Abstract
Biofilms consist of groups of bacteria attached to surfaces and encased in a hydrated polymeric matrix. Bacteria in biofilms are more resistant to the immune system and to antibiotics than their free-living planktonic counterparts. Thus, biofilm-related infections are persistent and often show recurrent symptoms. The metal chelator EDTA is known to have activity against biofilms of gram-positive bacteria such as Staphylococcus aureus. EDTA can also kill planktonic cells of Proteobacteria like Pseudomonas aeruginosa. In this study we demonstrate that EDTA is a potent P. aeruginosa biofilm disrupter. In Tris buffer, EDTA treatment of P. aeruginosa biofilms results in 1,000-fold greater killing than treatment with the P. aeruginosa antibiotic gentamicin. Furthermore, a combination of EDTA and gentamicin results in complete killing of biofilm cells. P. aeruginosa biofilms can form structured mushroom-like entities when grown under flow on a glass surface. Time lapse confocal scanning laser microscopy shows that EDTA causes a dispersal of P. aeruginosa cells from biofilms and killing of biofilm cells within the mushroom-like structures. An examination of the influence of several divalent cations on the antibiofilm activity of EDTA indicates that magnesium, calcium, and iron protect P. aeruginosa biofilms against EDTA treatment. Our results are consistent with a mechanism whereby EDTA causes detachment and killing of biofilm cells.
Biofilms consist of groups of bacteria attached to surfaces and encased in a hydrated polymeric matrix. Bacterial biofilms are abundant in the environment and are involved in several human bacterial infections (reviewed in references 11, 14, and 31). Of medical importance, biofilms can withstand host immune responses (19-21) and are much more resistant to antibiotic treatments than their nonattached, individual, free-living (planktonic) counterparts (28, 36). For these reasons, biofilm infections are persistent, and individuals often show recurring symptoms following antibiotic therapy. One of the best-studied models for biofilm formation is the bacterium Pseudomonas aeruginosa (reviewed in references 27 and 30), which causes many types of infections, including biofilm-associated chronic lung infections in cystic fibrosis patients, acute ulcerative keratitis in users of extended-wear soft contact lenses, and bacteremia in severe-burn victims.
The metal chelator EDTA has been shown to cause lysis, loss of viability, and increased sensitivity of planktonic Proteobacteria to a variety of antibacterial agents (reference 13; reviewed in references 25, 29, and 40). This has led to the use of EDTA as a preservative in many products. Little is known about the influence of EDTA on biofilms of Proteobacteria. Raad et al. (32, 33) have shown that EDTA combined with minocycline is an effective treatment for microorganisms embedded in biofilms on catheter surfaces. Their studies focused on Staphylococcus epidermidis, Staphylococcus aureus, and Candida albicans; however, they also reported two cases of P. aeruginosa-infected catheters where the EDTA-minocycline treatment caused a large decrease in the number of viable biofilm cells (32). Recently, Kite et al. (23) reported that tetrasodium EDTA could be used to eradicate biofilms on catheters. Ayres et al. (3) have examined the effects of permeabilizing agents on antibacterial activity against a P. aeruginosa biofilm grown on a metal disk. Their results further suggest increased anti-P. aeruginosa biofilm activity for several antibiotics when combined with EDTA (3).
We have further characterized the activity of EDTA against P. aeruginosa biofilms. We show that EDTA treatment of Pseudomonas biofilms results in 1,000-fold greater killing than treatment with gentamicin, an antibiotic commonly used to treat P. aeruginosa infections. Furthermore, a combination of EDTA and gentamicin can result in eradication of P. aeruginosa in our model biofilms. We present evidence that, in addition to killing, EDTA causes a rapid dispersion of P. aeruginosa cells from biofilms. Our data suggest that magnesium, calcium, and iron are involved in P. aeruginosa biofilm maintenance.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
We used P. aeruginosa PAO1 (17). For the flow cell experiments, we used PAO1 containing pMRP9-1. The strain constitutively expresses green fluorescent protein (GFP) when carrying this plasmid (12). Both flow cell and disk reactor biofilms were grown in 1% tryptic soy broth (TSB) (Becton Dickinson, Sparks, MD). All cultures were incubated at 37°C unless otherwise indicated.
Disk reactor biofilm experiments.
The rotating disk reactor was similar to that described previously (16). Reactors were inoculated with stationary-phase cultures (1%, vol/vol). After overnight growth, a flow of fresh medium was initiated (dilution rate, 0.7 h−1). After 24 h in a flow of medium, the polycarbonate chips with attached biofilm bacteria were removed from the spinning disk and washed three times in phosphate-buffered saline (PBS). We assessed the resistance of biofilm cells to EDTA or antibiotics as follows. Washed biofilms were incubated in either 1 ml of PBS (pH 7.4) or 20 mM Tris buffer (pH 7.4). EDTA (0.1 to 50 mM), gentamicin (1, 10, and 50 μg/ml), or a combination of the two was added as indicated. The chips were incubated for 1 or 24 h in 24-well tissue culture plates (Falcon no. 353047; Becton Dickinson Labware, Franklin Lakes, NJ) as indicated. Cells that detached from the biofilm during the treatment were enumerated by plating on LB agar. To estimate the number of remaining attached biofilm cells, we placed the disks in 1 ml PBS and dispersed the cells by using a tissue homogenizer (Brinkmann Instruments, Westbury, NY). Total CFU were determined by dilution and plating on LB agar.
Flow cell biofilm experiments.
We used a flow cell system for microscope examination of biofilms (30). The flow cells were inoculated with a 1:50 dilution (in 1% TSB) of a P. aeruginosa stationary-phase culture, and flow was initiated after 1 h. The flow rate was 0.17 per min. After 6 days, biofilms were stained with propidium iodide (4 or 30 μM, as indicated; Sigma Chemical Co., St. Louis, MO). We used the lower propidium iodide concentration to counterstain the biofilm matrix (4, 42) and the higher concentration to detect nonviable cells within the biofilm (37). After propidium iodide staining, EDTA was added to the growth medium (final concentration, 50 mM) and flow was resumed. Detachment of cells from the biofilm was determined by plating dilutions of effluent collected at 10-min intervals. Total counts in the effluent were determined by using a phase-contrast microscope and a hemocytometer.
For imaging of biofilms we used confocal scanning laser microscopy (CSLM) as described elsewhere (42) except for the results presented in Fig. 2. For these experiments we used an LSM 510 confocal microscope (Carl Zeiss, Germany) instead of a Bio-Rad confocal system. The excitation wavelength used for GFP was 488 nm, and emission wavelengths at 515 ± 15 nm were collected. To detect propidium iodide fluorescence, excitation was at 488 nm and emission wavelengths of >660 nm were collected with a 660LP filter. For time course experiments, flow cells were incubated on the microscope stage and z-series images were acquired at 2.5-min intervals. The biofilms were exposed to the scanning laser for approximately 30 s at each interval. After acquisition, images were processed using Volocity (Improvision, Lexington, MA) software.
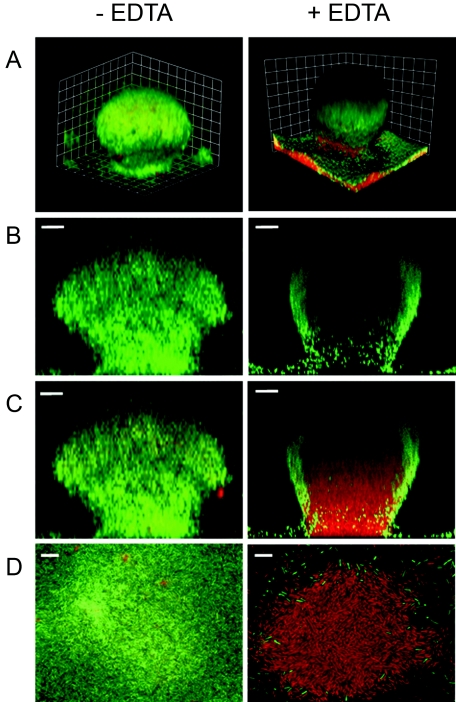
FIG. 2.
Effect of EDTA on P. aeruginosa biofilm structure. GFP-labeled P. aeruginosa biofilms were grown in flow cells for 6 days. Biofilms were grown at room temperature and treated with 50 mM EDTA for 2.5 h. The biofilm matrix and dead cells were counterstained with propidium iodide (30 μM) prior to EDTA treatment. (Left) Biofilm prior to EDTA treatment. (Right) Biofilm after EDTA treatment. The images were acquired by CSLM. (A) Three-dimensional reconstruction. The combined green (GFP) and red (propidium iodide) channels are presented. The squares are 15 μm on each side. (B) Sagittal views of the green channel only. (C) Sagittal view showing the combined red and green channels. Bars, 20 μm. (D) A 0.5-μm slice of the internal region of the biofilm. The combined red and green channels are presented. Bars, 10 μm.
RESULTS
EDTA enhances loss of P. aeruginosa biofilm-associated cells.
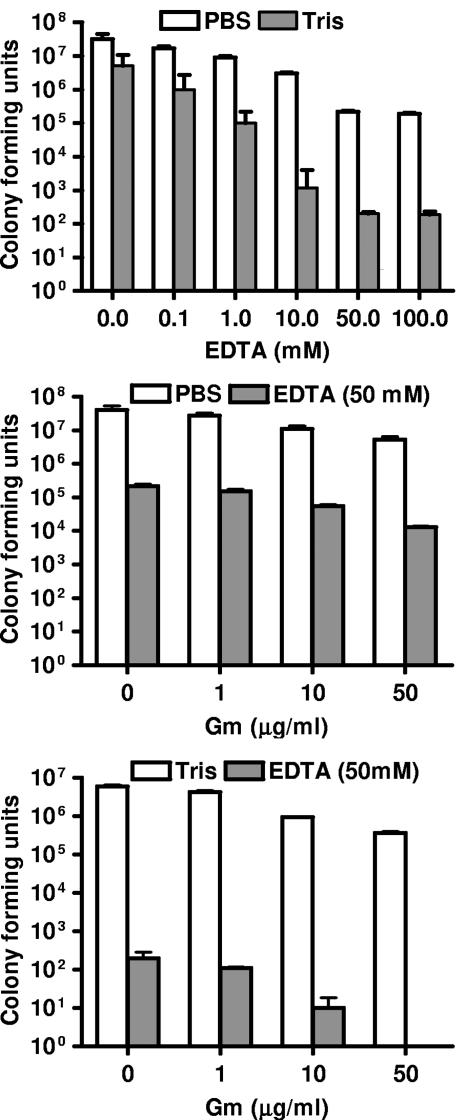
We examined the reduction in biofilm cells in response to EDTA treatment. Biofilms grown in a spinning disk reactor were exposed to various concentrations of EDTA, gentamicin, or both, and cell viability was measured (Fig. 1). In PBS, 50 mM EDTA reduced the number of biofilm-associated cells by >99%, while gentamicin (50 μg per ml, a concentration well over 10 times higher than the MIC for planktonic P. aeruginosa PAO1) caused a reduction of <10% in the number of biofilm cells. Furthermore, treatment with EDTA (50 mM) and gentamicin (50 μg per ml) together was more effective than EDTA alone (Fig. 1). Because Tris and EDTA can work synergistically to permeabilize planktonic Proteobacteria (25), we examined the effect of EDTA on P. aeruginosa biofilms in Tris buffer and found that all treatments were more effective in Tris buffer than in PBS (Fig. 1). In fact, the combination of EDTA (50 mM) and gentamicin (50 μg/ml) in Tris buffer completely eradicated biofilm-associated cells.
FIG. 1.
Treatment of P. aeruginosa biofilms with EDTA and gentamicin (Gm). Spinning disk reactor biofilms of P. aeruginosa PAO1 were treated for 24 h at 37°C. (Top) EDTA dose response in PBS, pH 7.4 (white), or 20 mM Tris buffer, pH 7.4 (gray). (Middle) PBS with the indicated concentrations of Gm with (gray) or without (white) 50 mM EDTA. (Bottom) Tris with the indicated concentrations of Gm with (gray) or without (white) 50 mM EDTA. Levels of viable bacteria remaining after treatment were determined by plating. Data are means from two disks from each of two spinning reactors. Error bars, standard deviations.
Effect of EDTA on P. aeruginosa biofilm structure.
To examine the effect EDTA has on P. aeruginosa biofilm architecture, we used a flow cell system. As expected, P. aeruginosa biofilms developed mushroom-like structures in our experiments (Fig. 2). Addition of 50 mM EDTA resulted in preferential killing of cells inside the mushroom-like structures. The structures remained intact, as indicated by a shell of green fluorescent cells around the edge. Dead cells stained with propidium iodide (red) within the shell (Fig. 2). We chose 50 mM EDTA based on previous experiments (Fig. 1), which indicate that this is close to the minimal EDTA concentration for maximal killing of P. aeruginosa.
EDTA induces dispersal of cells from biofilms.
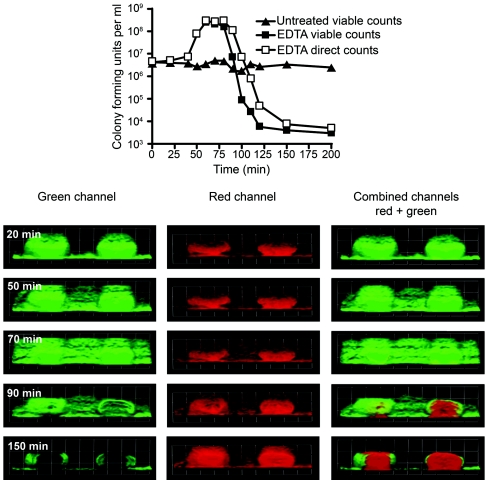
Imaging of bacteria in flow cell biofilms over time suggested that EDTA caused not only killing but also dispersal of cells from the biofilm. To further examine the EDTA effect, we collected time lapse images by CSLM (see movies S1 to S3 in the supplemental material), and we determined total and viable cell numbers in the flow cell effluent at 10-min intervals (Fig. 3). Whereas an untreated flow cell showed a constant level of viable, dispersed cells in the effluent, an increase in the number of cells in the effluent was detected 50 min after addition of EDTA to the medium reservoir, corresponding to the time at which EDTA reached the flow cell (Fig. 3, top). This increase in the number of cells in the effluent correlated with a dispersion of green fluorescence (i.e., cells) in the flow cell as observed by CSLM (Fig. 3, green channel, 50 to 90 min). After 90 min, the cell numbers in the effluent decreased. We believe this decrease is due to washout of detached cells from the flow cell and EDTA killing of cells, as can be seen by a difference of approximately 100-fold between the direct and viable counts (Fig. 3, top). The interior of the biofilm is affected at this time as dying cells lose their GFP and the propidium iodide staining is exposed. Here we used a low concentration of propidium iodide (4 μM versus 30 μM for the dead-cell staining) that stains the extracellular matrix but not P. aeruginosa cells (4, 37). With further incubation in the presence of EDTA, the decrease in cell numbers progresses and the internal regions of the mushroom-like biofilm structures become devoid of viable cells (Fig. 3, 150 min).
FIG. 3.
EDTA facilitates biofilm detachment and lysis. A GFP-labeled P. aeruginosa biofilm was grown in a flow cell for 6 days and then treated with 50 mM EDTA. The effluent was sampled at the indicated intervals. EDTA was added to the medium reservoir at time zero and reached the flow cell after 45 to 50 min. (Top) CFU and direct counts of the effluent cells. CFU from an untreated control biofilm effluent are shown for comparison. (Bottom) Time lapse microscope images taken at the indicated times during treatment with EDTA. The biofilm matrix was counterstained with 4 μM propidium iodide (red) prior to treatment. The green (GFP), red (propidium iodide), and combined channels are presented. The squares are 60 μm on each side.
Role of divalent cations in detachment and lysis of biofilms.
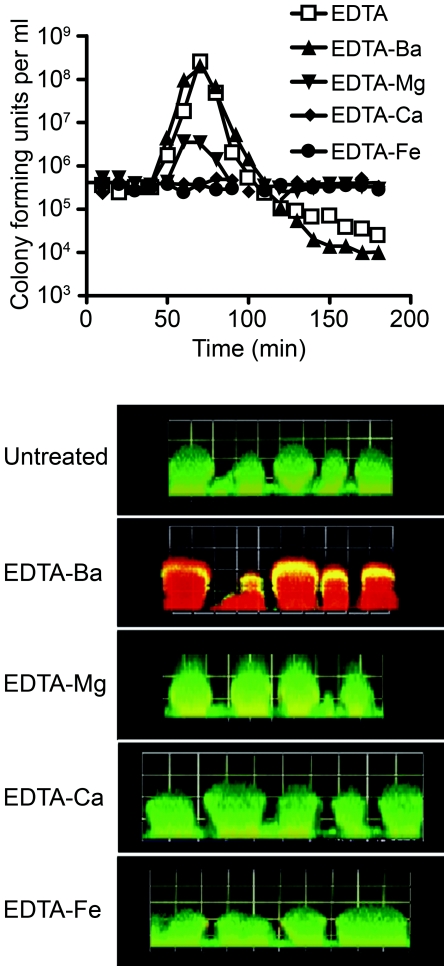
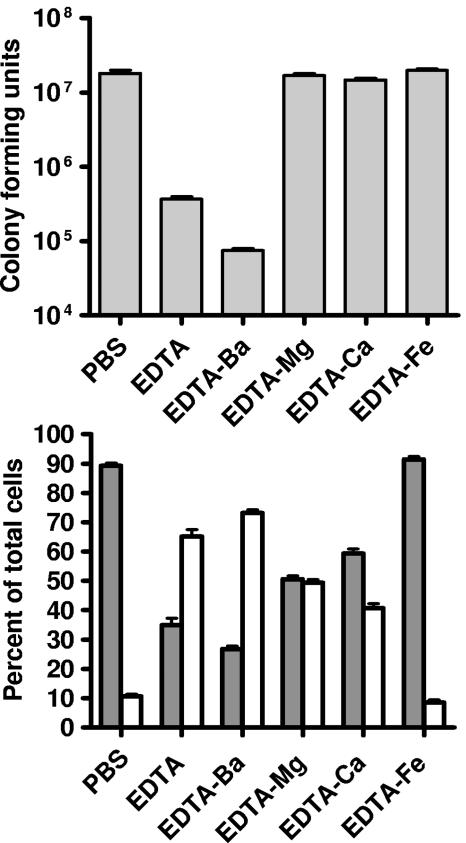
Previous work on EDTA-treated planktonic P. aeruginosa focused on cell lysis. Our results suggest that treatment of biofilms with EDTA facilitates two processes: cell detachment and killing (Fig. 2 and 3). To gain insight into which divalent cations are involved in cell detachment and lysis, we utilized the difference in the stability constant [log(Kc)] that EDTA has for various divalent cations (barium [Kc = 7.78], magnesium [Kc = 8.83], calcium [Kc = 10.61], and iron [Kc = 25.0]) (35). We reasoned that any EDTA effect blocked by the addition of a specific cation (at a concentration that will completely saturate EDTA) is due to the role that cation (or a cation for which EDTA has lower affinity) plays in stabilizing the biofilm. In flow cell experiments, the addition of barium had no effect on EDTA-mediated killing and detachment. Addition of magnesium appears to block killing (GFP is not lost in the internal region of the mushroom), but some detachment is evident (Fig. 4). When calcium or iron is added, killing and detachment are completely blocked (Fig. 4). Similar results were obtained in spinning disk reactor experiments (Fig. 5), although in this system addition of calcium led to some detachment (40% of the cells detached and were in the planktonic state [Fig. 5, bottom]). Detachment was effectively blocked by addition of iron. This suggests that both calcium and iron may be important in stabilizing biofilms.
FIG. 4.
Role of divalent cations in EDTA-mediated detachment and killing. GFP-labeled P. aeruginosa biofilms grown in flow cells were treated with EDTA saturated with the indicated divalent cations (50 mM). (Top) CFU in the effluent determined by plate counts. (Bottom) Microscope images taken 3 h after EDTA treatment. The biofilm matrix was counterstained with propidium iodide (4 μM) prior to treatment. The squares are 60 μm on each side.
FIG. 5.
Treatment of P. aeruginosa biofilms with EDTA and divalent cations. Spinning disk reactor biofilms of P. aeruginosa PAO1 were treated for 24 h at 37°C. (Top) Total (planktonic plus biofilm) viable counts. (Bottom) Percentage of viable cells present in the biofilm (gray) or the planktonic phase (white). Treatments were PBS alone or PBS plus EDTA (50 mM), EDTA-Ba (50 mM), EDTA-Mg (50 mM), EDTA-Ca (50 mM) or EDTA-Fe (50 mM). The viable cells, which remained attached to the disks after the treatment, were counted as biofilm cells. The viable planktonic cells were the detached cells in suspension in the treated wells. Data are means from two disks from each of two spinning reactors. Error bars, standard deviations.
DISCUSSION
EDTA has a detrimental effect on the outer membrane permeability of free-living planktonic Proteobacteria (15, 25, 29, 40). By chelating divalent cations from their binding sites in lipopolysaccharide (LPS), EDTA facilitates the release of a significant proportion of LPS from the cell (26). Although prolonged treatments with EDTA are lethal, short treatments increase the permeability of the outer membrane to hydrophobic molecules (25, 29). Thus, there can be synergy between EDTA and other antibacterial agents (2, 8, 24). In this study we report that EDTA not only kills P. aeruginosa planktonic cells but also affects P. aeruginosa biofilms (Fig. 1 and 2).
Exposure of P. aeruginosa biofilms to EDTA killed P. aeruginosa cells and triggered detachment of cells from biofilms (Fig. 3 to 5). CSLM revealed that the majority of the cell population affected by the EDTA treatment resides in the inner regions of the mushroom-like structures. This type of killing or detachment pattern has been observed in P. aeruginosa biofilms exposed to various conditions (6, 34, 41). We note that sloughing of cells from the outer regions of the biofilms might also contribute to the detachment process. Chen and Stewart (9) have previously tested the abilities of various chemical treatments to remove mixed P. aeruginosa-Klebsiella pneumoniae biofilms. They reported that EDTA treatment (10 mM) resulted in a 49% reduction in cell counts, and they presented some evidence that this was due to dispersal of biofilm bacteria. The authors hypothesized that calcium was important for stabilizing the biofilm matrix (9). Other studies have also suggested a role for calcium in stabilizing biofilms of bacteria (18, 22, 39).
To better understand how P. aeruginosa biofilms are affected by EDTA treatment, we examined the abilities of different divalent cations to block EDTA-induced detachment and killing. Barium addition did not block killing, but the addition of magnesium, calcium, or iron did (Fig. 4 and 5). The relative stability constants of EDTA for the divalent cations may be ranked in ascending order as follows: barium, magnesium, calcium, and iron. Thus, our data support previous conclusions that magnesium can block lysis of planktonic P. aeruginosa by EDTA (1, 7). EDTA is thought to chelate stabilizing magnesium ions from the LPS, causing release of LPS from the outer membrane (5, 26). Magnesium did not completely block EDTA-induced detachment, but the addition of either calcium or iron did (Fig. 4 and 5). Based on previous work, one might have anticipated an involvement of iron and calcium in biofilm maintenance. In P. aeruginosa, addition of calcium to growth media increased biofilm cohesiveness, resulting in decreased detachment (38). Turakhia et al. (39) demonstrated that addition of EGTA (a calcium-specific chelator) to a mixed aerobic sewage sludge biofilm resulted in immediate detachment of cells from the biofilm. We found similar EGTA effects on detachment from the biofilm, but killing was fivefold lower than that found with EDTA (data not shown). Chen and Stewart (10) have tested the viscosity of a mixed P. aeruginosa-Klebsiella pneumoniae biofilm suspension following addition of various cations. They report that addition of iron salts significantly increased biofilm viscosity. The authors concluded that electrostatic interactions contribute to biofilm cohesion and that iron cations are potent cross-linkers of the biofilm matrix (10).
The use of EDTA to treat biofilm-related infections is being evaluated by several groups, with promising results (23, 32, 33); however, little is known about how EDTA causes increased killing of biofilm cells. The results of this study suggest that the activity of EDTA against biofilm cells is mediated by chelation of several divalent cations that are required to stabilize the biofilm matrix. Future work will be required to determine their specific role in this process. Our results imply that EDTA chelation of magnesium, calcium, and iron can enhance detachment of cells from the biofilm. EDTA also facilitates the killing of biofilm cells by chelating magnesium associated with the LPS. This dispersal process and the increased cell permeability facilitated by EDTA may also explain the enhanced killing observed in combined EDTA and gentamicin treatment (Fig. 1). This combination may have therapeutic utility.
Supplementary Material
Acknowledgments
We thank Amy L. Schaefer for critical reading of the manuscript.
This work was supported by grants from the W. M. Keck Foundation and the National Institute of General Medical Sciences (GM59026) to E.P.G. E.B. was partially funded by the Fulbright program.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Ayres, H. M., J. R. Furr, and A. D. Russell. 1998. Effect of divalent cations on permeabilizer-induced lysozyme lysis of Pseudomonas aeruginosa. Lett. Appl. Microbiol. 27:372-374. [DOI] [PubMed] [Google Scholar]
- 2.Ayres, H. M., J. R. Furr, and A. D. Russell. 1999. Effect of permeabilizers on antibiotic sensitivity of Pseudomonas aeruginosa. Lett. Appl. Microbiol. 28:13-16. [DOI] [PubMed] [Google Scholar]
- 3.Ayres, H. M., D. N. Payne, J. R. Furr, and A. D. Russell. 1998. Effect of permeabilizing agents on antibacterial activity against a simple Pseudomonas aeruginosa biofilm. Lett. Appl. Microbiol. 27:79-82. [DOI] [PubMed] [Google Scholar]
- 4.Banin, E., M. L. Vasil, and E. P. Greenberg. 2005. Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA 102:11076-11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boggis, W., M. A. Kenward, and M. R. Brown. 1979. Effects of divalent metal cations in the growth medium upon sensitivity of batch-grown Pseudomonas aeruginosa to EDTA or polymyxin B. J. Appl. Bacteriol. 47:477-488. [DOI] [PubMed] [Google Scholar]
- 6.Boles, B. R., M. Thoendel, and P. K. Singh. 2005. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 57:1210-1223. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M. R., and J. Melling. 1968. Loss of sensitivity to EDTA by Pseudomonas aeruginosa grown under conditions of Mg-limitation. J. Gen. Microbiol. 54:439-444. [DOI] [PubMed] [Google Scholar]
- 8.Brown, M. R., and R. M. Richards. 1965. Effect of ethylenediamine tetraacetate on the resistance of Pseudomonas aeruginosa to antibacterial agents. Nature 207:1391-1393. [DOI] [PubMed] [Google Scholar]
- 9.Chen, X., and P. S. Stewart. 2000. Biofilm removal caused by chemical treatments. Water Res. 34:4229-4233. [Google Scholar]
- 10.Chen, X., and P. S. Stewart. 2002. Role of electrostatic interactions in cohesion of bacterial biofilms. Appl. Microbiol. Biotechnol. 59:718-720. [DOI] [PubMed] [Google Scholar]
- 11.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 12.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 13.Gray, G. W., and S. G. Wilkinson. 1965. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J. Gen. Microbiol. 39:385-399. [DOI] [PubMed] [Google Scholar]
- 14.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95-108. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, R. E. 1984. Alterations in outer membrane permeability. Annu. Rev. Microbiol. 38:237-264. [DOI] [PubMed] [Google Scholar]
- 16.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183:5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, J., and K. L. Pinder. 1995. Effect of calcium on development of anaerobic acidogenic biofilms. Biotechnol. Bioeng. 45:212-218. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, E. T., A. Kharazmi, P. Garred, G. Kronborg, A. Fomsgaard, T. E. Mollnes, and N. Hoiby. 1993. Complement activation by Pseudomonas aeruginosa biofilms. Microb. Pathog. 15:377-388. [DOI] [PubMed] [Google Scholar]
- 20.Jensen, E. T., A. Kharazmi, N. Hoiby, and J. W. Costerton. 1992. Some bacterial parameters influencing the neutrophil oxidative burst response to Pseudomonas aeruginosa biofilms. APMIS 100:727-733. [PubMed] [Google Scholar]
- 21.Jesaitis, A. J., M. J. Franklin, D. Berglund, M. Sasaki, C. I. Lord, J. B. Bleazard, J. E. Duffy, H. Beyenal, and Z. Lewandowski. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J. Immunol. 171:4329-4339. [DOI] [PubMed] [Google Scholar]
- 22.Kierek, K., and P. I. Watnick. 2003. The Vibrio cholerae O139 O-antigen polysaccharide is essential for Ca2+-dependent biofilm development in sea water. Proc. Natl. Acad. Sci. USA 100:14357-14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kite, P., K. Eastwood, S. Sugden, and S. L. Percival. 2004. Use of in vivo-generated biofilms from hemodialysis catheters to test the efficacy of a novel antimicrobial catheter lock for biofilm eradication in vitro. J. Clin. Microbiol. 42:3073-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert, R. J., G. W. Hanlon, and S. P. Denyer. 2004. The synergistic effect of EDTA/antimicrobial combinations on Pseudomonas aeruginosa. J. Appl. Microbiol. 96:244-253. [DOI] [PubMed] [Google Scholar]
- 25.Leive, L. 1974. The barrier function of the gram-negative envelope. Ann. N. Y. Acad. Sci. 235:109-129. [DOI] [PubMed] [Google Scholar]
- 26.Leive, L. 1965. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem. Biophys. Res. Commun. 21:290-296. [DOI] [PubMed] [Google Scholar]
- 27.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 28.Nickel, J. C., I. Ruseska, J. B. Wright, and J. W. Costerton. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsek, M. R., and E. P. Greenberg. 1999. Quorum sensing signals in development of Pseudomonas aeruginosa biofilms. Methods Enzymol. 310:43-55. [DOI] [PubMed] [Google Scholar]
- 31.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 32.Raad, I., I. Chatzinikolaou, G. Chaiban, H. Hanna, R. Hachem, T. Dvorak, G. Cook, and W. Costerton. 2003. In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob. Agents Chemother. 47:3580-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raad, I., R. Hachem, R. K. Tcholakian, and R. Sherertz. 2002. Efficacy of minocycline and EDTA lock solution in preventing catheter-related bacteremia, septic phlebitis, and endocarditis in rabbits. Antimicrob. Agents Chemother. 46:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skoog, A. D., D. M. West, and F. J. Holler. 1996. Fundamentals of analytical chemistry, 7th ed. Saunders College Publishing, Fort Worth, Tex.
- 36.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 37.Teitzel, G. M., and M. R. Parsek. 2003. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 69:2313- 2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turakhia, M. H., and W. G. Characklis. 1989. Activity of Pseudomonas aeruginosa in biofilms: effect of calcium. Biotechnol. Bioeng. 33:405-414. [DOI] [PubMed] [Google Scholar]
- 39.Turakhia, M. H., K. E. Cooksey, and W. G. Characklis. 1983. Influence of calcium-specific chelant on biofilm removal. Appl. Environ. Microbiol. 46:1236-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb, J. S., M. Lau, and S. Kjelleberg. 2004. Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 186:8066-8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarwood, J. M., D. J. Bartels, E. M. Volper, and E. P. Greenberg. 2004. Quorum sensing in Staphylococcus aureus biofilms. J. Bacteriol. 186:1838-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.