Abstract
rRNA internal transcribed spacer phylogeny showed that Chesapeake Bay is populated with diverse Synechococcus strains, including members of the poorly studied marine cluster B. Marine cluster B prevailed in the upper bay, while marine cluster A was common in the lower bay. Interestingly, marine cluster B Synechococcus included phycocyanin- and phycoerythrin-rich strains.
Phototrophic picoplankton (<3 μm) play an important role in the ocean's carbon cycle (18, 22, 33, 34). Synechococcus strains, which are small (1- to 3-μm) unicellular cyanobacteria, are a major component of marine picophytoplankton (32). New ecotypes and genotypes continue to emerge as the diversity of Synechococcus from different ecosystems is explored (3, 4, 6-10, 12-14, 20, 21, 24, 25, 30, 31), but in general, less is known about Synechococcus living in coastal and estuarine regions than about that in offshore regions (26). In Chesapeake Bay, picophytoplankton contribute 10 to 20% of total primary production during summer (1, 19, 23). Picophytoplankton can reach levels of 106 cells/ml and account for 56% of primary production in the lower bay (2). Recently, picocyanobacterial strains isolated from the bay were found to be dominated by marine Synechococcus (6), but knowledge about the diversity and distribution of picocyanobacterial populations in different Chesapeake habitats remains unclear.
Marine Synechococcus strains have been classified into three major clusters, i.e., marine clusters A, B, and C (MC-A, MC-B, and MC-C) (32). The MC-A cluster contains diverse Synechococcus strains isolated from coastal and open oceans, and its classification is supported by 16S rRNA and internal transcribed spacer (ITS) phylogeny (17, 19, 24). The MC-C cluster contains four closely related marine Synechococcus strains (11). In contrast to MC-A and MC-C, the phylogenetic position of MC-B is less understood.
Chesapeake Bay, the largest estuary in the United States, provides strong hydrological gradients and diverse habitats for picophytoplankton. In this study, we investigated the population structure of picocyanobacteria in Chesapeake Bay, based on the ITS sequences of isolates and environmental clones of picocyanobacteria.
Isolation and cultivation of Chesapeake Bay Synechococcus strains were as previously described (6). Water samples for DNA (2-m depth) were collected from three Chesapeake Bay stations (Table 1), using Niskin bottles, on board the R/V Cape Henlopen on 26 to 30 September 2002 and 4 to 8 March 2003. To concentrate microbial cells, 250 ml of water was filtered through 0.2-μm-pore-size filters (15). Nucleic acids from isolates and microbial communities were extracted using a method described elsewhere (27). ITS fragments of Synechococcus isolates were amplified as described by Rocap et al. (25). Clone libraries containing a large portion of the rRNA operon (16S rRNA-ITS-23S rRNA) from bacterioplankton within six surface water samples were constructed as previously described (28) with the following changes: (i) platinum HIFI polymerase mix (Invitrogen, Carlsbad, CA) was used to provide hot-start amplification, (ii) PCR products were A tailed using the QIAGEN A addition kit (QIAGEN, Chattsworth, CA), and (iii) products were cloned using the TOPO TA cloning kits for sequencing (Invitrogen). A minimum of 82 clones from each library were screened by a novel screening method adapted from the ITS-length heterogeneity-PCR method, which measures the length variation of two fragments amplified by PCR with fluorescence-labeled primers (29). Clones were screened based on the lengths of two regions of the ITS (SSU1406-tRNAala and SSU1406-LSU66). Representative clones were also sequenced to confirm the prescreening results. Plasmids were purified using the FastPlamid (Eppendorf, Westbury, NY) and Montage Miniprep96 (Millipore, Billerica, MA) kits. Sequencing was performed on an ABI Prism 3100 genetic analyzer using Big Dye V3.1 chemistry (Applied Biosystems, Foster City, CA). Phylogenetic analyses were conducted using the MacVector 7.2 program (Accelrys Software Inc., San Diego, CA) and the Molecular Evolutionary Genetics Analysis software, MEGA 3.1 (16).
TABLE 1.
Characteristics of three rrn operon clone libraries constructed from water samples collected in the upper, middle, and lower Chesapeake Bay in 2002a
| Characteristic | Value for clone library
|
||
|---|---|---|---|
| CB01 (upper bay) | CB11 (mid-bay) | CB22 (lower bay) | |
| Station name | 908 | 818 | 707 |
| Location | 39.0800N, 76.2000W | 38.1800N, 76.1700W | 37.0700N, 76.0700W |
| Water temp (°C) | 23.3 | 23.9 | 24.2 |
| Salinity (ppt) | 15.5 | 19.4 | 27 |
| Bacterial count (106 cells ml−1) | 6.42 | 2.91 | 2.57 |
| Synechococcus count (106 cells ml−1) | 0.23 | 0.29 | 0.36 |
| Synechococcus in total bacteria (%) | 3.58 | 9.97 | 14.01 |
| PC type in total Synechococcus (%) | 86.7 | 47.8 | 18.4 |
| Total clones | 91 | 84 | 88 |
| No. (%) of cyanobacterial clones | 4 (4.4) | 12 (14.3) | 7 (8.0) |
| Prescreening size (bp) by FAM/HEXb of: | |||
| 943/458 | CB22A09 | ||
| 954/464 | CB11C11, CB11D02 | ||
| 964/457 | CB11B02, CB11E03, CB11H03 | CB22A07 | |
| 974/464 | CB01C11, CB01E02, CB01C12 | CB11C04, CB11D06 | |
| 996/460 | CB11F09, CB11H07 | CB22D04, CB22G11 | |
| 1,007/460 | CB11D12, CB11G04 | CB22H05 | |
| 1,056/486 | CB01F08 | CB22C09 | |
| 1,123/487 | CB11G10 | ||
Twenty-three of 263 clones were identified as cyanobacteria based on prescreening of ITS length. Relevant physical, chemical, and biological characteristics at these stations are shown.
See reference 29 for an explanation of FAM/HEX.
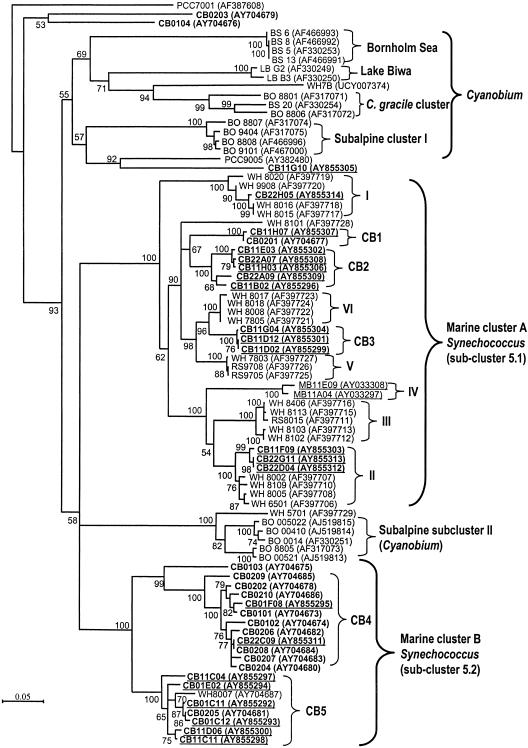
Phylogenetic analysis of 82 picocyanobacterial ITS sequences (Fig. 1) included picocyanobacteria from freshwater lakes, brackish or estuarine waters, and coastal and oceanic waters. The majority of Chesapeake Bay cyanobacterial ITS sequences were affiliated with either MC-A or MC-B. Eleven of 14 Chesapeake isolates clustered with WH8007 (MC-B cluster). Among 22 environmental clones putatively identified as cyanobacteria, 13 clustered within MC-A, 9 clustered within MC-B, and 1 clone (CB11G10) formed a deep branch within the Cyanobium cluster. The discrepancy in MC-A distribution between culture and culture-independent methods is likely due to the salinity of media used for isolation (10 to 20 ppt) favoring the growth of estuarine MC-B rather than MC-A strains. MC-B strains are known to have an elevated salt requirement for growth. Regardless, both approaches confirmed that freshwater Synechococcus strains are rare in the bay, even in the upper bay where the salinity is in the range of 5 to 10 ppt. At least 16 subclusters (>95% sequence identity; bootstrap value, 100) could be identified across all the picocyanobacteria included in this study (Fig. 1). Eleven subclusters overlap with previously reported subclusters (8, 25), while at least four new subclusters (CB1 to CB4) were novel and unique to the Chesapeake Bay.
FIG. 1.
Neighbor-joining tree based on ITS sequences (786 positions) of picocyanobacterial isolates (58 sequences, not underlined) and environmental clones (24 sequences, underlined) collected from lakes and from brackish, estuarine, coastal, and oceanic waters. The tree was rooted with PCC7001. Numbers at tree branches indicated the bootstrap values from 1,000 resamplings. Bootstrap values of less than 50 are not shown. The scale bar is equivalent to 0.05 substitution per site. Names in boldface represent the sequences from this study. Prefixes for the Synechococcus strains or environmental clones are as follows: CB, Chesapeake Bay; WH, Woods Hole; RS, Red Sea; MB, Monterey Bay; LB, Lake Biwa; BO, Lake Constance (Bodensee); and BS, Baltic Sea. The strains with the PCC prefix are the picocyanobacterial isolates collected by the Pasteur Culture Collection. The GenBank accession numbers are given in parentheses.
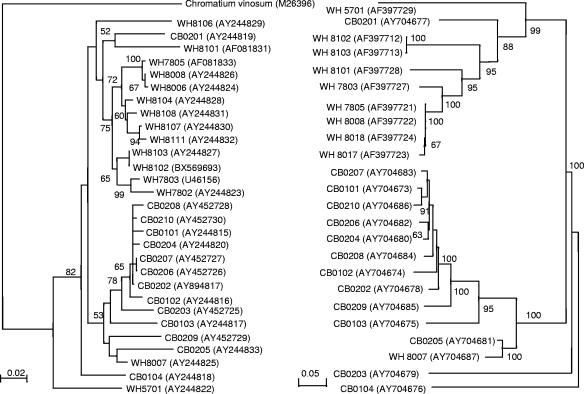
MC-B is a polyphyletic group containing both phycocyanin- and phycoerythrin-rich Synechococcus strains. At least two subclusters (CB4 and CB5) could be defined within the MC-B cluster. Within subcluster CB4, five phycoerythrin-rich Synechococcus strains (CB0206, CB0207, CB0208, CB0209, and CB0210) were closely related to four phycocyanin-rich Synechococcus strains (CB0101, CB0102, CB0202, and CB0204) (Fig. 1). A close relationship between phycocyanin- and phycoerythrin-rich Synechococcus strains in MC-B was also evident based on rbcL phylogeny (Fig. 2). The separation of MC-A and MC-B was also supported by rbcL phylogeny (Fig. 2).
FIG. 2.
Comparison of phylogenetic trees constructed from rbcL gene (left panel) and ITS (right panel) sequences from Chesapeake Bay and Woods Hole isolates. Bootstrap values were calculated based on 1,000 resamplings. Values lower than 50 are not shown. The scale bar is equivalent to 0.02 substitution per site for the rbcL phylogenetic tree and to 0.05 substitution per site for the ITS phylogeny.
Among six rRNA operon clone libraries, only three, constructed from the September samples, contained cyanobacterial sequences. The absence of cyanobacteria in the March clone libraries reflects a low abundance (typically, <103 cells/ml) of picocyanobacteria in the cold season. In the September clone libraries, all four clones from the upper bay were MC-B members, while only one of seven clones in the lower bay was an MC-B member. The mid-bay contained a mixture of both MC-A and MC-B members (Fig. 1 and Table 1). Despite the wide range of salinity along the bay, marine Synechococcus (MC-A and MC-B), not Cyanobium, dominated the Chesapeake picocyanobacterial community.
The ITS length among Chesapeake picocyanobacterial isolates and environmental clones varied widely, from 753 to 875 nucleotides and 606 to 913 nucleotides, respectively (see Table S1 in the supplemental material). The length heterogeneity of ITS is sufficient to differentiate various Synchococcus strains. Interoperon variation is not a concern for Synechococcus, which contains two identical rRNA operons (5). The average percent G+C of the ITS sequence for MC-B isolates and clones is 48.8% ± 2.5% (n = 19), which is lower than that for Cyanobium gracile PCC6307 (54%) and higher than those for Prochlorococcus (38.6% ± 2.0%) (25) and MC-A isolates and clones (44.1% ± 1.3%, n = 21) (see Table S1 in the supplemental material). Based on the ITS phylogeny and GC content, we suggest that WH8007, rather than WH5701 (10, 31), should be the reference strain for MC-B (or Synechococcus cluster 5.2 [11]).
Nucleotide sequence accession numbers.
GenBank accession numbers are shown in Fig. 1.
Supplementary Material
Acknowledgments
We thank the crew of the R/V Cape Henlopen.
This research was supported by National Science Foundation grants MCB-0132070 to K.E.W., F.C., and D. W. Coats; MCB-0238515 to R. Hill, F.C., and C. Fuqua; and MCB-0537041 to F.C.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Contribution no. 05-139 from the Center of Marine Biotechnology, University of Maryland Biotechnology Institute.
REFERENCES
- 1.Affronti, L. F., Jr., and H. G. Marshall. 1993. Diel abundance and productivity patterns of autotrophic picoplankton in the lower Chesapeake Bay. J. Plankton Res. 15:1-8. [Google Scholar]
- 2.Affronti, L. F., Jr., and H. G. Marshall. 1994. Using frequency of dividing cells in estimating autotrophic picoplankton growth and productivity in the Chesapeake Bay. Hydrobiologia 284:193-203. [Google Scholar]
- 3.Becker, S., M. Fahrbach, and A. Ernst. 2002. Quantitative tracing, by Taq nuclease assays, of a Synechococcus ecotype in a highly diversified natural population. Appl. Environ. Microbiol. 68:4486-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, S., A. K. Singh, C. Postius, P. Böger, and A. Ernst. 2004. Genetic diversity and distribution of periphytic Synechococcus spp. in biofilms and picoplankton of Lake Constance. FEMS Microb. Ecol. 49:181-190. [DOI] [PubMed] [Google Scholar]
- 5.Boyer, S. L., V. R. Flechtner, and J. R. Johansen. 2001. Is the 16S-23S rRNA internal transcribed spacer region a good tool for use in molecular systematics and population genetics? A case study in cyanobacteria. Mol. Biol. Evol. 18:1057-1069. [DOI] [PubMed] [Google Scholar]
- 6.Chen, F., K. Wang, J. Kan, D. S. Bachoon, J. Lu, S. Lau, and L. Campbell. 2004. Phylogenetic diversity of Synechococcus in the Chesapeake Bay revealed by ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCO) large subunit gene (rbcL) sequences. Aquat. Microb. Ecol. 36:153-164. [Google Scholar]
- 7.Crosbie, N. D., M. Pöckl, and T. Weisse. 2003. Dispersal and phylogenetic diversity of nonmarine picocyanobacteria, inferred from 16S rRNA gene and cpcBA-intergenic spacer sequence analyses. Appl. Environ. Microbiol. 69:5716-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, A., S. Becker, U. I. A. Wollenzien, and C. Postius. 2003. Ecosystem-dependent adaptive radiations of picocyanobacteria inferred from 16S rRNA and ITS-1 sequence analysis. Microbiology 149:217-228. [DOI] [PubMed] [Google Scholar]
- 9.Ferris, M. J., M. Kuhl, A. Wieland, and D. M. Ward. 2003. Cyanobacterial ecotypes in different optical microenvironments of a 68°C hot spring mat community revealed by 16-23S rRNA internal transcribed spacer region variation. Appl. Environ. Microbiol. 69:2893-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuller, N. J., M. D. Marie, F. Partensky, D. Vaulot, A. F. Post, and D. J. Scanlan. 2003. Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl. Environ. Microbiol. 69:2430-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herdman, M., R. W. Castenholz, I. Iteman, J. B. Waterbury, and R. Rippka. 2001. Subsection I (formerly Chroococcales Wettstein 1924, emend. Rippka, Deruelles, Waterbury, Herdman and Stanier 1979), p. 493-514. In D. R. Boone, R. W. Castenholz, and G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer Publishers, New York, N.Y. [Google Scholar]
- 12.Honda, D., A. Yokota, and J. Sugiyama. 1999. Detection of seven major evolutionary lineages in cyanobacteria based on the 16S rRNA gene sequence analysis with new sequences of five marine Synechococcus strains. J. Mol. Evol. 48:723-739. [DOI] [PubMed] [Google Scholar]
- 13.Iteman, I., R. Rippka, N. Tandeau de. Marsac, and M. Herdman. 2000. Comparison of conserved structural and regulatory domains within divergent 16S rRNA-23S rRNA spacer sequences of cyanobacteria. Microbiology 146:1275-1286. [DOI] [PubMed] [Google Scholar]
- 14.Janse, I., M. Meima, W. Edwin, A. Karkinaal, and G. Zwart. 2003. High-resolution differentiation of cyanobacteria by using rRNA-internal transcribed spacer denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 69:6634-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kan, J., K. Wang, and F. Chen. Temporal variation and detection limit of an estuarine bacterioplankton community analyzed by denaturing gradient gel electrophoresis (DGGE). Aquat. Microb. Ecol., in press.
- 16.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinformatics 5:150-163. [DOI] [PubMed] [Google Scholar]
- 17.Laloui, W., K. A. Palinska, R. Rippka, F. Partensky, N. Tandeau de Marsac, M. Herdman, and I. Iteman. 2002. Genotyping of axenic and non-axenic isolates of the genus Prochlorococcus and the OMF-‘Synechococcus’ clade by size, sequence analysis or RFLP of the internal transcribed spacer of the ribosomal operon. Microbiology 148:453-465. [DOI] [PubMed] [Google Scholar]
- 18.Li, W. K. W., D. V. Subba Rao, W. G. Harrison, J. C. Smith, J. J. Cullen, B. Irwin, and T. Platt. 1983. Autotrophic picoplankton in the tropical ocean. Science 219:292-295. [DOI] [PubMed] [Google Scholar]
- 19.Malone, T. C., H. W. Ducklow, E. R. Peele, and S. E. Pike. 1991. Picoplankton carbon flux in Chesapeake Bay. Mar. Ecol. Prog. Ser. 78:11-22. [Google Scholar]
- 20.Moore, L. R., G. Rocap, and S. W. Chisholm. 1998. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature 393:464-467. [DOI] [PubMed] [Google Scholar]
- 21.Palenik, B. 2001. Chromatic adaptation in marine Synechococcus strains. Appl. Environ. Microbiol. 67:991-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platt, T., D. V. S. Rao, and B. Irwin. 1983. Photosynthesis of picoplankton in the oligotrophic ocean. Nature 301:702-704. [Google Scholar]
- 23.Ray, R. T., L. W. Haas, and M. E. Sieracki. 1989. Autotrophic picoplankton dynamics in a Chesapeake Bay sub-estuary. Mar. Ecol. Prog. Ser. 52:273-285. [Google Scholar]
- 24.Robertson, B. R., N. Tezuka, and M. M. Watanabe. 2001. Phylogenetic analyses of Synechococcus strains (cyanobacteria) using sequences of 16S rDNA and part of the phycocyanin operon reveal multiple evolutionary lines and reflect phycobilin content. Int. J. Syst. Evol. Microbiol. 51:861-871. [DOI] [PubMed] [Google Scholar]
- 25.Rocap, G., D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 2002. Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl. Environ. Microbiol. 68:1180-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scanlan, D. J., and N. J. West. 2002. Molecular ecology of the marine cyanobacterial genera Prochlorococcus and Synechococcus. FEMS Microbiol. Ecol. 40:1-12. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt, T. M., E. F. DeLong, and N. R. Pace. 1991. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 173:4371-43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki, M. T., O. Beja, L. T. Taylor, and E. F. Delong. 2001. Phylogenetic analysis of ribosomal RNA operons from uncultivated coastal marine bacterioplankton. Environ. Microbiol. 3:323-331. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki, M. T., C. M. Preston, O. Beja, J. R. de la Torre, G. F. Steward, and E. F. DeLong. 2004. Phylogenetic screening of ribosomal RNA gene-containing clones in bacterial artificial chromosome (BAC) libraries from different depths in Monterey Bay. Microb. Ecol. 48:473-488. [DOI] [PubMed] [Google Scholar]
- 30.Toledo, G., B. Palenik, and B. Brahamsha. 1999. Swimming marine Synechococcus strains with widely different photosynthetic pigment ratios form a monophyletic group. Appl. Environ. Microbiol. 65:5247-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbach, E., D. J. Scanlan, D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 1998. Rapid diversification of marine picophytoplankton with dissimilar light-harvesting structures inferred from sequences of Prochlorococcus and Synechococcus (cyanobacteria). J. Mol. Evol. 46:188-201. [DOI] [PubMed] [Google Scholar]
- 32.Waterbury, J. B., and R. Rippka. 1989. The order Chroococcales, p. 1728-1746. In N. R. Krieg and J. B. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 3. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 33.Waterbury, J. B., S. W. Watson, R. R. L. Guillard, and L. E. Brand. 1979. Widespread occurrence of a unicellular, marine, planktonic cyanobacterium. Nature 277:293-294. [Google Scholar]
- 34.Waterbury, J. B., S. W. Watson, F. W. Valois, and D. G. Franks. 1986. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can. Bull. Fish. Aquat. Sci. 214:71-120. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.