 h. This procedure was repeated over a period of 13.5 day, which corresponded to approximately 90 generations. After 0, 30, 60, and 90 generations, samples were taken and dilutions were plated on solid YEB medium. One hundred colonies from each time point were tested for spectinomycin resistance. The transformants generated with pPZP211 were highly stable; we did not observe any loss of spectinomycin resistance, even when cells were grown without selection.
Transformants of M. loti carrying the pPZP211 plasmid were tested for the ability to form nodules. Nodulation tests were performed with Lupinus angustifolius L. cv. Uniharvest (22). The presence of the binary vector did not prevent M. loti from forming effective nodules on L. angustifolius plants. To confirm the stability of the plasmid in the bacteria during nodulation, bacteria and bacteroids were isolated from three randomly selected nodules per plant (three plants per treatment) 4 weeks after inoculation and characterized. The bacteria were isolated on nonselective (YEB) medium from the surface-sterilized nodules. The colonies were tested for Specr, and the restriction pattern of the purified plasmid was analyzed. We found no loss of antibiotic resistance, and the transformant isolated from the nodules harbored the pPZP211 vector. Taken together, these lines of evidence indicated that the vector was stably inherited by the bacteria during nodulation.
Our aim was to develop a simple, rapid method to facilitate the introduction of binary vectors into rhizobia, thus helping to extend the use of rhizobia for crop improvement. We tested rhizobial species with different growth rates (Table 1) and found that the transformation method described here was suitable for all of the strains tested. The sizes of the plasmids used ranged between 9.3 and 10.9 kb and showed no correlation with transformation frequencies (Table 3). Electroporation experiments with Azotobacter vinelandii with plasmid sizes between 4.8 and 24 kb gave similar results (18). The plasmids used in our experiments had low or medium copy numbers (9, 11, 14); such plasmids, especially derivatives with an RK2 replication origin, could be very useful for cloning and expressing toxic gene products in bacterial systems due to their reduced, leaky expression (1). The vectors used belong to different compatibility groups and target independent cellular locations in E. coli (15, 21). It is conceivable that the same would occur in rhizobia. These indirect features of the transformation system might allow a greater range of genetic manipulations of rhizobia. For example, multiple plasmids bearing different genes could be assembled in one strain.
Host-specific restriction-modification is also an important factor determining transformation efficiency. We used DNA isolated from E. coli in our transformation experiments because manipulation of plasmid DNA is routine in this species. Although plasmid DNA isolated from E. coli yields fewer transformants compared to plasmid DNA isolated from the same species in the cases of Mesorhizobium huakuii (13) and Bradyrhizobium japonicum (10), transformation efficiencies are not related to the methylation state of plasmid DNAs isolated from different E. coli strains (13).
Further optimization of the protocol could be achieved by altering the drug selection prior to plating (4).
The protocol presented here provides the opportunity to create rhizobial transformants rapidly and simply. These transformants carry binary vectors, which can be engineered to carry genes of interest, offering the opportunity to modulate the rhizobium-plant interaction in a novel way. Rhizobia engineered with T-DNAs and a disarmed Ti plasmid could potentially transfer the T-DNA to the host cell during the infection-establishment process. These genes are transiently expressed and could therefore confer a localized advantage on the symbiosis without creating a stable transgenic plant. Such a technique could open up possibilities for novel plant host-rhizobium interaction studies; the presence of T-DNA in rhizobia might be a potential tool to examine aspects of the role of the host plant during symbiosis.
Acknowledgments
We thank K. B. Nellerup for technical support, J. Reeves (Horticulture Research Institute, Palmerston North, New Zealand) for providing the L. angustifolius seeds, I. Dusha (Biological Research Centre, Szeged, Hungary) for the S. meliloti and S. fredii strains, and G. Lovei (Department of Integrated Pest Management, Flakkebjerg, Denmark) for critical discussion.
REFERENCES
- 1.Anthony, L. C., H. Suzuki, and M. Filutowicz. 2004. Tightly regulated vectors for the cloning and expression of toxic genes. J. Microbiol. Methods 58:243-250. [DOI] [PubMed] [Google Scholar]
- 2.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broothaerts, W., H. J. Mitchell, B. Weir, S. Kaines, L. M. A. Smith, W. Yang, J. E. Mayer, C. Roa-Rodrigez, and R. A. Jefferson. 2005. Gene transfer to plants by diverse species of bacteria. Nature 433:629-633. [DOI] [PubMed] [Google Scholar]
- 4.Chen, H., R. S. Nelson, and J. L. Sherwood. 1994. Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. BioTechniques 16:664-668. [PubMed] [Google Scholar]
- 5.Courtois, J., B. Courtois, and J. Guillaume. 1988. High-frequency transformation of Rhizobium meliloti. J. Bacteriol. 170:5925-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galibert, F., et al. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 8.Gard, B., R. C. Dogra, and P. K. Sharma. 1999. High-efficiency transformation of Rhizobium leguminosarum by electroporation. Appl. Environ. Microbiol. 65:2802-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleave, A. P. 1992. A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol. 20:1203-1207. [DOI] [PubMed] [Google Scholar]
- 10.Guerinot, M. L., B. A. Morlsseau, and T. Klapatch. 1990. Electroporation of Bradyrhizobium japonicum. Mol. Gen. Genet. 221:287-290. [Google Scholar]
- 11.Hajdukiewicz, P., Z. Svab, and P. Maliga. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25:989-994. [DOI] [PubMed] [Google Scholar]
- 12.Hatterman, D. R., and G. Stacey. 1990. Efficient DNA transformation of Bradyrhizobium japonicum by electroporation. Appl. Environ. Microbiol. 56:833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, M., Y. Maeda, Y. Hashimoto, and Y. Murooka. 2000. Efficient transformation of Mesorhizobium huakuii subsp. rengei and Rhizobium species. J. Biosci. Bioeng. 89:550-553. [DOI] [PubMed] [Google Scholar]
- 14.Hellens, R. P., E. A. Edwards, N. R. Leyland, S. Bean, and P. M. Mullineaux. 2000. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42:819-832. [DOI] [PubMed] [Google Scholar]
- 15.Ho, T. Q., Z. Zhong, S. Aung, and J. Pogliano. 2002. Compatible bacterial plasmids are targeted to independent cellular location in Escherichia coli. EMBO J. 21:1864-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holsters, M., D. de Waele, A. Depicker, E. Messens, M. Van Montagu, and J. Schell. 1978. Transfection and transformation of A. tumefaciens. Mol. Gen. Genet. 163:181-187. [DOI] [PubMed] [Google Scholar]
- 17.Jones, W. T., T. Al-Samarrai, J. M. Reeves, G. B. Ryan, C. A. Kirk, E. Vincze, D. Harvey, M. McCambridge, D. Greenwood, and P. H. S. Reynolds. 2004. The trans-acting protein interacting with the DNA motif proximal to the transcriptional start site of plant l-asparaginase is bacterial sarcosine oxidase. J. Bacteriol. 186:811-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koranyi, E., K. Burg, and M. Berenyi. 1998. Stable electrotransformation of symbiont candidate diazotrophic bacterium with plasmids carrying selectable and screenable marker genes. Res. Microbiol. 149:361-372. [DOI] [PubMed] [Google Scholar]
- 19.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moulin, L., A. Munive, B. Dreyfus, and C. Boivin-Masson. 2001. Nodulation of legumes by members of the beta-subclass of Proteobacteria. Nature 411:948-950. [DOI] [PubMed] [Google Scholar]
- 21.Pogliano, J. 2002. Dynamic cellular location of bacterial plasmids. Curr. Opin. Microbiol. 5:586-590. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds, P. H. S., L. A. Smith, J. M. J. J. Dikson, W. J. Jones, S. D. Jones, K. A. Rodber, A. Carne, and C. P. Liddane. 1992. Molecular cloning of acDNA encoding aspartate amonotransferase-P2 from lupine nodules. Plant Mol. Biol. 19:465-472. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Scholla, M. H., and G. H. Elkan. 1984. Rhizobium fredii sp. nov., a fast-growing species that effectively nodulates soybeans. Int. J. Syst. Bacteriol. 34:484-486. [Google Scholar]
- 25.Selvaraj, G., and V. N. Lyer. 1981. Genetic transformation of Rhizobium meliloti by plasmid DNA. Gene 15:279-283. [DOI] [PubMed] [Google Scholar]
- 26.van Berkum, P., and B. D. Eardly. 1998. Molecular evolutionary systematics of the Rhizobiaceae, p. 1-24. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae. Molecular biology of plant-associated bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 27.Wood, D. W., et al. 2001. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 294:2317-2323. [DOI] [PubMed] [Google Scholar]
- 28.Young, J. M., L. D. Kuykendall, E. Martınez-Romero, A. Kerr, and H. Sawada. 2003. Classification and nomenclature of Agrobacterium and Rhizobium. Int. J. Syst. Evol. Microbiol. 53:1689-1695. [DOI] [PubMed] [Google Scholar]