Abstract
Intact and decorticated single-celled Ascaris suum eggs were exposed to UV radiation from low-pressure, germicidal lamps at fluences (doses) ranging from 0 to 8,000 J/m2 for intact eggs and from 0 to 500 J/m2 for decorticated eggs. With a UV fluence of 500 J/m2, 0.44- ± 0.20-log inactivation (mean ± 95% confidence interval) (63.7%) of intact eggs was observed, while a fluence of 4,000 J/m2 resulted in 2.23- ± 0.49-log inactivation (99.4%). (The maximum quantifiable inactivation was 2.5 log units.) Thus, according to the methods used here, Ascaris eggs are the most UV-resistant water-related pathogen identified to date. For the range of fluences recommended for disinfecting drinking water and wastewater (200 to 2,000 J/m2), from 0- to 1.5-log inactivation can be expected, although at typical fluences (less than 1,000 J/m2), the inactivation may be less than 1 log. When the eggs were decorticated (the outer egg shell layers were removed with sodium hypochlorite, leaving only the lipoprotein ascaroside layer) before exposure to UV, 1.80- ± 0.32-log reduction (98.4%) was achieved with a fluence of 500 J/m2, suggesting that the outer eggshell layers protected A. suum eggs from inactivation by UV radiation. This protection may have been due to UV absorption by proteins in the outer layers of the 3- to 4-μm-thick eggshell. Stirring alone (without UV exposure) also inactivated some of the Ascaris eggs (∼20% after 75 min), which complicated determination of the inactivation caused by UV radiation alone.
Ascaris lumbricoides is the most prevalent of the parasitic intestinal worms; an estimated 1.4 billion people are infected worldwide, mostly in developing countries (7). Helminth infections like ascariasis lead to a host of physical and mental disabilities, including cognitive and societal impairment, higher susceptibility to infection, decreased responsiveness to vaccination, and malnutrition (6, 7), which impair the development of several hundred million children in developing countries (7). An adult female A. lumbricoides worm sheds up to 200,000 eggs daily (37); these eggs are passed in the feces of the infected individual and are thus present in wastewater, contaminated soil, and, in some cases, contaminated drinking water sources. Under favorable environmental conditions, the eggs may remain viable for up to 15 years (32).
Ascaris eggs have a 3- to 4-μm thick, four-layer shell that consists of an inner lipoprotein layer (ascaroside layer), a thicker chitin/protein layer, a lipoprotein vitelline layer, and an outer acid mucopolysaccharide/protein uterine layer (43). The inner lipoprotein layer consists of a unique mixture of 25% protein and 75% lipid-containing ascarosides and is responsible for the impermeability of the shell (43). The chitinous layer, which provides structural strength, contains chitin spindles in a protein matrix (11). The compositions of the vitelline and uterine layers are not well characterized, but both contain protein (43). The outer three layers can be removed by soaking the eggs in a solution of hypochlorite, leaving only the inner lipoprotein layer (2, 19); this process is referred to as “decortication.”
Ascaris eggs are more resistant than other water-related pathogens to most types of inactivation; the only exceptions are some viruses and bacterial endospores that are more resistant to very high temperatures. Ascaris eggs can be inactivated in minutes by temperatures above 60°C, but they can survive for more than 1 year at 40°C (10). Disinfection with chlorine at commonly applied doses is ineffectual (20). The impermeability of the inner ascaroside membrane also protects the eggs from a variety of strong acids, strong bases, oxidants, reductants, protein-disrupting agents, and surface-active agents (2). Although the use of UV disinfection is becoming more common in wastewater and drinking water treatment systems, the effect of UV radiation on Ascaris eggs has not been adequately studied yet.
Previous reports on UV irradiation of Ascaris eggs present conflicting evidence. Using data reported by de Lemos Chernicharo et al. (8), we calculated that 0.77-log inactivation was achieved for unembryonated (single-celled) A. lumbricoides eggs subjected to a 254-nm UV fluence (dose) of 200 J/m2 in a small photoreactor. In contrast, based on the data reported by Tromba (40), we calculated that infective (containing a larva) Ascaris suum eggs exposed to UV fluences between 240 and 960 J/m2 were inactivated by between 1.9 and 2.3 logs, although a fluence-response relationship was not observed. A complete fluence (dose)-response curve for Ascaris egg inactivation by low-pressure UV, using standardized procedures and a collimated light source, has not been reported. Such bench-scale experiments have become the standard for determining the fluence response of microorganisms to UV radiation (4, 29, 30).
The objectives of the research presented here were (i) to determine the inactivation of A. suum eggs exposed to various fluences of UV light in a bench-scale experiment and (ii) to determine the contribution of the outer layers of the eggshell to the resistance of the eggs to UV exposure.
MATERIALS AND METHODS
A. suum eggs purchased from Excelsior Sentinel Inc. (Ithaca, NY) were used for all experiments; the eggs were obtained from the intestines of infected pigs and were shipped at a concentration of 2 × 106 eggs/ml in a 0.5% formalin storage solution. Storage and incubation of Ascaris eggs in formalin is a common procedure for reducing the growth of fungi and bacteria (5). All experiments were completed within 3 months after we received the eggs, which were stored at 4°C prior to use. A. suum eggs are commonly used as a model for A. lumbricoides because it is easier to obtain large numbers of eggs of A. suum than to obtain large numbers of A. lumbricoides eggs and the eggs can be concentrated without subjecting them to chemical treatments that might alter their characteristics. It has not yet been established that A. suum, which infects pigs, and the human parasite A. lumbricoides are distinct species (32). Although evidence of genetic differences has been reported, so far no morphological or physiological differences have been found (1, 45). Ascaris eggs obtained from the uteruses of adult worms have been shown to have infectivity and inactivation characteristics similar to those of eggs obtained from infected feces (14, 31); however, there is some evidence that the resistance of the egg shells to environmental conditions is increased by a quinone-tanning process that occurs after they leave the uterus (11, 43). Also, when eggs from worm uteruses are dissected, it is difficult to ensure that only completely mature eggs (with complete eggshells) are collected. For these reasons, eggs were collected from pig intestines rather than worm uteruses. The eggs were in the unembryonated (single-cell) stage, which is often the dominant developmental stage of eggs in wastewater (unpublished data) or drinking water sources, although more developed eggs are present if they spend significant time in an aerobic environment prior to disinfection.
Preparation of working suspensions of Ascaris eggs.
A working suspension of eggs in phosphate-buffered saline (PBS) (21) was prepared by adding 0.5 ml of the purchased stock egg suspension to 40 ml PBS in a 50-ml centrifuge tube. After room temperature was reached, the tube was centrifuged in a Diamond/IEC model K centrifuge (Thermo Electron Corporation, Woburn, MA) to separate the eggs. This and all subsequent centrifugations were performed at 1,000 × gfor 5 min. The supernatant was aspirated to 5 ml to remove the formalin, and the tube was refilled to 35 ml with PBS. Each 35 ml of working suspension supplied eggs for five samples, which were processed within 30 h of the rinsing step.
The procedure used for preparing a decorticated egg working suspension (removing the outer eggshell layers) was the same as the procedure used for preparing the intact egg working suspension, except that following the rinsing step, a pipette was used to carefully remove all but 1 ml of the supernatant and the tube was refilled with a 10% household bleach solution until the volume was 10 ml. After 9 min (concentration × time, ∼37,500 mg OCl−-min/liter), the bleach solution was diluted with 30 ml of deionized water. The contents of the tube were mixed and centrifuged. The supernatant was aspirated until the volume was 5 ml, and a pipette was used to carefully remove all but 1 ml of the remaining supernatant. The tube was then filled with PBS until the volume was 35 ml. The dilution steps were sufficient to prevent further oxidation of the eggshells by HOCl.
A sample of each working suspension was diluted to 60% in PBS (to obtain the same concentration that was exposed to UV radiation), and a Lambda 14 UV/VIS spectrophotometer (Perkin-Elmer, Fremont, CA) was used to determine the absorbance at 254 nm (1-cm path length) of the egg suspension in reference to deionized water.
Sample preparation and UV exposure.
Each sample of eggs was prepared just before UV exposure. For each sample, 6 ml of the working suspension and 4 ml of PBS were added to a 60-mm glass petri dish while the preparation was being stirred with a magnetic stirrer. The stir speed was adjusted to maintain the eggs in suspension without causing a significant vortex. The total number of eggs in each sample was approximately 1.7 × 104.
Samples in the petri dishes were exposed to UV fluences at 254 nm ranging from 0 to 8,000 J/m2 (500, 1,000, 2,000, 3,000, 4,000, 5,000, 6,000, 7,000, and 8,000 J/m2) for intact eggs and from 0 to 500 J/m2 (100, 200, 300, 400, and 500 J/m2) for decorticated eggs using a bench-scale quasi-parallel beam apparatus. The quasi-parallel beam was constructed using the guidelines established by Bolton and Linden (4). Two 25-W General Electric low-pressure germicidal lamps (G25T8) were suspended above a 30.5-cm collimating box. Two 6.5-cm concentric apertures cut in the top and the bottom of the box blocked nonparallel rays emanating from the lamps. For UV exposure, each petri dish containing a sample was placed on a magnetic stirrer. The water surface was 1 cm below the lower aperture and 35 cm below the lamps. The incident irradiance at the center of the surface of each sample was measured using a Spectroline DM254XA UV meter (Spectronics Corporation, Westbury, NY) and was constant at 1.1 W/m2 throughout the experiments. The petri factor, which accounted for variation in the irradiance across the surface of the sample, the reflection factor, the water factor, which accounted for the absorbance of the sample, the divergence factor, and the average germicidal irradiance throughout the sample volume for each batch of working suspension were calculated as described by Bolton and Linden (4) using a modified version of the spreadsheet “Germicidal Fluence (UV Dose) Calculations for a Low Pressure UV Lamp” obtained from Bolton Photosciences Inc. (Edmonton, Alberta, Canada). The desired fluence was achieved by controlling the exposure time for each sample using a manual shutter. The exposure times ranged from 0 to 153 min for intact eggs and from 0 to 10.5 min for decorticated eggs. During and after exposure, all samples were kept in the dark to prevent potential photoreactivation. Samples were exposed to a random order of fluences, and each fluence was repeated in triplicate.
Two types of controls were prepared to assess the impact of stirring on the viability of the A. suum eggs. Samples that were not exposed to UV either were taken directly from the working suspension of A. suum eggs or were stirred without exposure to UV.
Recovery of eggs and incubation.
Following UV exposure and while the sample was being stirred, the contents of a petri dish were transferred with a pipette to a foil-covered 50-ml centrifuge tube. The dish was rinsed twice with 10 ml PBS, which was also recovered. The tube was then centrifuged, and the supernatant was removed by aspiration and pipetting. The remaining 1-ml egg suspension was recovered in a 1.5-ml, foil-covered microcentrifuge tube. The 50-ml tube was rinsed with 0.5 ml PBS, which was also recovered. The microcentrifuge tube was then centrifuged in an IEC Micromex RF centrifuge (International Equipment Co., Needham Heights, MA). The supernatant was removed with a pipette, and the eggs were resuspended in 1.5 ml of 0.1 M H2SO4 for incubation (in the same tube). Samples were incubated with loose caps at 28°C for 30 ± 3 days (41).
Enumeration.
After incubation, the 1.5-ml tube was centrifuged, and the supernatant was removed. To facilitate viewing of the internal structure, the eggs that had not been decorticated previously were treated with sodium hypochlorite to remove the outer shells, as follows. A 1.5-ml portion of 10% household bleach was added to the egg suspension. After 4 min, the tube was centrifuged, and the bleach solution was removed with a pipette (total exposure time, 9 min). The tube was refilled with deionized water to dilute the remaining bleach and centrifuged, and the supernatant was removed, which left 0.3 ml, including the pellet containing eggs. Decorticated eggs were not subjected to bleaching and rinsing steps since their outer shells had been removed previously.
Using a pipette, 60 μl of a well-mixed egg suspension was placed on a microscope slide and covered with a coverslip. The slide was viewed with an Olympus BH-2 phase-contrast microscope (Scientific Instrument Co., Sunnyvale, CA) at a magnification of ×100, and at least 300 eggs were counted in each sample. Eggs were categorized as follows: embryonated (eggs containing a fully developed larva), unembryonated (eggs without a larva but with an internal structure), or empty eggshells.
RESULTS
Effect of stirring on Ascaris eggs.
The results for stirred and unstirred samples that were not exposed to UV light are shown in Table 1. In addition to embryonated eggs (with larvae) and unembryonated eggs (without larvae but with internal structure), empty eggshells were observed in some of the samples. The percentages of embryonated eggs in the intact and decorticated egg suspensions that were not stirred were approximately 95 and 97%, respectively. Stirring for 10 or 75 min had only a minor effect on the percentage of embryonated eggs in the decorticated egg suspensions. However, in the suspensions of intact eggs stirred for 75 min, a significant decrease in viability, to 73%, was observed. Significant numbers of both unembryonated eggs and empty eggshells were present in these stirred samples. Thus, intact eggs appeared to have been damaged by stirring, whereas the decorticated eggs were not damaged.
TABLE 1.
Effect of stirring and UV exposure on intact and decorticated A. suum eggs
| Eggs | Time stirred (min) | UV fluence (J/m2) | n | No. embryonated (mean) | No. unembryonated (mean) | No. with empty shells (mean) | % Embryonated | % Embryonated not including empty eggshells | Log inactivation (mean) | 95% confidence interval |
|---|---|---|---|---|---|---|---|---|---|---|
| Intact | 0 | 0 | 1 | 313 | 11 | 5 | 95.14 | 96.60 | ||
| 75 | 0 | 3 | 331 | 49 | 72 | 73.21 | 87.09a | |||
| 10 | 500 | 3 | 167 | 362 | <1 | 31.57 | 31.57 | 0.44 | 0.20 | |
| 19.5 | 1,000 | 3 | 45 | 474 | <1 | 8.61 | 8.61 | 1.00 | 0.06 | |
| 37.5 | 2,000 | 3 | 10 | 405 | <1 | 2.41 | 2.41 | 1.56 | 0.11 | |
| 56.5 | 3,000 | 3 | 3.7 | 339 | <1 | 1.07 | 1.07 | 1.91 | 0.28 | |
| 75.5 | 4,000 | 3 | 2.7 | 523 | <1 | 0.51 | 0.51 | 2.23 | 0.43b | |
| 94 | 5,000 | 3 | 0 | 414 | <1 | 0.00 | 0.00 | >2.53 | ||
| 112 | 6,000 | 3 | 0 | 307 | <1 | 0.00 | 0.00 | >2.43 | ||
| 133 | 7,000 | 3 | 0 | 304 | <1 | 0.00 | 0.00 | >2.42 | ||
| 150 | 8,000 | 3 | 0.07 | 463 | <1 | 0.14 | 0.14 | >2.53 | ||
| Decorticated | 0 | 0 | 3 | 296 | 7.3 | 1.3 | 97.15 | 97.58 | ||
| 10 | 0 | 3 | 300 | 10.3 | <1 | 96.57 | 96.67a | |||
| 75 | 0 | 2 | 294 | 14.5 | 2.0 | 94.69 | 95.30 | |||
| 2.5 | 100 | 3 | 258 | 51 | <1 | 83.57 | 83.57 | 0.06 | 0.02 | |
| 4.5 | 200 | 3 | 90 | 215 | <1 | 29.51 | 29.51 | 0.52 | 0.03 | |
| 6.5 | 300 | 3 | 29 | 275 | <1 | 9.55 | 9.55 | 1.01 | 0.16 | |
| 8.5 | 400 | 3 | 9 | 295 | <1 | 2.86 | 2.86 | 1.53 | 0.15 | |
| 10.5 | 500 | 3 | 5 | 297 | <1 | 1.55 | 1.55 | 1.80 | 0.32 |
Value used in the equation in the text for Pst.
Eggs were recovered in only two of three samples. Confidence intervals were calculated as if one egg was also recovered in the third sample and, therefore, may be underestimated.
Interestingly, empty eggshells were rarely observed in the samples exposed to UV radiation; it is possible that empty eggshells were produced by the stirring but were destroyed during the simultaneous UV treatment or the subsequent incubation and chlorine treatment. Unfortunately, it was not possible to independently investigate the effects of stirring and UV exposure because stirring was necessary to prevent the eggs from settling so that a consistent fluence of UV could be applied throughout each sample suspension. Nonetheless, it was necessary to choose an appropriate control with which to calculate inactivation due to UV. Because the levels of recovery of the empty eggshells were different for the different treatments (e.g., much higher for the non-UV-exposed samples) and appeared to be an effect of stirring rather than UV exposure, empty eggshells were excluded from the log inactivation calculation. Thus, the log inactivation was calculated using the following equation: log inactivation = −log (Puv/Pst), where Pst is the percentage of embryonated eggs in the non-UV-exposed, stirred sample (not including empty eggshells) and Puv is the percentage of embryonated eggs in the UV-exposed, stirred sample (not including empty eggshells).
For the intact eggs, the control that was used (Pst = 87.09% in Table 1) had a significant effect on the inactivation that was attributed to UV. If the effect of stirring had not been accounted for (e.g., by using 95.14% in Table 1) the inactivation attributed to UV would have been overestimated. On the other hand, if the empty eggshells had been included in the total egg counts (e.g., by using 73.21% in Table 1), the inactivation attributed to UV may have been underestimated.
UV inactivation of A. suum eggs.
The results obtained when intact and decorticated A. suum eggs were exposed to a range of UV fluences are shown in Table 1. The percentage of embryonated eggs, the log inactivation (calculated using the equation given above), and the 95% confidence intervals are shown. For samples in which no embryonated eggs were recovered, the minimum possible inactivation was determined by calculating the value for inactivation if one embryonated egg had been present in the eggs counted. Some embryonated eggs were recovered in all of the decorticated egg samples and in all but one of the intact egg samples exposed to 4,000 J/m2 or less. Two embryonated eggs were recovered in an intact egg sample exposed to 8,000 J/m2, but this result was considered to be an outlier, possibly due to contamination.
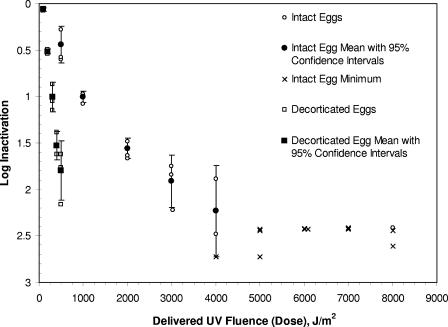
The decorticated eggs were much more susceptible to UV than the intact eggs; at a UV fluence of 500 J/m2, 1.80- ± 0.32-log inactivation (mean ± 95% confidence interval) of decorticated eggs was observed, whereas the inactivation of intact eggs was only 0.44 ± 0.20 log. The maximum log inactivation of intact eggs was 2.23 ± 0.49 at a fluence of 4,000 J/m2; higher log inactivation values could not be detected with the sample size of approximately 300 eggs. The log inactivation curves for the intact and decorticated eggs are compared in Fig. 1.
FIG. 1.
Fluence (dose)-response relationship for inactivation of intact and decorticated A. suum eggs subjected to low-pressure UV light.
DISCUSSION
Effect of stirring on Ascaris eggs.
As shown in Table 1, stirring intact Ascaris eggs for 75 min resulted in more than a 20% decrease in the percentage of embryonated eggs recovered after incubation. A possible explanation is that the intact eggs, with their rigid eggshells, were more susceptible to damage from the physical forces created by the stir bar, whereas the decorticated eggs, which had only a flexible lipid membrane, were less susceptible to such damage. Alternatively, it is possible that stirring decorticated eggs also produced empty eggshells that subsequently disintegrated during incubation in H2SO4. Similarly, it is possible that empty eggshells were not recovered from the UV-exposed samples because UV damage made the eggshells more susceptible to further degradation by H2SO4 during incubation or by chlorine during decortication. Because it was not possible to separate the effects of stirring and UV exposure (with a nonstirred, UV-exposed control), there is some uncertainty about whether the method we used is the most appropriate way to determine log inactivation. Unfortunately, other types of mixing that might cause less damage to the eggs (e.g., mechanical, recirculating flow, or aeration) also cause shading, making it more difficult to calculate the fluence of UV radiation delivered. Nonetheless, it is recommended that future experiments be conducted in a way that stirring can be eliminated or minimized as a source of error.
UV inactivation of A. suum eggs.
Intact Ascaris eggs were highly resistant to UV radiation; a fluence of 1,000 J/m2 was required to achieve 1-log inactivation. This fluence is two to four times higher than the fluences required to inactivate adenoviruses (13, 24, 36, 39), which are the most UV resistant of the human enteric viruses that have been studied. Thus, according to the methods used in our research, Ascaris eggs are the most UV-resistant water-related pathogen identified to date. Compared to intact eggs, the decorticated Ascaris eggs were more susceptible to UV radiation, and a fluence of only 300 J/m2 was required to achieve 1-log inactivation. The improved efficacy of UV for inactivation of decorticated eggs compared with the inactivation of intact eggs suggests that the outer layers of the eggshell provide protection from UV radiation. All three of these layers (chitin/protein layer, lipoprotein vitelline layer, and acid mucopolysaccharide protein uterine layer) contain protein. Although proteins absorb 254-nm UV radiation much less efficiently than nucleic acids absorb this radiation (15), the intact Ascaris eggshell is quite thick (3 to 4 μm), and we hypothesize that proteins in the outer layers absorbed some of the UV radiation, protecting the nucleic acids inside.
Although we did not measure the UV absorption of the Ascaris egg membrane directly, three lines of reasoning support this hypothesis. First, if the 4-μm eggshell has an absorption coefficient of 1,300 cm−1, it would be sufficient to decrease the UV intensity by two-thirds across the eggshell, which could explain the difference between the UV fluence needed to achieve 1-log inactivation of the intact eggs and the UV fluence needed to achieve 1-log inactivation of the decorticated eggs. An adsorption coefficient of this order of magnitude seems reasonable based upon previously reported values for particles in secondary wastewater, which are predominately composed of bacterial cells and had adsorption coefficients (at 254 nm) ranging from 3,300 to 74,300 cm−1 (22). Second, the molar extinction coefficient of cellular proteins at 254 nm is reported to be about 100 M−1 cm−1 (23); if there is one protein every 1 nm, then the resulting reduction in UV intensity would also be about two-thirds across the eggshell. Finally, we also measured the absorbance of our egg suspensions, although we could not estimate the amount of light that was scattered versus the amount of light that was absorbed because our spectrophotometer did not have integrating-sphere capability. Nonetheless, the absorbance measurements (e.g., for 3,500 eggs/ml, the absorbance was about 1 for a 1-cm path length) were consistent with absorption coefficients of about 1,000 cm−1 or more for the eggshell. Thus, while decortication significantly improved the efficacy of UV radiation for inactivating Ascaris eggs, it is not a practical treatment step because the majority of eggs in drinking water supplies and treated wastewater are intact, and decortication requires extremely high hypochlorite concentrations.
The UV inactivation of both intact and decorticated eggs (Fig. 1) followed roughly first-order kinetics, although a significant amount of tailing was observed with the intact eggs. Part of the tailing may have been an artifact of the detection method used, because very low numbers of embryonated eggs were observed at the higher UV fluences (10/405 eggs at 2,000 J/m2, 3.7/339 eggs at 3,000 J/m2, and 2.7/523 eggs at 4,000 J/m2 [Table 1]). An enumeration method that was more amenable to analysis of larger numbers of eggs may reduce the variability associated with higher fluences (Fig. 1). Tailing could also be due to a distribution of UV resistance within the Ascaris population (a small number of eggs that are highly UV resistant) (15) or due to development of increasing protection from UV as a consequence of UV exposure (for example, by aggregation) (3).
Our results indicate that the level of inactivation of Ascaris eggs by UV is lower than the level measured previously. Considering only embryonated eggs (the eggs containing fully developed larvae), we calculated that de Lemos Chernicharo et al. (8) observed a 0.78- ± 0.26-log reduction for A. lumbricoides subjected to a fluence of 200 J/m2, whereas we measured only a 0.44- ± 0.20-log reduction for A. suum at a fluence of 500 J/m2. A possible explanation for the difference is that de Lemos Chernicharo et al. used eggs dissected from worm uteruses, whereas we used eggs isolated from host intestines. Eggs collected from worm uteruses may not be as resistant to environmental conditions as eggs exposed to host intestinal contents (11, 43), and all of the eggs may not be fully mature (with complete eggshells) when they are collected. Another difference between the studies is that we used A. suum and de Lemos Chernicharo et al. used A. lumbricoides; however, to date, no morphological or physiological differences have been found between these species (1, 32, 45). It is also important to note that it is not clear how the applied fluence was determined by de Lemos Chernicharo et al. in their pilot-scale photoreactor; therefore, it is possible that the mean received fluence was even lower than the reported applied fluence. Finally, de Lemos Chernicharo et al. observed a significant number of eggs in intermediate stages of development after incubation, whereas we observed very few eggs in intermediate stages. The U.S. Environmental Protection Agency's standard procedure for determining viability defines only eggs that are fully embryonated after 28 days of incubation as viable (41). Even if all of the eggs at intermediate stages in the study of de Lemos Chernicharo et al. had fully developed to contain larvae after a longer incubation time, the log inactivation would have been 0.43 ± 0.12 at 200 J/m2, which is still significantly higher than the value observed in our research.
The level of UV inactivation reported by Tromba (40) was even higher. Based on our calculations (including larvae recovered from the lungs and intestines 7 to 18 days postinfection), between 1.9- and 2.3-log inactivation of A. suum was observed with fluences between 240 and 960 J/m2. Again, one of the reasons for the difference may be the use of eggs collected from worm uteruses rather than pig intestines. Another important difference is that embryonated eggs, rather than single-celled eggs, were exposed to UV radiation. Further research is needed to determine how susceptibility to UV varies during the developmental stages of the eggs.
A final important difference between Tromba's research and ours is the use of pig infectivity to determine viability. Our method of determining viability by embryonation in 28 days is consistent with existing regulations for determination of Ascaris egg viability in biosolids (41). To understand the difference between the use of incubation and the use of infectivity for determining viability, it is useful to review the Ascaris life cycle, which has been described by various authors (9, 17, 27, 34). Briefly, when Ascaris eggs are excreted in the feces of a host, each of them contains a single cell, which requires 6 to 12 weeks of aerobic incubation in the environment (or less time in the laboratory under ideal conditions) to divide and grow into an infective, third-stage larva (12). Once an infective egg is ingested by a host, the larva hatches in the intestine, penetrates the wall of the cecum or upper large intestine, and migrates to the liver. In the liver, it sheds the L2 cuticle and migrates to the lungs, where it penetrates the alveoli. From the lungs it moves up the trachea, is swallowed again by the host, and returns to the small intestine, where it develops into an adult.
Assuming that most of the damage caused to the eggs by 254-nm UV radiation is damage to the nucleic acids (15) and recognizing that sections of the UV-damaged Ascaris genome may not be expressed until later in the life cycle, it is reasonable to assume that fewer eggs complete development if the endpoint is infectivity of the liver, lung, or small intestine (in that order) than if the endpoint is growth to the third-stage larva. However, it is important to point out that during Ascaris egg development to the third-stage larva, the Ascaris egg grows from one cell to about 600 cells (35), which is significantly different from what happens in the life cycle of the single-celled prokaryotic protozoans Giardia and Cryptosporidium. For protozoans exposed to UV, infectivity studies or cell cultures are necessary to measure viability, because the DNA is not fully replicated until the organisms reproduce, after infection (16, 18, 33, 38). In Ascaris, however, the complete DNA of the organism is replicated when the single cell in the egg undergoes the first two cell divisions before it differentiates into the germ and somatic cell lines that form the larva (26).
Another important consideration is that because Ascaris larval migration through the liver causes rupture of the portal vessel and lesions (34) and migration through the lungs can cause pneumonitis, asthma, dyspnea, cough, and substernal pain (7), once a third-stage larva hatches and penetrates the intestinal lining, it is a health risk to its host. Therefore, in the interest of protecting public health, we believe that development to the third-stage larva is a better endpoint than pig infectivity. Additionally, from a practical perspective, infectivity measures are not suited to establishing a fluence-response relationship for UV-exposed Ascaris eggs, because techniques for larval isolation from liver and lung tissues result in only incomplete recovery and the recovery is not proportional to the number of embryonated eggs in the inoculant (17, 34, 40). Alternatively, a most-probable-number approach could be used (25), but this would require inoculating a large number of pigs.
Based on our results, we conclude that some inactivation of Ascaris eggs occurs in most UV disinfection systems. In drinking water, the required fluences proposed in the draft UV guidance manual for the long-term enhanced surface water treatment rule range from 15 to 1,860 J/m2, depending on the log inactivation of Giardia, Cryptosporidium, and viruses that is required (42). Over this range, Ascaris egg inactivation may range from 0 to almost 1.5 logs. At a fluence of 400 J/m2, which is the required fluence for drinking water disinfection units that meet NSF/ANSI Standard 55 (30), significantly less than 1-log removal is expected. In wastewater, a typical fluence recommended for disinfecting tertiary effluent from granular filtration is 1,000 J/m2 (28); at this fluence an approximately 1-log reduction in the number of Ascaris eggs may occur. Thus, the contribution of UV to the overall removal and inactivation of Ascaris eggs in a treatment process may or may not be significant, depending on the influent concentrations and the desired effluent quality (e.g., the World Health Organization recommendations for reuse of wastewater for agricultural irrigation require less than 0.1 or 1 intestinal nematode egg/liter, depending on the application [44]). Compared to chlorine, UV provides a more effective barrier in the treatment process under typical conditions. Nonetheless, physical methods (e.g., sedimentation, filtration, and membranes) should be used if significant removal of Ascaris eggs is required.
Acknowledgments
This work was supported in part by the University of California at Berkeley Chancellor's Opportunity Fellowship.
We are grateful to Jim Bolton of Bolton Photosciences for his advice regarding building the quasi-collimated beam apparatus and generating UV fluence-response curves and to Brian Pecson for his help developing the Ascaris incubation and enumeration procedures.
REFERENCES
- 1.Anderson, T. J. C., and J. Jaenike. 1997. Host specificity, evolutionary relationships and macrogeographic differentiation among Ascaris populations from humans and pigs. Parasitology 115:325-342. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, J. 1976. Studies on the induction of permeability in Ascaris lumbricoides eggs. Parasitology 73:109-121. [DOI] [PubMed] [Google Scholar]
- 3.Blatchley, E. R., N. Dumoutier, T. N. Halaby, Y. Levi, and J. M. Laine. 2001. Bacterial responses to ultraviolet irradiation. Water Sci. Technol. 43:179-186. [PubMed] [Google Scholar]
- 4.Bolton, J. R., and K. G. Linden. 2003. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J. Environ. Eng. 129:209-215. [Google Scholar]
- 5.Bowman, D. D., M. D. Little, and R. S. Reimers. 2003. Precision and accuracy of an assay for detecting Ascaris eggs in various biosolid matrices. Water Res. 37:2063-2072. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, P. J., M. Chico, C. Sandoval, I. Espinel, A. Guevara, M. M. Levine, G. E. Griffin, and T. B. Nutman. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 69:1574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crompton, D. W. T. 2001. Ascaris and ascariasis. Adv. Parasitol. 48:285-375. [DOI] [PubMed] [Google Scholar]
- 8.de Lemos Chernicharo, C. A., J. C. de Castro Silva, A. M. Zerbini, and V. M. Godinho. 2003. Inactivation of E. coli and helminth eggs in aerobic and anaerobic effluents using UV radiation. Water Sci. Technol. 47:185-192. [PubMed] [Google Scholar]
- 9.Fagerholm, H.-P., P. Nansen, A. Roepstorff, F. Frandsen, and L. Eriksen. 2000. Differentiation of cuticular structures during the growth of the third-stage larva of Ascaris suum (Nematoda, Ascaridoidea) after emerging from the egg. J. Parasitol. 86:421-427. [DOI] [PubMed] [Google Scholar]
- 10.Feachem, R. G., D. J. Bradley, H. Garelick, and D. D. Mara. 1980. Appropriate technology for water supply and sanitation: health aspects of excreta and sullage managment—a state-of-the-art review, vol. 3. The World Bank, Washington, D.C.
- 11.Gamble, H. R., R. H. Fetterer, and J. J. F. Urban. 1995. Reproduction and development in helminths, p. 289-305. In J. J. Marr and M. Muller (ed.), Biochemistry and molecular biology of parasites. Academic Press, London, United Kingdom.
- 12.Geenen, P. L., J. Bresciani, J. Boes, A. Pedersen, L. Eriksen, H.-P. Fagerholm, and P. Nansen. 1999. The morphogenesis of Ascaris suum to the infective third-stage larvae within the egg. J. Parasitol. 85:616-622. [PubMed] [Google Scholar]
- 13.Gerba, C. P., D. M. Gramos, and N. Nwachuku. 2002. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl. Environ. Microbiol. 68:5167-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiglietti, R., P. Rossi, M. Ramsan, and A. Colombi. 1995. Viability of Ascaris suum, Ascaris lumbricoides, and Trichuris muris eggs to alkaline pH and different temperatures. Parassitologia 37:229-232. [PubMed] [Google Scholar]
- 15.Harm, W. 1980. Biological effects of ultraviolet radiation. Cambridge University Press, Cambridge, United Kingdom.
- 16.Johnson, A. M., K. G. Linden, K. M. Ciociola, R. De Leon, W. Giovanni, and P. A. Rochelle. 2005. UV inactivation of Cryptosporidium hominis as measured in cell culture. Appl. Environ. Microbiol. 71:2800-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungersen, G., H.-P. Fagerholm, P. Nansen, and L. Eriksen. 1999. Development of patent Ascaris suum infections in pigs following intravenous administration of larvae hatched in vitro. Parasitology 119:503-508. [DOI] [PubMed] [Google Scholar]
- 18.Keegen, A. R., S. Fanok, P. T. Monis, and C. P. Saint. 2003. Cell culture-Taqman PCR assay for evaluation of Cryptosporidium parvum disinfection. Appl. Environ. Microbiol. 69:2505-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy, M. W., and F. Qureshi. 1986. Stage-specific secreted antigens of the parasitic larval stages of the nematode Ascaris. Immunology 58:515-522. [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnaswami, S. K., and F. J. Post. 1968. Effect of chlorine on Ascaris (Nematoda) eggs. Health Lab. Sci. 5:225-232. [PubMed] [Google Scholar]
- 21.Lauderdale, T.-L., K. C. Chapin, and P. R. Murray. 1999. Reagents, p. 1670. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology. American Society for Microbiology, Washington, D.C.
- 22.Loge, F. J., R. W. Emerick, D. E. Thompson, D. C. Nelson, and J. L. Darby. 1999. Factors influencing ultraviolet disinfection performance. Part I. Light penetration to wastewater particles. Water Environ. Res. 71:377-381. [Google Scholar]
- 23.Masschelein, W. J. 2002. Ultraviolet light in water and wastewater sanitation. CRC Press LLC, Boca Raton, FL.
- 24.Meng, Q. S., and C. P. Gerba. 1996. Comparative inactivation of enteric adenovirus, poliovirus, and coliphages by ultraviolet irradiation. Water Res. 30:2665-2668. [Google Scholar]
- 25.Mofidi, A. A., E. A. Meyer, P. M. Wallis, C. I. Chou, B. P. Meyer, S. Ramalingam, and B. M. Coffey. 2002. The effect of UV light on the inactivation of Giardia lamblia and Giardia muris cysts as determined by animal infectivity assay (P-2951-01). Water Res. 36:2098-2108. [DOI] [PubMed] [Google Scholar]
- 26.Muller, F., and H. Tobler. 1999. Chromatin diminution in the parasitic nematodes Ascaris suum and Parascaris univalens. Int. J. Parasitol. 30:391-399. [DOI] [PubMed] [Google Scholar]
- 27.Murrell, D., L. Eriksen, P. Nansen, H. C. Slotved, and T. Rasmussen. 1997. Ascaris suum: a revision of its early migratory path and implications for human ascariasis. J. Parasitol. 83:255-260. [PubMed] [Google Scholar]
- 28.National Water Research Institute. 2003. Ultraviolet disinfection guidelines for drinking water and water reuse, 2nd ed. National Water Research Institute and AWWA Research Foundation, Fountain Valley, CA.
- 29.National Water Research Institute and AWWA Research Foundation. 2000. Ultraviolet disinfection: guidelines for drinking water and water reuse. National Water Research Institute and AWWA Research Foundation, Fountain Valley, CA.
- 30.NSF Joint Committee on Drinking Water Treatment Units. 2002. NSF international standard/American national standard for drinking water treatment units—ultraviolet microbiological water treatment systems, NSF/ANSI 55-2002. National Sanitation Foundation International, Ann Arbor, MI.
- 31.Oksanen, A., L. Eriksen, A. Roepstorff, B. Ilsøe, P. Nansen, and P. Lind. 1990. Embryonation and infectivity of Ascaris suum eggs. A comparison of eggs collected from worm uteri with eggs isolated from pig faeces. Acta Vet. Scand. 31:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Lorcain, P., and C. V. Holland. 2000. The public health importance of Ascaris lumbricoides. Parasitology 121:S51-S71. [DOI] [PubMed] [Google Scholar]
- 33.Rochelle, P. A., M. M. Marshall, J. R. Mead, A. M. Johnson, D. G. Korich, J. Rosen, and R. De Leon. 2002. Comparison of in vitro cell culture and a mouse assay for measuring infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 68:3809-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roepstorff, A., L. Eriksen, H. C. Slotved, and P. Nansen. 1997. Experimental Ascaris suum infection in the pig: worm population kinetics following single inoculations with three doses of infective eggs. Parasitology 115:443-452. [DOI] [PubMed] [Google Scholar]
- 35.Roussell, D., M. Gruidl, and K. Bennett. 1994. Germ-line determination in Caenorhabditis and Ascaris—will a helicase begin to unravel the mystery. Parasitol. Today 10:110-113. [DOI] [PubMed] [Google Scholar]
- 36.Shin, G. A., K. G. Linden, and M. D. Sobsey. 2005. Low pressure ultraviolet inactivation of pathogenic enteric viruses and bacteriophages. J. Environ. Eng. Sci. 4:S7-S11. [Google Scholar]
- 37.Sinniah, B. 1982. Daily egg production of Ascaris lumbricoides: the distribution of eggs in the faeces and the variability of egg counts. Parasitology 84:167-175. [DOI] [PubMed] [Google Scholar]
- 38.Slifko, T. R., D. E. Huffman, K. Salisbury, and J. B. Rose. 2002. Comparison of tissue culture and animal models for assessment of Cryptosporidium parvum infection. Exp. Parasitol. 101:97-106. [DOI] [PubMed] [Google Scholar]
- 39.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tromba, F. G. 1978. Effect of ultraviolet radiation on the infective stages of Ascaris suum and Stephanurus dentatus with a comparison of the relative susceptibilities of some parasitic nematodes to ultraviolet. J. Parasitol. 64:245-252. [PubMed] [Google Scholar]
- 41.U.S. Environmental Protection Agency. 1999. Control of pathogens and vector attraction in sewage sludge. EPA/625/R-92/013 (revised edition). U.S. Environmental Protection Agency, Washington, D.C.
- 42.U.S. Environmental Protection Agency. 2003. Ultraviolet disinfection guidance manual, proposal draft EPA 815-D-03-007. U.S Environmental Protection Agency, Washington, D.C.
- 43.Wharton, D. 1980. Nematode egg-shells. Parasitology 81:447-463. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 1989. Health guidelines for the use of wastewater in agriculture and aquaculture. Report of a WHO scientific group. WHO Tech. Rep. Ser. 778:1-74. [PubMed] [Google Scholar]
- 45.Zhu, X., N. B. Chilton, D. E. Jacobs, J. Boes, and R. B. Gasser. 1999. Characterization of Ascaris from human and pig hosts by nuclear ribosomal DNA sequences. Int. J. Parasitol. 29:469-478. [DOI] [PubMed] [Google Scholar]