Abstract
Influenza vaccination with current inactivated vaccines homologous to the prevalent wild-type virus can reduce influenza illness in 75%–80% of healthy adults. Vaccine is recommended for all individuals with chronic underlying diseases and for those aged 65 years or older. Although influenza vaccination is still advocated for patients with blunted immunity, protection rates are not as high, running at 40% for frail institutionalized elderly people. The influenza antiviral agents amantadine or rimantadine, zanamivir and oseltamivir can modify the severity of illness and reduce the duration of illness by about 1.5–2.5 days. Amantadine inhibits only influenza A. Resistant virus may emerge in up to 33% of amantadine-treated patients in the first 5 days of treatment and be transmitted to susceptible close contacts. Side effects are usually mild in short courses of treatment. The neuraminidase inhibitor drugs zanamivir and oseltamivir act on both influenza A and B. Treatment is most effective when given within 30–36 hours after the onset of illness, and the earlier the better. Influenza should be treated with antiviral drugs in unvaccinated and vaccinated high-risk patients, as well as immunosuppressed patients with influenza-like illness, in periods of confirmed influenza prevalence. These drugs may be of great value in the event of a major viral antigenic shift that causes pandemic influenza, if an adequate supply can be sustained.
Influenza is responsible for more morbidity and mortality each year in North America than all other respiratory diseases combined, and it results in tremendous economic costs both from admissions to hospital and loss of productivity. Influenza and its attendant complication of pneumonia constitute the sixth leading cause of death overall in Canada.1 Preventive strategies are the key to reducing the impact of influenza on our communities, and effective vaccination strategies have been in place for half a century and continue to be improved upon. However, for the individual physician faced with a severely ill patient during an influenza epidemic, effective treatment is required. This need has led to the development of antiviral agents that halt or impede the ability of the virus to infect respiratory epithelial cells.
Two main classes of drug interfere with influenza virus infection (Table 1). The first drug to be developed was amantadine, which belongs to the ion channel blocker group of anti-influenza drugs. It has been used to treat and prevent influenza A since the mid-to-late 1960s.2 In the United States, rimantadine, an agent with fewer side effects than amantadine is more commonly used, but has never been licensed in Canada. The second class of agents consists of the viral neuraminidase inhibitors (NAIs), zanamivir (Relenza) and oseltamivir (Tamiflu).3
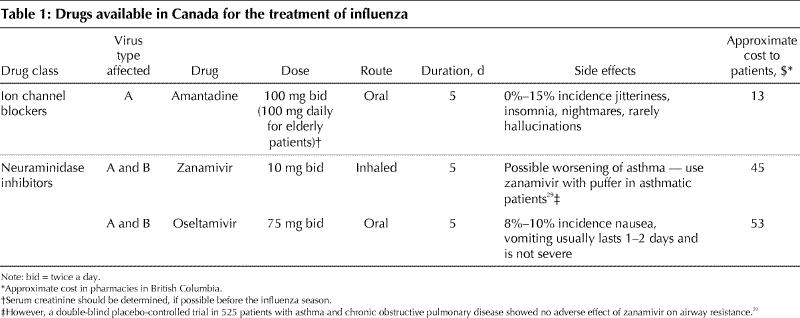
Table 1
Biology of influenza and mechanism of action of anti-influenza drugs
In order to understand the action of the antiviral drugs, a quick overview of the influenza virus is in order. Influenza belongs to the orthomyxovirus group of RNA viruses. Influenza is a relatively large virus that is essentially confined to infecting cells of the upper and lower respiratory tract. Viremia has rarely been detected. The infection cycle of the influenza virus is depicted in Fig. 1. There are 2 major proteins on the surface of the influenza virus, the hemagglutinin (HA) and the neuraminidase (NA). The hemagglutinin mediates attachment of the virus particles to the respiratory epithelial cells via specific receptors. Once the virus has bound to its host cell, it is transported into the cytoplasm in an endosome. The acid pH in the endosome activates or opens an ion channel called the M2 protein, permitting hydrogen ions to enter the virion. The resulting acidification of the virus is necessary for viral uncoating, another essential step in viral replication. Amantadine inhibits the function of the M2 protein and thus stops the replication process. Amantadine is only effective against influenza A, and not influenza B, because influenza B does not have an M2 protein, but a substitute protein called NB that is not affected by amantadine.4
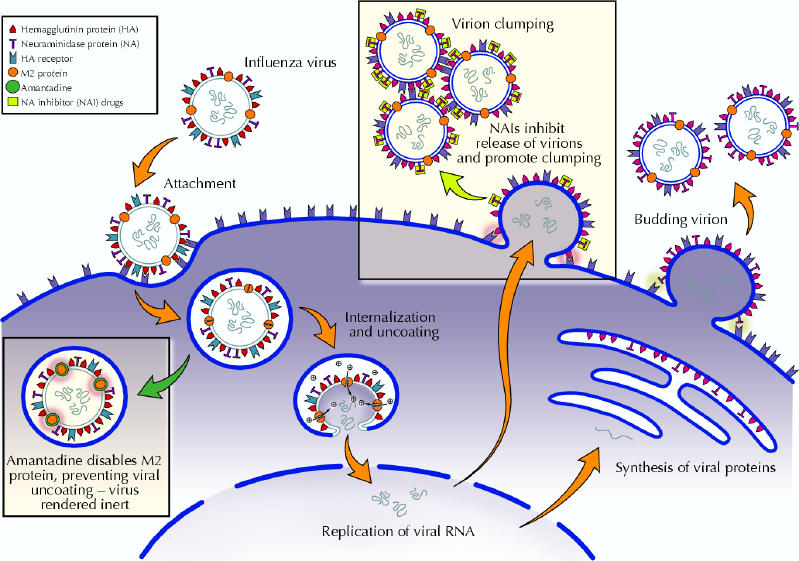
Fig. 1: Schematic representation of influenza virus attachment, internalization, replication and exit from the host respiratory cell and steps inhibited by antiviral drugs. Amantadine blocks viral internalization and uncoating. Neuraminidase inhibitors prevent the neuraminadase from releasing budding viruses and dispersing virions. Photo: Myra Rudakewich
Neuraminidase is a viral enzyme that cleaves the neuraminic acid component of sialic acid in the respiratory epithelial cell hemagglutinin receptors. After replication, in order to exit the cell and infect other cells, influenza virus particles bud off the host cell membrane. The viral neuraminidase is required to release the budding virus particles by digesting the hemagglutinin receptors holding the viruses to the cell. The virus particles thus released still have hemagglutinin receptors from the cell membrane coating them, and the hemagglutinins of other newly released viruses bind to these causing clumping. The neuraminidase cleaves these residues, allowing the viruses to disperse, enhancing their ability to infect other cells. The third function of the neuraminidase is to digest neuraminic acid in respiratory mucus, perhaps facilitating viral spread.5 The NAI drugs, zanamivir and oseltamivir, bind to the active site on the viral neuraminidase, blocking its activity. Thus, virus particles cannot exit the cells as easily, and they tend to clump and not disperse. This impedes their ability to infect more cells and attenuates the patient's infection. However, there is still subclinical or mild infection that actively immunizes the patient against that strain. The NAIs are active against both influenza A and B.
Why does influenza require treatment?
Many people do not require treatment for influenza, however, treatment may be of significant benefit to those at high risk of complications or those who for whatever reason need to minimize their absence from work. High-risk individuals are those over the age of 65 years and those with chronic underlying diseases such as chronic obstructive pulmonary disease (COPD), asthma, chronic heart disease, diabetes, chronic renal or hepatic disease, neoplastic disease and chronic connective tissue disease. They may have increased morbidity and mortality from influenza virus infection, usually as a result of bacterial superinfection, the most common being pneumonia. Influenza vaccine should be given to all such patients, but in reality this does not always happen. Moreover, some patients may refuse vaccination, especially if they have had previous side effects, or have developed a respiratory viral infection (that may have been caused by some noninfluenza respiratory virus) after receiving an influenza vaccine in previous years. Influenza vaccine is only 40%–60% effective in elderly patients,6 especially nursing home residents, and in patients with immunosuppression caused by underlying disease or by the drugs they are taking, because they may not form sufficient antibodies.6,7 Antiviral treatment of influenza in these types of individuals may attenuate the infection and reduce morbidity and mortality. Strategies for the use of influenza antiviral drugs should complement the preventive benefits of vaccination.
In what circumstances should influenza antiviral drugs be used?
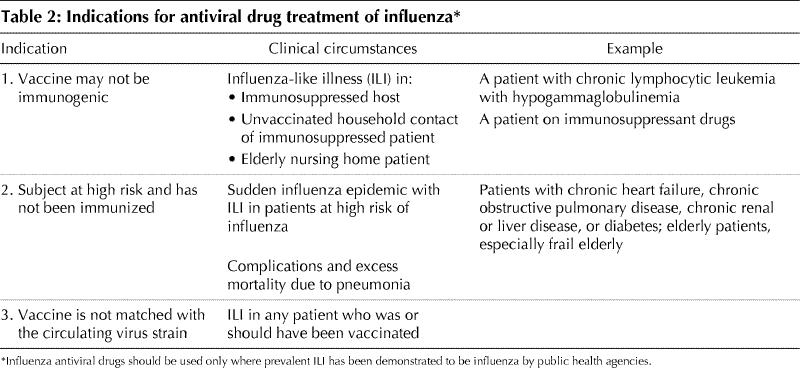
These clinical indications are listed in Table 2 and range from presumptive treatment in nursing home outbreaks to treatment in the immunocompromised patient. The categories listed may also apply to prevention of influenza with antiviral agents, the effectiveness of which has been confirmed for both amantadine and the NAIs.8,9,10,11,12 Influenza antivirals may also be used to prevent influenza in the event that the epidemic strain has undergone such a significant change in its antigenic structure (antigenic shift or antigenic drift) that it is no longer covered by the current vaccine, as occurred in January of 1997 when the epidemic strain suddenly changed from A/Wuhan to A/Sydney.
Table 2
Clinical trials with influenza antiviral drugs
Descriptions of comparative studies of the influenza antiviral drugs are listed in Table 3. All but one of these studies were double-blind and placebo-controlled. The older trials of amantadine for the treatment of influenza demonstrated a reduction in febrile influenza illness of about 1–1.6 days compared with placebo.13,14,15 Interestingly, one study showed no reduction in the rates of virus-positive nasal culture when using amantadine compared with placebo over the 5 days of treatment.13 Whether a component of this was related to acquired amantadine resistance was not tested. Another small family-based double-blind trial of amantadine in young adults and children with influenza showed a reduction of 1.5 days of reduced activity, 2.6 days to 50% fewer symptoms and 1.6 days fewer of fever. However, 33% of recipients began excreting amantadine-resistant virus by day 5 of treatment.15 In a recent report of the apparent failure of amantadine prophylaxis to curtail influenza outbreaks in Ontario nursing homes, institution of treatment with oseltamavir was found to be associated with the cessation of further cases.12 However, isolates were not tested for amantadine resistance. Because of this concern, it has been suggested that if one is going to treat the index case in a family with amantadine, other at-risk family members should receive an NAI rather than amantadine, for prophylaxis.
Table 3
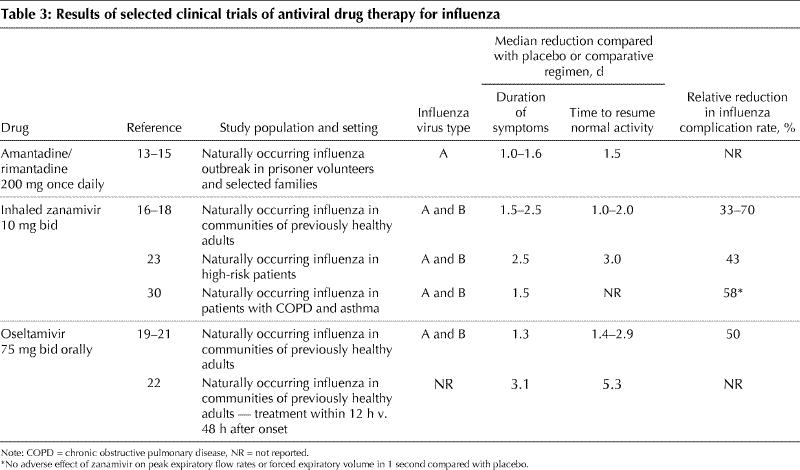
In more recent trials of the NAIs, patients were recruited by media advertisements, as well as emergency departments and general practice clinics. The major end points of the studies were cessation of symptoms of feverishness, cough, nasal obstruction, sore throat, myalgias, headache and fatigue. These trials showed a statistically significant reduction in these acute symptoms of between 1 and 2.5 days, depending on the subgroup analyzed, compared with placebo. This clinical effect was coincident with a decrease in the titre of viral shedding. Secondary benefits included 50%–70% fewer complications, 30%–50% fewer courses of antibiotics compared with placebo and earlier return to normal activities by 1–3 days. In these trials, all patients received an anti-pyretic/analgesic such as acetaminophen or paracetamol for symptomatic relief, and there was less use of these medications in the drug-treated patients, reflecting less severe disease. The symptom scores in the MIST zanamivir trial (in Australia, New Zealand and South Africa)16 and US trials with zanamivir,17,18 as well as the US and international trials with oseltamivir,19,20 indicated that illness in patients treated with the NAI was less severe, allowing an earlier return to normal function. A European trial in Scandinavia and the United Kingdom demonstrated that 46% more patients in the zanamivir group were afebrile by 24 hours after the start of treatment compared with placebo.21 In most of these studies, the earlier the antiviral was started after the onset of symptoms of influenza-like illness (ILI), the greater the difference between drug and placebo. For example, with zanamivir, although patients were enrolled within 48 hours of the onset of symptoms, the significant differences in symptom resolution were only seen in those patients treated within 30 hours of onset.17,18 There are further data from the IMPACT trial (1428 patients treated) showing that oseltamivir treatment within 12 hours of symptom onset further reduces the duration of illness and accelerates return to normal activities by 3.1 days and 5.3 days respectively, compared with starting treatment 48 hours after the start of symptoms.22 In all of the trials, the viral shedding in nasal secretions was significantly reduced in quantity and duration. Although not tested, this presumably would lessen the exposure risk for close contacts.
It is important to remember that in evaluating the specific efficacy of influenza antiviral drugs, the true test of the drug's inherent potency is its performance in ILI proven to be influenza. In intention-to-treat analyses, all patients who received at least one day of drug, whether they turned out to have influenza or ILI caused by another virus, are included in the analysis of efficacy. Although this may reflect the “real life” use of antivirals, it is really a test of effective strategy in using the drug, not the inherent efficacy of the drug against influenza virus. The more intense the influenza epidemic is, that is, the higher the attack rate, the better the efficacy will be in intention-to-treat studies, as the effect will not be as diluted by noninfluenza ILI. Most of the patients entered into the phase III trials were previously healthy. We still need more data on the performance of antiviral agents for the treatment of influenza in high-risk patients. One such study that examined the efficacy of zanamivir in high-risk patients from 6 placebo-controlled trials demonstrated that patients had 2.5 fewer days of symptoms, and returned to their previous baseline activities 3 days earlier.23 Only one of the trials (with zanamivir) featured significantly high attack rates of influenza B,16 and more information is required on treating this virus type in adults.
Diagnosing influenza and initiating treatment
The first caution is not to prescribe anti-influenza drug therapy unless one is sure there is an influenza outbreak in the community. One cannot diagnose influenza virus infections clinically with confidence, because many respiratory viruses such as parainfluenza, respiratory syncytial virus, adenovirus and coxsackievirus cause similar symptoms and signs. When evaluating an adult patient in a situation in which there is influenza and other respiratory viruses causing ILI in the community, the presence of fever (oral temperature ≥ 37.8° C) early on in the illness suggests influenza rather than another virus.17 Currently, in Canada there are several influenza surveillance programs operating, and updates can be obtained from most provincial health agency Web sites as well as from television broadcasts. In an average influenza prevalence period, clinical ILI has been shown to be true influenza in about 50%–70% of cases. Rapid enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) diagnostic tests for influenza A and B are available. ELISA tests are more sensitive in children than adults (over 80% sensitive for children less than 4 years of age versus 25% for adults over 40 years of age) and have not been widely used in the office setting.24 PCR-based tests are more sensitive (over 90%) for all age groups but are not widely used for primary diagnosis in commercial laboratories. In Canada, there is no reimbursement made to the clinician of the cost of purchasing or interpreting office-based tests, and they take extra time. On the other hand, when 30%–50% of individuals with ILI may not have influenza and will not benefit from the drugs, rapid diagnosis is cost-effective. A second caution is not to prescribe an influenza antiviral drug if symptoms have been present for longer than 36–48 hours, and the most benefit occurs when drug therapy is started earlier than 30 hours after the onset of symptoms, at least for the NAIs.18,22
These data raise questions about the best logistics for physicians to follow in prescribing influenza antivirals. Should all patients be seen in the office first? In a severe epidemic, this could flood the office possibly to the exclusion of other patients. Should influenza antivirals be prescribed over the telephone after a brief history is taken? The chances are that if there is an ongoing influenza epidemic, the latter strategy would often work well. However, there have been concerns about bacterial pneumonia being misdiagnosed as influenza during an epidemic and treated with an NAI, resulting in a poor outcome. Therefore, best practice may dictate that at least in those patients at high risk of pneumonia as a complication of influenza, an examination is necessary before deciding to treat with amantadine, oseltamivir or zanamivir. Guidelines such as those created for the treatment of community-acquired pneumonia25 should be formulated for treating influenza. A suggested algorithm is depicted in Fig. 2.
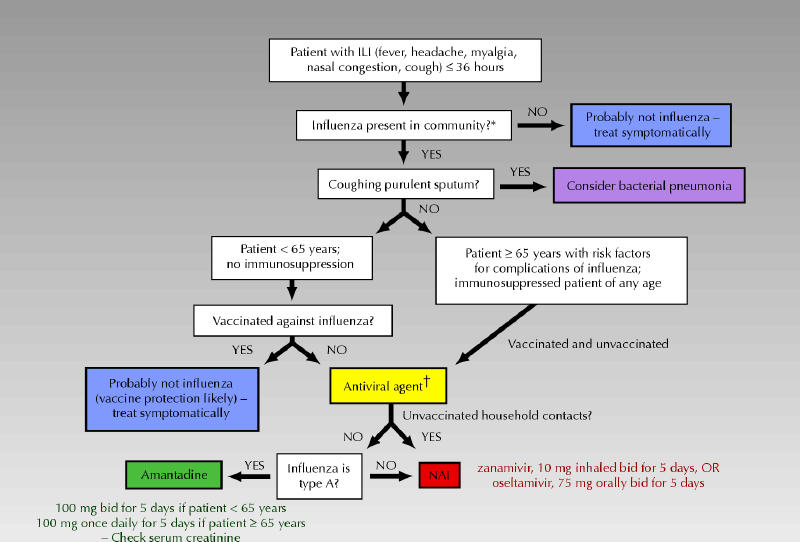
Fig. 2: An algorithm for the treatment of acute influenza-like illness (ILI). *Influenza virus outbreak confirmed by public health authorities. †Influenza antiviral treatment could be considered. It could also be considered in relatively healthy unvaccinated people aged less than 65 years to minimize absence from work. Photo: Myra Rudakewich
Which antiviral drug is best — amantadine, zanamivir or oseltamivir?
The information needed to answer this question with assurance is lacking. No published trials have compared these agents head to head. Any preferences therefore must be based on each drug's antiviral spectrum, side effects and ease of administration, which might affect compliance, as well as affordability for patients.
The side effects of amantadine have been a potential limitation to its use. The most frequent reactions are nausea, dizziness and insomnia. These have been reported to occur in 5%–15% of patients taking amantadine for prophylaxis over 6 weeks,8 but other studies of amantadine treatment (lasting up to 10 days) have documented no difference in side effects compared with placebo.9,10,26 The major side effects are almost an amphetamine-like effect, including jitteriness, anxiety, nightmares and, rarely, hallucinations. These effects appear to be due to an effect of amantadine, similar to amphetamines, of stimulation of the central nervous system. The symptoms usually resolve once the medication is stopped. For current treatment courses of 5 days, these side effects are less likely to occur. In patients with impaired renal function and elderly people, the dose should be reduced. Amantadine plasma concentrations of 300 ng/mL prevent influenza infection, and in elderly patients 1.4 mg/kg daily (about 100 mg daily for a 70-kg person) achieves this level.27 In elderly people, regardless of serum creatinine, and in those individuals with creatinine clearances of 30–50 mL/minute, treatment should be started with one dose of 200 mg, then reduced to 100 mg daily for 4 days. An NAI would be preferable if the serum creatinine level is greater than 200 mg/L. Another drawback of amantadine for the treatment of influenza is that within 5 days of treatment up to one-third of patients may begin to shed resistant virus due to a mutation in the amantadine target, the M2 protein. In family settings, family members of the person being treated with amantadine can be infected by these amantadine-resistant strains.28 The spectrum of amantadine, as previously mentioned, covers only influenza A, although A strains cause most epidemics.
The NAIs may be used to treat both A and B influenza and have relatively fewer side effects. A caution that the inhaled zanamivir powder may be associated with bronchospasm and may exacerbate asthma in some patients29 appears in the product monograph, although one double-blind placebo-controlled study of zanamivir treatment of acute influenza in patients with asthma and COPD showed no difference in airway resistance.30 The oral agent oseltamivir causes mild nausea and vomiting (in about 7% of cases) that is relatively short-lived (first day or two of therapy), and in the published trials this did not affect compliance.19,20 Oseltamivir may be easier to administer to frail or confused elderly patients who may have trouble using the zanamivir inhalation device. Zanamivir, however, may be better tolerated by patients with a lot of nausea.
Although NAI–resistant isolates have been described, they are at present uncommon (about 1% of treated adults).31,32 Evidence suggests that the mutation in the neuraminidase required for resistance to the NAIs may also make the virus less successful at infecting, but this requires more clinical study. There have been no documented secondary cases caused by the spread of an NAI–resistant virus in families in which one family member was being treated with an NAI for influenza, as there have been with amantadine-resistant strains during amantadine treatment.
How much does it cost?
The cost–benefit ratio determines which drug costs are reimbursed by today's provincially funded drug benefit programs. At a retail pharmacy in British Columbia, the cost to the patient of a 5-day course of amantadine, which has generic status, is about $13 including the dispensing fee. The same course of oral oseltamivir, 75 mg twice daily, is about $53, and 5 days of inhaled zanamivir, 10 mg twice daily, is $45. Cost–benefit analyses have been published comparing treating acute influenza with NAIs to prevention by vaccination. If one considers vaccination of all individuals aged 15–65 years, representing predominantly individuals at low risk of complications, vaccination or supportive care only are more cost-effective than treatment with the the NAIs.33 Amantadine treatment was not compared. If one models the cost–benefit ratio of treating high-risk patients (these data come from the high-risk patients treated in the MIST trial with zanamivir), treatment becomes cost-effective, largely because of the reduced influenza complications such as pneumonia and reduced exacerbation of underlying conditions such as congestive heart failure or diabetes, with their attendant requirement for more expensive medical interventions, especially admission to hospital.34 Most of the available cost–benefit studies have used quality-adjusted life years or QALYs as the end point. The potential additional economic benefit of the indirect cost–benefit ratio of earlier return to work productivity has not been extensively studied, but it is estimated that the indirect costs of epidemic influenza may be 5 to 10 times higher than direct costs.35 This should be born in mind when assessing the cost–benefit ratio of influenza antiviral drugs.
Summary
Influenza is a major public health problem causing clinical morbidity, mortality and major economic losses each year that there is an epidemic. Up until now, vaccination strategies, which constitute the mainstay of influenza control, have been directed at preventing morbidity and mortality in high-risk groups. Diligent efforts to optimize vaccine coverage must be maintained and strengthened. Unvaccinated individuals in critical occupations and those high-risk individuals who do not mount an effective protective antibody level to influenza vaccine may develop influenza and may benefit from one of the 3 available antiviral agents in Canada, amantadine, zanamivir or oseltamivir. Limiting factors for amantadine are side effects and the need to check renal function especially in elderly patients, as well as the rapid appearance of resistant virus that can be spread to close contacts. The limiting factor for the NAIs is cost. We have yet to design the optimal logistics for effectively and efficiently using antivirals in the face of an epidemic. “The earlier the better” seems to be the important concept for treating each individual. Influenza antiviral drugs may be of life-saving importance if and when the next worldwide pandemic hits, and supply will be a critical issue. Stockpiling influenza antiviral drugs of all types to deal with this latter eventuality is likely to be crucial.
Table

Footnotes
A patient information sheet appears on page 57
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. Grant Stiver, Division of Infectious Diseases, University of British Columbia, D-Floor, 2733 Heather St., Vancouver BC V5Z 3J5; fax 604 875-4013; gstiver@interchange.ubc.ca
References
- 1.Selected leading causes of death, by sex, 1997. Ottawa: Statistics Canada; 2002. Available: www.statcan.ca/english/Pgdb/health36.htm (accessed 2002 Nov 20).
- 2.Aoki FY. Amantadine and rimantadine. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of influenza. London: Blackwell Science; 1998. p. 457-76.
- 3.Laver WG, Bischofberger N, Webster RG. Disarming the flu viruses. Sci Am 1999;280:78-87. [DOI] [PubMed]
- 4.Betakova T, Nermut MV, Hay AJ. The NB protein is an integral component of the membrane of influenza B virus. J Gen Virol 1996;77:21689-94. [DOI] [PubMed]
- 5.Calfee DP, Hayden FG. New approaches to influenza chemotherapy: neuraminidase inhibitors. Drugs 1998;56:537-53. [DOI] [PubMed]
- 6.Govaert TME, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA, et al. The efficacy of influenza vaccination in elderly individuals. JAMA 1994;272:1661-5. [PubMed]
- 7.Stiver HG, Weinerman BH. Impaired antibody response to inactivated influenza A and B vaccine in cancer patients. CMAJ 1978;119:733-8. [PMC free article] [PubMed]
- 8.Dolin R, Reichman RC, Madore HP, Maynard R, Linton PN, Webber-Jones J, et al. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med 1982;307:580-4. [DOI] [PubMed]
- 9.Hayden FG, Atmar RL, Schilling M, Johnson C, Poretz D, Paar D, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med 1999;341:1336-42. [DOI] [PubMed]
- 10.Monto AS, Robinson DP, Herlocher L, Hinson JM Jr, Elliott MJ, Crisp A, et al. Zanamivir in the prevention of influenza among healthy adults. JAMA 1999; 282:31-6. [DOI] [PubMed]
- 11.Welliver R, Monto AS, Carewicz O, Schatteman E, Hassman M, Hedrick J, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA 2001;285:748-54. [DOI] [PubMed]
- 12.Bowles SK, Lee W, Seymor AE, Vearncombe M, Loeb M, Tamblyn S, et al. Use of oseltamivir during outbreaks in Ontario nursing homes, 1999–2000. Am J Geriatr Soc 2002;50:608-16. [DOI] [PubMed]
- 13.Togo Y, Hornick RB, Felitti VJ, Kaufman ML, Dawkins AT Jr, Kilpe VE, et al. Evaluation of therapeutic efficacy of amantadine in patients with naturally occurring A2 influenza. JAMA 1970;211:1149-56. [PubMed]
- 14.Wingfield WL, Pollack D, Grunert RR. Therapeutic effect of amantadine HCl and rimantadine HCl in naturally occurring influenza A2 respiratory illness in man. N Engl J Med 1969;281:579-84. [DOI] [PubMed]
- 15.Hayden FG, Sperber SJ, Belshe RB, Clover RD, Hay AJ, Pyke S., et al. Recovery of drug-resistant influenza A during therapeutic use of rimantadine. Antimicrob Agents Chemother 1991;35:1741-7. [DOI] [PMC free article] [PubMed]
- 16.MIST Study Group. Randomised trial of efficacy and safety of inhaled zanamivir in treatment of infuenza. A and B infections. Lancet 1998;352:1877-81. [PubMed]
- 17.Monto AS, Fleming DM, Henry D, de Groot R , Makela M, Klein T, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J Infect Dis 1999;180(2):254-61. [DOI] [PubMed]
- 18.Hayden FG, Osterhaus AD, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. N Engl J Med 1997;337:874-80. [DOI] [PubMed]
- 19.Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza. JAMA 2000;283:1016-24. [DOI] [PubMed]
- 20.Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomized controlled trial. Lancet 2000;355:1845-50. [DOI] [PubMed]
- 21.Makela MJ, Paukens K, Rostila T, Fleming DM, Man CY, Keene ON, et al. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J Infection 2000;40:42-8. [DOI] [PubMed]
- 22.Aoki FY, Macleod MD, Paggiaro P, Carewicz O, El Sawy A, Wat C, et al. Early administration of oral oseltamivir maximises the benefits of influenza treatment. J Antimicrob Chemother. In press. [DOI] [PubMed]
- 23.Lazelari J, Campion K, Keene O, Silagy C. Zanamivir for the treatment of influenza A and B infection in high risk patients. Arch Intern Med 2001;161:212-7. [DOI] [PubMed]
- 24.Steininger C, Kundi M, Aberle SW, Aberle JH, Popow-Kraupp T. Effectiveness of reverse transcription-PCR virus isolation and enzyme linked immunosorbent assay diagnosis of influenza A virus infection in different age groups. J Clin Microbiol 2002;40:2051-6. [DOI] [PMC free article] [PubMed]
- 25.Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian guidelines for the initial management of community-acquired pneumonia. Clin infect Dis 2000;31:383-421. [DOI] [PubMed]
- 26.Demicheli V, Jefferson T, Rivetti D, Deeks J. Prevention and early treatment of influenza in healthy adults. Vaccine 2000;18:957-1030. [DOI] [PubMed]
- 27.Aoki FY, Sitar DS. Amantadine pharmacokinetics in healthy elderly men: implications for influenza prevention. Clin Pharmacol Ther 1985;37:137-44. [DOI] [PubMed]
- 28.Hayden FG, Belshe RB, Clover RD, Hay AJ, Oaks MG, Soo W. Emergence and apparent transmission of rimantadine-resistant influenza virus in families. N Engl J Med 1989;321:1696-702. [DOI] [PubMed]
- 29.Neuraminidase inhibitors for treatment of influenza A and B infections. MMWR Morb Mortal Wkly Rep 1999;48(RR14):1-7. [PubMed]
- 30.Murphy KR, Eivindson A, Paukens K, Stein WJ, Pellier G, Watts R, et al. Efficacy and safety of inhaled zanamivir for the treatment of influenza in patients with asthma or chronic obstructive pulmonary disease. Clin Drug Invest 2000;20:337-49.
- 31.Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet 2000;533:827-35. [DOI] [PubMed]
- 32.Aoki FY, Doucette KE. Oseltamivir: a clinical and pharmacological perspective. Expert Opin Pharmacother 2001;2:1671-83. [DOI] [PubMed]
- 33.Muennig PA, Khan K. Cost-effectiveness of vaccination versus treatment of influenza in healthy adolescents and adults. Clin Infect Dis 2000;33:1897-85. [DOI] [PubMed]
- 34.Mauskopf JA, Cates SC, Griffin AD, Neighbors DM, Lamb SC, Rutherford C, et al. Cost effectiveness of zanamivir for the treatment of influenza in a high risk population in Australia. Pharmacoeconomics 2000;17:6 11-20. [DOI] [PubMed]
- 35.Szucs T. The socioeconomic burden of influenza. JAntimicrob Chemother 1999;44:(Suppl B):11-5. [DOI] [PubMed]


