Abstract
Two LysR-type transcriptional regulators, BenM and CatM, control benzoate consumption by the soil bacterium Acinetobacter baylyi ADP1. These homologs play overlapping roles in the expression of multiple genes. This study focuses on the benABCDE operon, which initiates benzoate catabolism. At this locus, BenM and CatM each activate transcription in response to the catabolite cis,cis-muconate. BenM, but not CatM, additionally responds to benzoate as an effector. Regulation by CatM alone is insufficient for growth on benzoate as the sole carbon source. However, three point mutations independently increased CatM-activated benA transcription and enabled growth on benzoate without BenM. Two mutations generate variants with one amino acid change in the 303-residue CatM, CatM(V158M) and CatM(R156H). These substitutions affected regulation of benA differently than that of catB, another CatM-regulated gene involved in benzoate catabolism. In relation to CatM, CatM(V158M) increased cis,cis-muconate-dependent transcription of benA but decreased that of catB. CatM(R156H) increased effector-independent expression of catB compared to CatM. In contrast, cis,cis-muconate was required with CatM(R156H) to activate unusually high benA expression. Thus, induction by cis,cis-muconate depends on both the sequence of CatM and the promoter. A point mutation at position −40 of the benA promoter enhanced CatM-activated gene expression and altered regulation by CatM(R156H). BenM and CatM bound to the same locations on ben region DNA. The frequency with which spontaneous mutations allow CatM to substitute for BenM might predict that one regulator would be sufficient for controlling benzoate consumption. This prediction is discussed in light of current and previous studies of the BenM-CatM regulon.
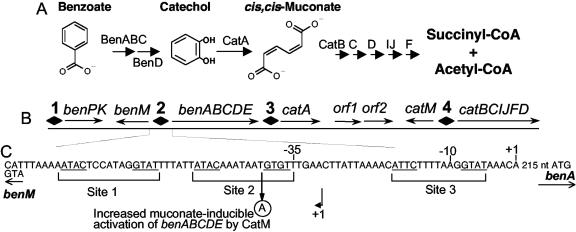
In the soil bacterium Acinetobacter baylyi ADP1 (formerly Acinetobacter sp. strain ADP1), BenM and CatM are homologous transcriptional regulators involved in aromatic compound degradation (Fig. 1) (8, 12, 29). These proteins are 59% identical in sequence, and both respond to a metabolite formed during benzoate consumption, cis,cis-muconate (referred to below as muconate) (Fig. 1A). However, unlike CatM, BenM also responds to benzoate as an effector. These two regulators jointly activate more than a dozen chromosomal ben and cat genes involved in benzoate catabolism (Fig. 1B). Furthermore, during growth with benzoate as a carbon and energy source, BenM and CatM repress genes needed to degrade alternative aromatic compounds (4).
FIG. 1.
BenM and CatM regulate benzoate degradation in ADP1. Catabolism depends on enzymes encoded by the ben and cat genes (A). These chromosomal genes are in an approximately 20 kbp cluster (B) (not drawn to scale). BenM and CatM regulate transcription initiation in four regions (diamonds 1 to 4). The functions of two open reading frames downstream of catA (orf1 and orf2) are unknown, but they are not expressed during growth on benzoate. In the intergenic benMA region (C), there are three potential binding sites for BenM and CatM, as described in the text. Site 1 exactly matches the consensus sequence (underlined) of LysR-type regulators within a subclass to which BenM and CatM belong (10, 31). The sequences in sites 2 and 3 differ from the consensus by a single nucleotide that reduces the extent of dyad symmetry. Above the DNA sequence, the transcription initiation site (+1) and promoter regions (−10 and −35) are shown for benA. The initiation site for the divergently transcribed benM is also indicated (+1 below an arrow). A point mutation (circled A) increases the ability of CatM to activate transcription of the benABCDE operon (12).
In mutants lacking catM or benM, there is little effect on the expression of some genes such as catA or benPK (8, 12). At these loci, one regulator compensates for the loss of the other. This redundancy raises questions about the need for both regulators. To understand the evolution and retention of the benM and catM paralogs, these studies focused on the benABCDE operon. At this locus the regulators exert markedly different effects. BenM represses benA transcription in the absence of its effectors (6, 12). In response to muconate or benzoate, BenM activates ben operon transcription. Furthermore, both effectors together cause a synergistic increase in BenM-activated transcription (6).
CatM activates benABCDE transcription in response to muconate but not benzoate (11, 12). As assessed with a benA::lacZ fusion in a benM mutant, CatM with muconate activates expression at a level 7-fold below that of BenM with muconate and 21-fold below that of BenM with both effectors (11, 12). Regulation by CatM is insufficient to support the growth of mutants lacking BenM on benzoate. However, CatM alone activates high-level transcription from the other promoters involved in benzoate consumption (benP, catA, catB) (Fig. 1). Therefore, mutations that increase ben operon expression permit benzoate to be consumed without BenM. For example, a point mutation in the −10 region of the benA promoter enables a benM mutant to grow on benzoate (12). Without inducers, this mutation increases benA expression to a level fourfold higher than that induced from the wild-type promoter by CatM with muconate (12).
Here, mutations that increase CatM-activated transcription from the benA promoter were studied in order to identify constraints that normally prevent CatM from serving as the sole regulator of benzoate catabolism in ADP1. In the absence of CatM, BenM permits benzoate to be consumed very slowly (29). However, regulation by BenM in a catM mutant causes abnormally high levels of muconate to accumulate (12, 15). This observation suggests limitations on the ability of BenM to activate transcription from the catB promoter. BenM and CatM are members of the LysR-type family, a large group of homologous regulators that control diverse functions (31). The ADP1 regulators belong to a subclass of this family involved in the catabolism of aromatic compounds and pollutants in numerous bacterial genera (16, 32). Thus, the BenM-CatM regulon may serve as a good model for understanding complex regulatory circuits involved in biodegradation.
This report describes a variant, CatM(V158M), that enables growth on benzoate without BenM. CatM(V158M) was compared to CatM(R156H), a variant that activates cat operon expression without muconate (23). Additionally, the interactions of CatM with the benA promoter region were compared to those of BenM at the same region. The importance of the DNA sequence was explored in further investigations of a point mutation in the benA promoter that increases CatM activation of benA. These experiments help elucidate the threshold level of benABCDE operon expression needed for growth on benzoate. Furthermore, they highlight the importance of balanced expression from multiple promoters within the pathway. The accumulation of toxic metabolites may be a key factor in the evolution of this regulatory scheme.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Acinetobacter strains (Table 1) were derived from ADP1, recently reclassified as an A. baylyi strain (33, 37). Bacteria were cultured in Luria-Bertani (LB) broth or minimal medium at 37°C (12). Escherichia coli DH5α (Invitrogen) was used as a plasmid host. E. coli BL21(DE3) (Stratagene) was used to express and purify BenM and CatM. Carbon sources were added at the following final concentrations: 3 mM benzoate, 3 mM muconate, or 10 mM succinate. Antibiotics were added as needed at the following final concentrations: ampicillin, 150 μg/ml for A. baylyi or 50 μg/ml for E. coli; kanamycin, 25 μg/ml; streptomycin, 13 μg/ml; spectinomycin, 13 μg/ml; tetracycline, 13 μg/ml. For growth curves, succinate-grown colonies were used to inoculate 5-ml cultures for overnight growth with succinate as the carbon source. In the morning, 500 μl of an overnight culture was used to inoculate 50 ml of minimal medium with benzoate or succinate as the sole carbon source. Cell growth was monitored turbidometrically with a Klett-Summerson colorimeter.
TABLE 1.
A. baylyi strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| A. baylyi strains | ||
| ADP1 | Wild type (BD413) | 18 |
| ISA36 | benM::ΩS4036 | 12 |
| ADP102 | catM3102b | 23 |
| ACN47 | benM::ΩS4036 benA::lacZ-Kmr5032 | 12 |
| ACN146 | benM::ΩS4036 benMA5146 | 12 |
| ACN153 | benM::ΩS4036 catM5153 [CatM(V158M)] | This study |
| ACN157 | benA::lacZ-Kmr5032 benM::ΩS4036 benMA5146 | 12 |
| ACN164 | benM::ΩS4036 benA::lacZ-Kmr5032 catM5153 [CatM(V158M)] | This study |
| ACN293 | benM::ΩS4036 benMA5147 ΔcatM5293 | 27 |
| ACN539 | benM::ΩS4036 benMA5147 ΔcatM5293 catB::lacZ-Kmr5534 | This study |
| ACN541 | benM::ΩS4036 benMA5146 catMΩK5541 | This study |
| ACN547 | benM::ΩS4036 benMA5146 catM3102 [CatM(R156H)] | This study |
| ACN548 | benM::ΩS4036 benMA5146 benA::lacZ-Kmr5032 catM3102 [CatM(R156H)] | This study |
| ACN549 | catM3102 [CatM(R156H)] | This study |
| ACN558 | benM::ΩS4036 catM3102 [CatM(R156H)] | This study |
| ACN559 | benM::ΩS4036 benA::lacZ-Kmr5032 catM3102 [CatM(R156H)] | This study |
| ACN560 | benM::ΩS4036 catB::lacZ-Kmr5534 catM5153 [CatM(V158M)] | This study |
| ACN561 | benM::ΩS4036 benMA5146 catB::lacZ-Kmr5534 catM3102 [CatM(R156H)] | This study |
| ACN585 | benM::ΩS4036 catB::lacZ-Kmr5534 | This study |
| Plasmids | ||
| PUC13, pUC19 | Apr; cloning vector | 36 |
| pRK415 | Tcr; broad-host-range cloning vector | 19 |
| pCR2.1-TOPO | Apr; PCR cloning vector | Invitrogen |
| pET-21b | Apr; T7 expression vector | Novagen |
| pHP45 | Apr Smr Spr; source of ΩS | 25 |
| pKOK6 | Apr Kmr; source of promoterless lacZ-Kmr cassette | 20 |
| pUI1637 | Apr Kmr; source of ΩK | 14 |
| pIB17 | Apr; catM3102 (11950-13205)c in pUC19 | 23 |
| PIGG4 | Apr; catM fragment (11950-12892)c in pUC19 | This study |
| pIGG14 | Apr Smr Spr; catBCIJFD (12892-18153)c in pUC13 with ΩS inserted in EcoRV site (15660)c of catJ | This study |
| pBAC6 | Apr Kmr; fragment (11950-12892)c containing catM in pUC19 (ΩK in HincII site [12687]c of catM) | This study |
| pBAC44 | Apr Smr Spr; benMABCDE (563-7876)c in pUC19 (ΩS in multiple cloning site of vector) | This study |
| pBAC54 | Apr Kmr; lacZ-Kmr cassette in NsiI site (3761)c in benA with adjacent ben region (2316-5663)c in pUC19 | 12 |
| pBAC200 | Tcr; cat region DNA (9819-10649 and 15951-18153)c in pRK415; used to isolate the chromosomal catMB region | 13 |
| pBAC234 | Tcr; ACN153 cat region DNA (9819-18153)c in pRK415; isolated with pBAC200, catM5153 mutation | This study |
| pBAC284 | Apr; catM segment (11950-12892)c from pBAC234 cloned in pUC19, catM5153 mutation | This study |
| pBAC364 | Apr; benMA5146 intergenic region (2293-2540)c,d in pUC19, DNase I footprinting, antisense strand labeling | This study |
| pBAC366 | Apr; wild-type benMA intergenic region (2293-2540)c,d in pUC19, DNase I footprinting, antisense strand labeling | 6 |
| pBAC371 | Apr; benMA5146 region (2316-2540),c,d HindIII deletion of pBAC364, DNase I footprinting, sense strand labeling | This study |
| pBAC373 | Apr; wild-type benMA region (2316-2540),c,d HindIII deletion of pBAC366, DNase I footprinting, sense strand labeling | 6 |
| pBAC383 | Apr; PCR fragment with catM5153 in pET-21b, for purification of CatM(V158M) | This study |
| pBAC669 | Apr; catBCIJ (12892-15658)c in pUC13 | This study |
| pBAC673 | Apr; catB (13205-14225)c in pUC19 | This study |
| pBAC674 | Apr Kmr; catB (13205-14225)c upstream of lacZ-Kmr cassette in SalI site (14225)c of catB in pUC19 | This study |
| pBAC675 | Apr Kmr; catB (13205-14225)clacZ-KmrcatJFD (15660-17347)c in pUC19 | This study |
| pBAC679 | Apr Kmr; PCR fragment with catM3102 in pCR2.1-TOPO | This study |
| pBAC684 | Apr; 0.91-kb catM3102 NdeI-XhoId fragment from pBAC679 in pET-21b, complete coding sequence | This study |
Apr, ampicillin resistant; Tcr, tetracycline resistant; Smr, streptomycin resistant; Spr, spectinomycin resistant; Kmr, kanamycin resistant; ΩS, omega cassette conferring Smr Spr; ΩK, omega cassette conferring Kmr.
The original strain isolated with the catM3102 allele has a mucoid colony morphology and is likely to carry additional uncharacterized mutations.
Position in the ben-cat sequence in GenBank entry (accession number AF009224).
Restriction sites added in primers used to generate PCR-amplified fragment for cloning.
DNA sequencing and plasmid construction.
Standard methods were used for DNA purification, digestion, ligation, electrophoresis, and bacterial transformation (30). Sequencing was carried out at the University of Georgia Integrated Biotech Laboratories. Plasmids are listed in Table 1. To purify CatM(V158M), pBAC383 was made in the same way as CatM-encoding pBAC381 (8) except that the catM5153 allele was the template for PCR amplification. To generate a catB::lacZ fusion, the promoterless lacZ-Kmr cartridge of pKOK6 (20) was inserted into the SalI site in catB of pBAC673 to form pBAC674. To enable allelic exchange with the chromosome, cat region DNA was inserted downstream of the lacZ-Kmr cartridge as follows. A catJFD fragment was isolated as a BamHI-Asp718 fragment from pIGG14 and cloned into pBAC674 digested with BamHI and Asp718 to form pBAC675. To construct plasmid pBAC679, the catM3102 allele was PCR amplified from the pIB17 template and cloned into pCR2.1-TOPO. At sites introduced by the amplification primers, the catM3102 allele fragment was excised from this plasmid by digestion with NdeI and XhoI and ligated into similarly digested pET21b (Novagen) to create pBAC684. A plasmid with a catM disruption, pBAC6, was constructed by inserting an omega cassette encoding kanamycin resistance (14) into the HincII site of pIGG4.
BenM-independent Ben+ mutants.
Spontaneous mutants of benM-disrupted ISA36 (Table 1) that grow on benzoate (Ben+) were selected after incubation on solid benzoate medium (12, 13). The catM-catB regions from Ben+ mutants were isolated by gap repair (17). DNA segments were tested for the ability to transform ISA36 to a Ben+ phenotype (12, 13). The cat region of ACN153 was isolated on pBAC234. The mutation conferring Ben+ growth was localized to the catM segment on pBAC284, which was subjected to DNA sequence analysis.
Generation of A. baylyi strains by allelic exchange.
Plasmid-borne alleles were introduced into the chromosome by methods that exploit the high efficiency of natural transformation and recombination in ADP1-derived strains (12, 23). Briefly, recipients were transformed with DNA, typically linearized plasmids, or crude cell-free lysates. Transformants in which homologous recombination had replaced the corresponding chromosomal region of the recipient with the donor DNA were initially identified by phenotypic changes. Strains generated for this study were tested for antibiotic resistance and carbon source utilization. Strains with the catM3102 allele were tested for the characteristic high CatA enzyme activity in succinate-grown cultures (12, 23). Genotypes were confirmed by Southern hybridization, analysis of PCR-generated fragment sizes, and/or DNA sequencing of chromosomal regions.
With these methods, ACN153 was transformed with pBAC54 digested with Asp718. In a transformant, ACN164, homologous recombination had replaced the chromosomal benA locus with the fragment-borne benA::lacZ fusion. Similarly, a DNA fragment with catB::lacZ was made by digesting pBAC675 with Asp718. This fragment replaced the chromosomal catB-to-catF region of ISA36 and ACN153 to make ACN585 and ACN560, respectively. With the exception that it has the catB::lacZ fusion, ACN539 is isogenic to ACN293.
Several strains were constructed in multiple steps. To facilitate the chromosomal introduction of the catM3102 allele, a drug resistance marker was first inserted into catM. Exchange of the catM alleles could then be assessed by the acquisition of drug sensitivity. Plasmid pBAC6, digested with XmnI, was used to introduce the drug resistance marker into the chromosome of ACN146 to generate ACN541. A DNA fragment carrying the catM3102 allele (pBAC684 digested with XhoI) was used to transform ACN541. In strain ACN547, the catM3102 allele replaced the marker-disrupted catMΩK5541of the recipient strain, ACN541. Strain ACN547 served as the recipient when transformed by a DNA fragment carrying the catB::lacZ fusion (pBAC675 digested with Asp718). Introduction of this lacZ reporter into the chromosome by allelic exchange yielded strain ACN561. Strain ACN547 was the recipient in a transformation with donor DNA from ACN157 (in the form of a cell-free lysate) used to introduce the benA::lacZ fusion into the chromosome. The resulting strain was ACN548.
ACN559 was also made in steps. ACN547 was transformed with wild-type benM-benA DNA (pBAC44 digested with XmnI). DNA sequencing confirmed that a transformant, ACN549, had wild-type ben region DNA. Next, the wild-type benM of ACN549 was replaced with the benM-disrupted allele of ISA36 by transforming the former strain with a cell-free lysate of the latter to generate ACN558. In the final step, a cell-free lysate of ACN47 was used to introduce the benA::lacZ fusion into the chromosome of ACN558 to generate ACN559.
β-Galactosidase (LacZ) assays.
To assay the benA::lacZ transcriptional fusion, cultures were grown in minimal medium with muconate or succinate as the carbon source. To assay the catB::lacZ transcriptional fusion, cultures were grown in minimal medium on succinate. To assess induction, muconate (1 mM final concentration) was added to the growth medium for half the cultures. Growth was monitored by optical density at 600 nm (OD600), and assays were done when cultures reached stationary phase. Culture samples (2 to 20 μl per assay) were lysed with sodium dodecyl sulfate and chloroform. Assay directions were followed for the FlourAce β-galactosidase reporter kit (Bio-Rad). The product of substrate hydrolysis, 4-methylumbelliferone (4MU), was detected with a TD-360 minifluorometer (Turner Designs). Relative fluorescence unit measurements enabled 4MU quantification by comparison with a standard curve.
Purification of CatM(V158M).
A 50-ml culture of BL21(DE3)(pBAC383) was grown overnight in LB medium with ampicillin and used to inoculate 1 liter of the same medium. After a 4-h incubation at 37°C on a shaking platform, isopropyl-β-d-thiogalactopyranoside (1 mM final concentration) was added as an inducer. Following further incubation for 4 h, cells were harvested by centrifugation (6,000 × g). The cell pellet was stored at −70°C. The pellet was suspended in30ml buffer A (50 mM Tris [pH 8], 5 mM dithiothreitol, 10% [vol/vol] glycerol), and a crude extract was prepared by sonication. Following centrifugation (6,000 × g), the supernatant fraction was filtered through a 0.22-μm syringe filter, and subsequent purification was carried out with a fast protein liquid chromatography system (Pharmacia). The CatM(V158M) protein failed to bind to a 5-ml Hi-Trap heparin column (Pharmacia) and was recovered from the flowthrough fraction after 35% ammonium sulfate precipitation. The precipitate, which was collected by centrifugation (12,000 × g), was suspended in 7 ml buffer A and passed through a 5-ml Hi-Trap desalting column (Pharmacia). The protein sample was next loaded onto a 5-ml Hi-Trap heparin column that was then washed with 75 ml buffer A. Protein was eluted with 75 ml of a 0 to 0.6 M NaCl gradient in buffer A. Fractions containing CatM(V158M) were pooled after identification by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. By using gels stained with Coomassie brilliant blue R-250 (Bio-Rad), the CatM(V158M) protein was estimated to be >90% pure. The Bradford method was used to assay protein concentrations with bovine serum albumin as the standard (3).
DNase I footprinting.
As previously described, pBAC366 and pBAC373 were used to generate antisense and sense fragments of the wild-type benMA region end labeled with γ-32P (6). Similarly, pBAC364 and pBAC371 were used to produce labeled fragments of the benMA5146 region. For use in footprint reactions, the CatM and BenM proteins were purified by cation-exchange and heparin-agarose chromatography as described elsewhere (6, 8). Various concentrations of BenM, CatM, or CatM(V158M) were incubated with the radiolabeled DNA probe (ca. 300 pM; 200,000 cpm) at 30°C for 30 min with or without benzoate or muconate (0 to 5 mM). The binding reaction (20 μl total volume) was carried out in the following buffer: 80 mM Tris-acetate (pH 8.0), 100 mM potassium acetate, 25 mM ammonium acetate, 5 mM magnesium acetate, 0.1 mM EDTA, 1.0 mM dithiothreitol, 1 mM calcium chloride, 2 μg/ml calf thymus DNA, 50 μg/ml bovine serum albumin. DNase I cleavage reactions were carried out for 1 min at 30°C and analyzed by the methods used by Wang and Hoover (34).
RESULTS
A catM point mutation increases expression of benA without BenM.
Sequence similarity between benM and catM suggested that CatM variants might substitute for BenM. To test this possibility, spontaneous mutants that grow on benzoate without BenM were isolated. Ten independent mutants derived from a benM-disrupted parent strain (ISA36) were selected on benzoate medium, as previously described (12, 13). From each mutant, chromosomal DNA in the catM region was isolated and tested for its ability to transform the parent strain to grow on benzoate. In one mutant (ACN153), the coding sequence of catM was responsible for conferring this Ben+ phenotype. Sequence analysis of the mutant allele, designated catM5153, identified a single mutation, a G-to-A transition at position 474 of catM with respect to its translational start site. In the deduced sequence of the variant protein, CatM(V158M), a methionine residue substitutes for valine at position 158 of the 303-residue protein.
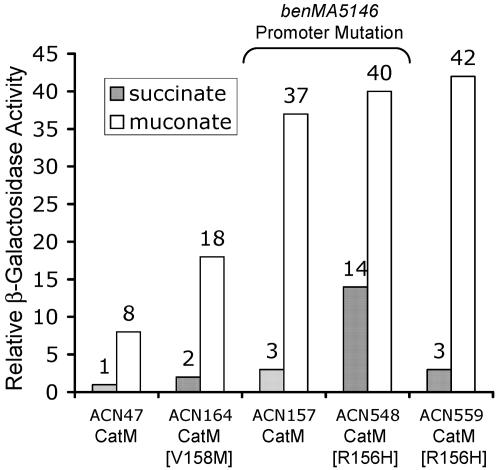
Since the primary obstacle to BenM-independent growth on benzoate is low expression of the benABCDE operon (12, 13), the effect of the catM mutation on ben gene expression was assessed. A benA::lacZ transcriptional fusion was introduced into the chromosome of ACN153, which carries the catM mutation. In the resulting strain, ACN164, the replacement of the wild-type benA allele by the transcriptional fusion prevents consumption of benzoate as the sole carbon source. This strain was grown on muconate or succinate as the carbon source, and the activity of the lacZ-encoded enzyme, β-galactosidase, was evaluated in relation to that of the comparable strain with a wild-type catM allele (ACN47). The benM gene is disrupted in all strains tested for expression of the benA::lacZ fusion. With either the wild-type or mutant catM allele, benA::lacZ expression was inducible by growth on muconate (Fig. 2). However, the mutant allele caused an approximately twofold increase in expression relative to that in ACN47. To test whether the variant CatM might recognize benzoate as an effector, lacZ activity in ACN164 was measured with benzoate added to the growth medium alone or in combination with muconate. However, no increase in gene expression was observed in response to benzoate (data not shown) (11).
FIG. 2.
Expression of a chromosomal benA::lacZ fusion in strains encoding CatM or variant regulators [CatM(V158M), encoded by catM5153, or CatM(R156H), encoded by catM3102]. All strains lack a functional benM, and two strains have a point mutation at position −40 relative to the benA transcription initiation site (benMA5146) (see Fig. 1). Cultures were grown on succinate or muconate as indicated. β-Galactosidase (LacZ) activity is shown relative to that measured for succinate-grown ACN47 (6.9 μmol/min/ml/OD600). Activities are averages of at least four repetitions, and standard deviations were <20% of the average value.
Effect of the catM5153 allele on catB expression.
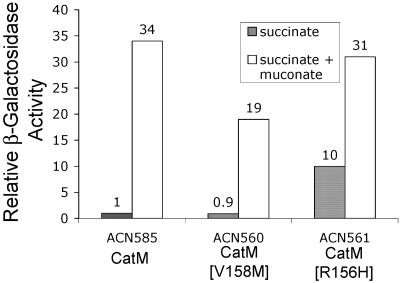
It was not clear whether the twofold change in muconate-inducible ben gene expression was responsible for the ability of ACN153, which has catM5153-encoded CatM(V158M), to form colonies on benzoate medium within 3 days. The comparable strain with wild-type catM does not form colonies on such a medium even after prolonged incubation. We tested whether catM5153 might additionally affect catB expression. Regulation of this gene was studied because CatM-dependent benA expression is augmented by decreased CatB enzyme activity (13). This augmentation may result from the transient accumulation of muconate, the coactivator of CatM, when there are reduced levels of CatB, the enzyme that degrades muconate (Fig. 1) (13). Effects related to internal muconate concentration during growth on benzoate would not be observed under our assay conditions, since the chromosomal benA::lacZ fusion prevents the conversion of benzoate to muconate (Fig. 1 and 2). To test the effect of CatM(V158M) on catB expression, a catB::lacZ transcriptional fusion was used. The activity of LacZ was compared between ACN585, with the wild-type catM, and ACN560, with catM5153. In both strains benM is disrupted. The fusion replaces catB on the chromosome, thereby preventing growth on muconate as the sole carbon source. Cultures were grown with succinate as the carbon source in the presence or absence of muconate as an effector.
Expression of the catB::lacZ fusion was induced by muconate in both strains (Fig. 3). Deletion of catM in a benM-disrupted strain (ACN539) eliminated the inducible β-galactosidase (LacZ) activity (data not shown). This result supports the conclusion that CatM and CatM(V158M) are the sole regulators of catB expression in ACN585 and ACN560, respectively. Although catB expression in ACN560 increased in response to muconate, the maximum level was approximately half that of ACN585. As noted previously, lowered catB expression may increase the amount of muconate available to serve as the CatM coactivator (13). Thus, during growth on benzoate, the catM5153 allele may increase benA expression to an extent greater than that measured by the benA::lacZ fusion (Fig. 2). Interestingly, the effect of catM5153 on catB expression differed from that on benA, a locus where the catM mutation caused expression levels to increase rather than decrease relative to those with wild-type catM (Fig. 2). These differences indicate that the improved ability of CatM(V158M) to activate benA expression is not due to a general increase in responsiveness to muconate. Nevertheless, the amino acid substitution at residue 158 altered the response of CatM to muconate in the regulation of both benA and catB. The position of this substitution was near that at residue 156 in a previously characterized variant, CatM(R156H), that activates cat gene transcription in the absence of effectors (23).
FIG. 3.
Expression of a chromosomal catB::lacZ fusion in strains encoding CatM or variant regulators [CatM(V158M), encoded by catM5153, or CatM(R156H), encoded by catM3102]. All strains lack a functional benM. Cultures were grown on succinate with or without muconate as an inducer, as indicated. β-Galactosidase (LacZ) activity is shown relative to that measured for succinate-grown ACN585 (3.96 μmol/min/ml/OD600). Activities are averages of at least three repetitions, and standard deviations were <20% of the average value.
Effect of CatM(R156H) on benA expression.
To improve our understanding of CatM-regulated benA expression, we reexamined catM3102, an allele with a point mutation encoding histidine rather than arginine at position 156 of CatM. This mutation was selected by its ability to confer high-level expression of the catIJF genes in the absence of muconate as the coactivator for CatM (23). When this catM mutation was isolated approximately 25 years ago, the benM and benA genes had not yet been identified in any organism.
The effect of this CatM(R156H) variant on benA expression had not previously been addressed. For this purpose we constructed ACN559, a benM-disrupted strain in which the catM3102 allele controls expression of the chromosomal benA::lacZ transcriptional fusion. As shown in Fig. 2, succinate-grown ACN559 expressed benA::lacZ at levels higher than those of ACN47, the comparable strain with wild-type catM. However, the catM mutation increased inducer-independent benA expression to a lesser extent than that observed for catB. Studies of the catB::lacZ fusion indicated a 10-fold increase in expression for succinate-grown strain ACN561 compared to ACN585 with wild-type catM (Fig. 3). Nevertheless, the CatM(R156H) variant significantly altered benA expression relative to that with wild-type CatM. In muconate-grown cells, the benA::lacZ expression level was approximately fivefold higher in ACN559 than in ACN47 (Fig. 2). This high level of benA expression in ACN559 raised the possibility that CatM(R156H) might substitute for BenM during growth on benzoate.
BenM-independent growth on benzoate.
To test the effect of catM3102 on benzoate catabolism, strain ACN558 was engineered to contain the wild-type benA region, the disrupted benM allele, and the catM mutation encoding CatM(R156H) (Table 1). ACN558 was able to grow with benzoate as the sole carbon source, although it grew more slowly than the wild type (ADP1), with both a longer generation time and a longer lag period (Table 2). This pattern of growth was nearly identical to that of ACN153, the strain with the CatM(V158M) variant (Table 2). These results demonstrate that a single CatM regulatory protein can control expression of both the cat and ben genes at levels sufficient to permit benzoate consumption without BenM.
TABLE 2.
Effects of catM and benA mutations on rates of BenM-independent growth on benzoatea
| Strain | Relevant characteristic(s)b | Generation time (min)c | Lag time (h)d |
|---|---|---|---|
| ADP1 | Wild type | 60 ± 10 | 7 ± 0.5 |
| ISA36 | No BenM, wild-type CatM | No growth | No growth |
| ACN558 | No BenM, CatM(R156H) | 96 ± 8 | 9 ± 0.5 |
| ACN153 | No BenM, CatM(V158M) | 93 ± 4 | 10 ± 0.5 |
| ACN146 | No BenM, −40 benA promoter mutation, wild-type CatM | 50 ± 11 | 7 ± 0.5 |
| ACN547 | No BenM, −40 benA promoter mutation, CatM(R156H) | 59 ± 8 | 4 ± 0.5 |
Provided as the sole carbon source. When succinate was provided as the carbon source, the growth rates of all these strains were comparable (data not shown).
See Table 1 for genotypes.
Averages of three or more determinations.
Time between inoculation and start of exponential growth.
To study BenM-independent regulation further, we used a previously isolated mutation that increases CatM-activated benA transcription (benMA5146, with a T-to-A transversion at −40 relative to the benA transcript start [Fig. 1C]) (12). The effect of this mutation on benA::lacZ expression was studied in succinate- and muconate-grown cultures (Fig. 2, strain ACN157). The mutation increased the level of muconate-inducible benA expression relative to that with the wild-type promoter (in ACN47). CatM-regulated benA expression patterns were similar to those of the CatM(R156H) variant at the wild-type benA promoter (Fig. 2, strain ACN559). Thus, a single mutation in either catM or the benA promoter is sufficient to increase CatM-activated ben gene expression. This benMA5146 promoter mutation in ACN146 did not noticeably affect growth. With benzoate as the carbon source, the growth curves for ACN146 and the wild type were nearly the same (Table 2). Therefore, mutations that allow CatM to regulate BenM-independent growth on benzoate do not necessarily cause slower growth.
Combined effects of point mutations in the benA promoter and the catM gene.
We tested the effect of the benMA5146 promoter mutation on the ability of CatM(R156H) to activate benA expression. In ACN548, which lacks BenM and carries the catM3102 allele, the benA::lacZ chromosomal fusion is under the control of the benMA5146 promoter. Interestingly, the combination of the promoter mutation and the catM mutation resulted in significantly increased levels of benA expression in succinate-grown cells (Fig. 2, ACN548). Expression of the benA::lacZ fusion in this strain was further induced by growth on muconate. This combination of two mutations enabled a benM mutant, ACN547, to grow on benzoate with a generation time comparable to that of the wild type yet with a shorter lag time than those for the other strains in Table 2. Rapid initiation of growth may reflect increased muconate-independent expression of the benABCDE operon (as indicated by succinate-grown ACN548 [Fig. 2]). High levels of ben gene expression prior to the metabolic formation of muconate may allow benzoate consumption to commence without delay. Moreover, the ability of the benMA5146 promoter mutation to alter CatM-regulated benA expression with both the wild-type and variant regulators raised questions about promoter-protein interactions.
CatM interactions with the benA promoter region.
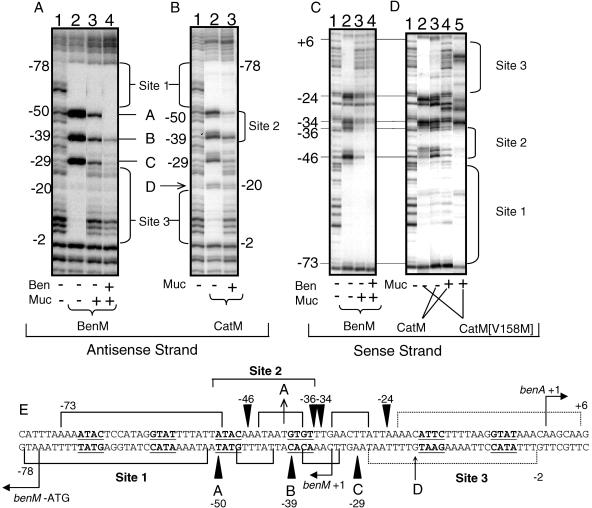
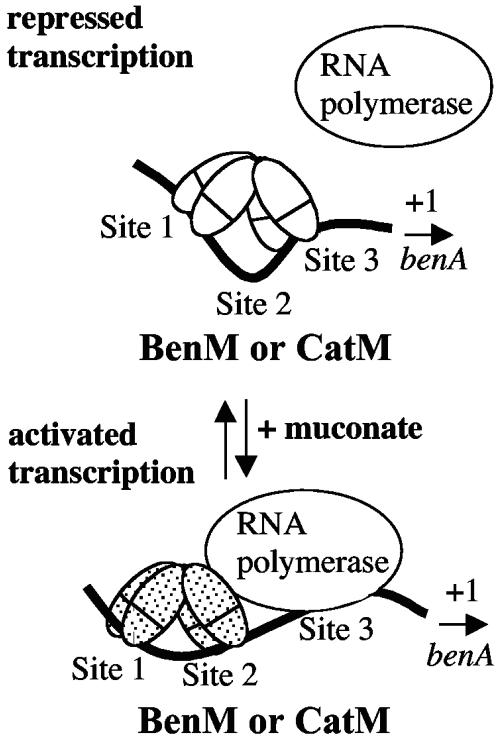
The N-terminal 58 amino acids of BenM are 85% identical to those of CatM. This resemblance predicts nearly identical DNA binding domains (22, 31, 32). To test whether BenM and CatM bind similarly to the benA promoter, DNase I footprinting was used. BenM, in the absence of effectors, binds to two regions upstream of the benA transcriptional start site (6). One region, designated site 3, overlaps the −10 region of the benA promoter. The second region, designated site 1, is adjacent to the −35 region of the benA promoter (Fig. 1C and 4E). Here, experiments with BenM (Fig. 4A and C, lanes 2) were used for comparison with experiments with CatM. Like BenM in the absence of effectors, the wild-type CatM (Fig. 4B, lane 2, and 4D, lane 3) or the CatM(V158M) variant (Fig. 4D, lane 2) protected the site 1 and 3 regions from DNase I cleavage compared to a reaction without any regulatory protein (Fig. 4, lane 1 in each panel). The binding of CatM or CatM(V158M) to the benA region is consistent with a previous model for BenM in which a regulatory tetramer, in the absence of effectors, represses benA transcription (Fig. 5, top).
FIG. 4.
DNase I footprinting of BenM, CatM, and CatM(V158M) at benA. DNase I-cleaved DNA was labeled on the antisense (A and B) or sense (C and D) strand of the benA promoter region. In lanes 2 to 5, a regulatory protein (0.15 μM) was present in the cleavage reaction, as indicated below panels A to D. The presence (+) or absence (−) of the effectors benzoate (Ben) and muconate (Muc) at a concentration of 1 mM in each reaction mixture is indicated. The binding sites labeled 1 to 3 are discussed in the text, and their positions are shown relative to the DNA sequence of the region (E). Nucleotides protected from DNase I digestion in both the absence and presence of inducers are indicated by solid brackets immediately above or below the benA sequence. Nucleotides protected from DNase I cleavage only in the absence of inducers are indicated by dotted brackets. Triangles and positions labeled A to D show sites that were hypersensitive to DNase I digestion. One hypersensitive site (site D) was evident in reactions with CatM but not BenM.
FIG. 5.
Model of regulated benA expression. Footprint data (Fig. 4) suggest that CatM binds to the same regions of the benA promoter DNA as does BenM in the absence or presence of muconate. Models of benA regulation by BenM have been presented elsewhere (6, 9). BenM mediates higher levels of muconate-activated benA transcription than does CatM, as discussed in the text.
With no effectors, BenM, CatM, or CatM(V158M) rendered some positions between the protected regions hypersensitive to DNase I cleavage (Fig. 4A and B, sites A to D, and Fig. 4E). These regularly spaced cleavage sites, on both the sense and antisense strands, are separated by the distance of one DNA helical turn (10 to 11 nucleotides). One site on the antisense strand was more sensitive to DNase I cleavage in the presence of CatM relative to BenM (position −20 [Fig. 4B and E, site D]). As interpreted for BenM, some sites may become more sensitive to DNase I cleavage than in the absence of protein via the formation of an exposed DNA loop when the tetrameric regulator binds. The spacing of the protein binding sites (sites 1 and 3) places them on the same side of the DNA helix (6).
Footprint changes in response to effectors.
The addition of effectors resulted in a loss of protection from DNase I cleavage in the site 3 region (Fig. 4A to C, lanes 3 and 4, and 4D, lanes 4 to 5). Benzoate did not affect the DNase I cleavage patterns in reactions containing wild-type or variant CatM protein with or without muconate (data not shown), consistent with previous conclusions that CatM does not respond to benzoate (12). Loss of protection in the site 3 region should improve the ability of RNA polymerase to access the −10 promoter region. These inducing conditions are associated with the transcriptional activation of benA.
In the site 2 region, the effectors altered the cleavage patterns. For example, the BenM protein with both muconate and benzoate caused the continuous region of protection starting at site 1 to extend in size on both strands (Fig. 4A and C, lanes 4). Notably, the hypersensitive sites at −50 on the antisense strand and −46 on the sense strand became protected. The cleavage sites at positions −39 and −29 on the antisense strand became much less enhanced. Comparable changes occurred in the corresponding regions on the sense strand. Transcriptional activation may occur when a regulatory tetramer binds to the site 1 and site 2 regions (Fig. 5, bottom).
With BenM and muconate alone (Fig. 4A and C, lanes 3), the DNase I cleavage patterns were intermediate between those with no effectors and those with both effectors (Fig. 4A and C, lanes 2 and 4). Intermediate results also occur with benzoate as the sole effector (6). Similarly, this intermediate pattern resulted from wild-type CatM in the presence of muconate (Fig. 4B and D, lanes 4). These conditions correspond to low-level benA transcription and may reflect a mixture of DNA fragments with protein bound to sites 1 and 3 and others with protein bound to sites 1 and 2. Increasing the muconate concentration up to 5 mM with CatM also increased protection of bands at −50 or −46 on the antisense or sense strand, respectively (data not shown) (11). This site 2 position was also protected in the presence of muconate and CatM(V158M) (Fig. 4D, lane 5), an arrangement that correlates with increased benA transcription (Fig. 5, bottom). During benA transcription, the site 3 promoter region should interact with RNA polymerase and be unprotected in the absence of the polymerase. Thus, the anomalous site 3 banding pattern with CatM(V158M) was surprising (Fig. 4D, lane 5). Occasionally, a similar result was observed in footprints with wild-type BenM and CatM. Therefore, while the significance of this pattern is unclear, it may not depend solely on the variant CatM(V158M) protein.
The benMA5146 DNA region was also used in DNase I footprints (data not shown) (11). No major differences occurred in the positions to which CatM binds the DNA. In the absence or presence of muconate, the cleavage patterns for CatM and the benMA5146 region were similar to those for the wild-type benA promoter with CatM or the CatM(V158M) variant (Fig. 4).
DISCUSSION
CatM-benA DNA interactions.
The overlapping DNA-binding functions of CatM and BenM reflect their sequence similarity, which is 85% overall and 98% in their N-terminal DNA binding domains. DNase I footprints suggested that without effectors, BenM, CatM, and CatM(V158M) recognized LysR-type binding sequences within site 1 and site 3 regions of benA DNA (Fig. 4A to C, lanes 2, and 4D, lanes 2 and 3). Such interactions should repress basal benA expression by blocking the −10 promoter region. Consistent with this interpretation, CatM-mediated repression occurs in vitro and in vivo in strains lacking BenM (6, 12).
Effector interactions with LysR-type proteins may cause global conformational changes in the tetramer (22). Effectors alter the number and/or the position of protein subunits bound to DNA (21, 31, 32). Effectors can also alter DNA bending and thereby impact transcription (1, 2). With benA DNA, muconate alleviated protection by BenM, CatM, or CatM(V158M) in the −10 (site 3) region of the promoter (Fig. 4A to C, lanes 3, and 4D, lane 4). With the variant protein in this region, alleviation of protection was accompanied by an altered DNase I cleavage pattern of unknown significance (Fig. 4D, lane 5 versus lane 1). Muconate also affected the site 2 region. With CatM, muconate reduced the extent of hypersensitivity in the cleavage of those positions indicated in Fig. 4E (Fig. 4B, lane 3, and 4D, lane 4). These patterns with CatM and muconate in the site 2 DNA were similar to those with BenM (Fig. 4A and C, lanes 3). The footprint patterns with muconate, which correlate with low-level benA transcription, may arise from a mixed population of DNA fragments in the active and repressed configurations (Fig. 5). Higher-level transcription correlates with protection of the cleavage sites at positions −50 on the antisense strand and −46 on the sense strand, as observed for BenM with muconate and benzoate (Fig. 4A and C, lanes 4) and for CatM(V158M) with muconate (Fig. 4D, lane 5).
Although there were some differences in the cleavage patterns, the overall benA regions to which the wild-type and variant CatM bound were similar to those of BenM. Therefore, the variations in benA expression levels could not be attributed to major shifts in the location of DNA-protein binding sites for BenM, CatM, CatM(V158M), and the mutant promoter benMA5146 (data not shown). Moreover, the extent of BenM and CatM similarity raised questions concerning the evolutionary retention of both paralogs.
Functional divergence of BenM and CatM.
CatM served as the sole ben-cat regulator when spontaneous mutations occurred in catM (catM5153 and catM3102) or the benA promoter (benMA5146). Although the mutants rapidly consumed benzoate (Table 2), the relative fitness of the mutant and wild-type strains was not evaluated. Mutations that increased CatM-mediated ben gene transcription altered the balance of ben and cat gene expression. CatM(V158M) and CatM(R156H) affected benA and catB differently (Fig. 2 and 3). During benzoate consumption (Fig. 1), the altered regulation of these genes should affect metabolite flow and the accumulation of muconate, a key effector that is also toxic at high concentrations (15).
This toxicity prevents growth on alternative carbon sources when muconate accumulates endogenously during the metabolism of aromatic precursors in a strain lacking catB (5, 35). Interestingly, exogenous muconate does not inhibit growth in the same fashion. Perhaps the coupled uptake and degradation of muconate prevent its intracellular accumulation (15). Further evidence that endogenous muconate is harmful comes from the analysis of spontaneous mutants. Those selected mutants that grow without catB in the presence of an appropriate aromatic precursor, such as benzoate, anthranilate, benzyl alcohol, or benzaldehyde, invariably acquire secondary mutations that block muconate formation (5, 35). By this method, numerous catA mutations have been obtained in which catechol accumulation is evident but not lethal (5, 35). Thus, the endogenous accumulation of muconate appears to be more toxic than that of catechol.
The slow growth of ACN558 and ACN153, which encode CatM variants, may reflect the fact that these mutants are less adept than the wild type at balancing ben and cat gene expression to optimize muconate concentrations during growth on benzoate (Table 2). The key role of muconate as an effector allows this compound to control the genes needed for its own formation and degradation. The importance of the muconate concentration is also suggested by the short lag time of ACN547, a strain with high inducer-independent expression of the ben genes (Table 2). In this strain, muconate should not need to accumulate in order to initiate pathway induction.
In the wild type, pathway induction is initiated by benzoate in conjunction with BenM. As assessed by transcriptional fusions (12), muconate alone causes BenM to activate higher benA expression levels than do the CatM variants (Fig. 2). Thus, muconate alone should be sufficient to induce benzoate consumption under laboratory conditions. Nevertheless, the intricate synergistic response of BenM to two effectors allows benzoate consumption to initiate quickly, to reach high levels in the presence of the substrate (benzoate) and a catabolite (muconate), and to decrease upon substrate depletion (6). During evolution, selection may favor regulatory schemes that optimize the ability to adapt to changing conditions (28). Furthermore, the use of two regulators could help balance ben and cat gene regulation when muconate is derived from substrates other than benzoate. Some aromatic compounds, such as anthranilate, are degraded via catechol such that cat but not ben gene expression is required (5, 15, 37). The complexity of the regulatory circuit provides the potential for very rapid and large variations in gene expression.
Promoter dependence of regulation.
An intriguing aspect of the complexity is that CatM and BenM function differently at multiple promoters. Studies of the CatM(R156H) variant emphasize the importance of specific promoter sequences. This variant activates high-level muconate-independent catB transcription (Fig. 3). Surprisingly, the main effect of the CatM(R156H) variant on benA transcription was to increase inducer-dependent gene expression (Fig. 2, ACN559 versus ACN47). The different effects on catB and benA (Fig. 2 and 3) suggest that protein-DNA interactions in the benA region do not properly situate CatM for optimal RNA polymerase contact. It may be that the affinity of the benA promoter for CatM is reduced relative to that for BenM and/or that the conformations of the two regulators in their activated states are sufficiently different to affect transcription.
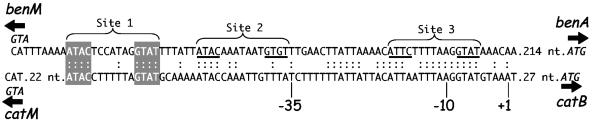
The benA and catB promoters have greater sequence differences between their site 2 regions than between their site 1 and 3 regions (Fig. 6). Our regulatory model predicts that transcriptional activation results when a regulatory tetramer binds site 1 and site 2. The CatM(R156H) variant may have a higher proclivity for binding the catB site 2 region in the absence of muconate than it has for the comparable region of benA. Consistent with this possibility, a mutation of T to A at position −40 in the site 2 region of benA increased inducer-independent transcriptional activation by CatM(R156H) (Fig. 2, ACN548 versus ACN559). This mutation also increased CatM-activated inducible benA transcription (Fig. 2). In comparison to the wild-type sequence, the mutated sequence, AGTGT, more closely resembles the corresponding portion of the site 1 region of catB, AGTAT, to which CatM binding has been demonstrated (Fig. 6) (29).
FIG. 6.
Comparison of sequences in the benA and catB promoter regions. The sequences are aligned relative to the transcription initiation site (+1), and identity is indicated (:). The significance of the ben region sites 1, 2, and 3 is discussed in the text. Gray shading indicates a consensus sequence (ATAC-N7-GTAT) used to bind CatM (29), BenM (6), and other related members in a subclass of LysR-type regulators.
It is not clear how the central portion of CatM affects protein binding to the benA site 2 region. Nevertheless, mutations at positions 156 and 158, in the effector-binding domain of CatM, increase transcriptional activation of benA (Fig. 2). In other LysR-type regulators, mutations have been identified that affect both inducer and DNA binding. For example, amino acid substitutions in a central position of the NahR protein affect both inducer response and DNA binding (24). The effector-binding domains of diverse LysR-type regulators may share a common Rossmann-fold topology characteristic of a family of periplasmic binding proteins (26). This topology involves two domains connected by a hinge that allows movement when an effector binds in the interdomain cavity. Residues 156 and 158 in CatM are predicted to be near this hinge-like region, and they could affect interdomain movement. Alternatively, these positions might affect the oligomerization of CatM. When mutations important to transcriptional activation by LysR regulators were mapped relative to the structure of CbnR, many corresponded to residues at the interface between subunits (22). It is also possible that the two CatM substitutions affect transcription by different mechanisms. More information about effector-induced conformational changes in CatM and its variants should be provided by current structural studies (7, 9). Structural investigations, which may also reveal the basis for the inability of CatM to respond to benzoate, will complement the physiological characterization of the regulatory mutations in this report.
Acknowledgments
We thank Nathaniel Cosper, Robert Scott, and Cory Momany for assistance with purification of the regulatory proteins. Timothy Hoover helped with the DNase I footprints and made many useful suggestions concerning the experiments and the manuscript. We also thank Ann Onyenwoke and Matthew Wisdom, who worked on this project as undergraduate students.
This research was supported by National Science Foundation grants MCB-0212604 and MCB-0516914 to E.L.N. with an REU supplement for H.A.D.
REFERENCES
- 1.Akakura, R., and S. C. Winans. 2002. Constitutive mutations of the OccR regulatory protein affect DNA bending in response to metabolites released from plant tumors. J. Biol. Chem. 277:5866-5874. [DOI] [PubMed] [Google Scholar]
- 2.Akakura, R., and S. C. Winans. 2002. Mutations in the occQ operator that decrease OccR-induced DNA bending do not cause constitutive promoter activity. J. Biol. Chem. 277:15773-15780. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Brzostowicz, P. C., A. B. Reams, T. J. Clark, and E. L. Neidle. 2003. Transcriptional cross-regulation of the catechol and protocatechuate branches of the β-ketoadipate pathway contributes to carbon source-dependent expression of the Acinetobacter sp. strain ADP1 pobA gene. Appl. Environ. Microbiol. 69:1598-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundy, B. M., A. L. Campbell, and E. L. Neidle. 1998. Similarities between the antABC-encoded anthranilate dioxygenase and the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADP1. J. Bacteriol. 180:4466-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bundy, B. M., L. S. Collier, T. R. Hoover, and E. L. Neidle. 2002. Synergistic transcriptional activation by one regulatory protein in response to two metabolites. Proc. Natl. Acad. Sci. USA 99:7693-7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, T., S. Haddad, E. Neidle, and C. Momany. 2004. Crystallization of the effector-binding domains of BenM and CatM, LysR-type transcriptional regulators from Acinetobacter sp. ADP1. Acta. Crystallogr. D 60:105-108. [DOI] [PubMed] [Google Scholar]
- 8.Clark, T. J., C. Momany, and E. L. Neidle. 2002. The benPK operon, proposed to play a role in transport, is part of a regulon for benzoate catabolism in Acinetobacter sp. strain ADP1. Microbiology 148:1213-1223. [DOI] [PubMed] [Google Scholar]
- 9.Clark, T. J., R. S. Phillips, B. M. Bundy, C. Momany, and E. L. Neidle. 2004. Benzoate decreases the binding of cis,cis-muconate to the BenM regulator despite the synergistic effect of both compounds on transcriptional activation. J. Bacteriol. 186:1200-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coco, W. M., M. R. Parsek, and A. M. Chakrabarty. 1994. Purification of the LysR family regulator, ClcR, and its interaction with the Pseudomonas putida clcABD chlorocatechol operon promoter. J. Bacteriol. 176:5530-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collier, L. S. 2000. Transcriptional regulation of benzoate degradation by BenM and CatM in Acinetobacter sp. strain ADP1. Ph.D. thesis. University of Georgia, Athens.
- 12.Collier, L. S., G. L. Gaines III, and E. L. Neidle. 1998. Regulation of benzoate degradation in Acinetobacter sp. strain ADP1 by BenM, a LysR-type transcriptional activator. J. Bacteriol. 180:2493-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosper, N. J., L. S. Collier, T. J. Clark, R. A. Scott, and E. L. Neidle. 2000. Mutations in catB, the gene encoding muconate cycloisomerase, activate transcription of the distal ben genes and contribute to a complex regulatory circuit in Acinetobacter sp. strain ADP1. J. Bacteriol. 182:7044-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eraso, J. M., and S. Kaplan. 1994. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J. Bacteriol. 176:32-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaines, G. L., III, L. Smith, and E. L. Neidle. 1996. Novel nuclear magnetic resonance spectroscopy methods demonstrate preferential carbon source utilization by Acinetobacter calcoaceticus. J. Bacteriol. 178:6833-6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerischer, U. 2002. Specific and global regulation of genes associated with the degradation of aromatic compounds in bacteria. J. Mol. Microbiol. Biotechnol. 4:111-121. [PubMed] [Google Scholar]
- 17.Gregg-Jolly, L. A., and L. N. Ornston. 1990. Recovery of DNA from the Acinetobacter calcoaceticus chromosome by gap repair. J. Bacteriol. 172:6169-6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juni, E., and A. Janik. 1969. Transformation of Acinetobacter calcoaceticus (Bacterium anitratum). J. Bacteriol. 98:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keen, T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 20.Kokotek, W., and W. Lotz. 1989. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene 84:467-471. [DOI] [PubMed] [Google Scholar]
- 21.McFall, S. M., S. A. Chugani, and A. M. Chakrabarty. 1998. Transcriptional activation of the catechol and chlorocatechol operons: variations on a theme. Gene 223:257-267. [DOI] [PubMed] [Google Scholar]
- 22.Muraoka, S., R. Okumura, N. Ogawa, T. Nonaka, K. Miyashita, and T. Senda. 2003. Crystal structure of a full-length LysR-type transcriptional regulator, CbnR: unusual combination of two subunit forms and molecular bases for causing and changing DNA bend. J. Mol. Biol. 328:555-566. [DOI] [PubMed] [Google Scholar]
- 23.Neidle, E. L., C. Hartnett, and L. N. Ornston. 1989. Characterization of Acinetobacter calcoaceticus catM, a repressor gene homologous in sequence to transcriptional activator genes. J. Bacteriol. 171:5410-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, H. H., H. Y. Lee, W. K. Lim, and H. J. Shin. 2005. NahR: effects of replacements at Asn 169 and Arg 248 on promoter binding and inducer recognition. Arch. Biochem. Biophys. 434:67-74. [DOI] [PubMed] [Google Scholar]
- 25.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 26.Quiocho, F. A., and P. S. Ledvina. 1996. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol. Microbiol. 20:17-25. [DOI] [PubMed] [Google Scholar]
- 27.Reams, A. B., and E. L. Neidle. 2003. Genome plasticity in Acinetobacter: new degradative capabilities acquired by the spontaneous amplification of large chromosomal segments. Mol. Microbiol. 47:1291-1304. [DOI] [PubMed] [Google Scholar]
- 28.Reams, A. B., and E. L. Neidle. 2004. Selection for gene clustering by tandem duplication. Annu. Rev. Microbiol. 58:119-142. [DOI] [PubMed] [Google Scholar]
- 29.Romero-Arroyo, C. E., M. A. Schell, G. L. Gaines III, and E. L. Neidle. 1995. catM encodes a LysR-type transcriptional activator regulating catechol degradation in Acinetobacter calcoaceticus. J. Bacteriol. 177:5891-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 32.Tropel, D., and J. R. van der Meer. 2004. Bacterial transcriptional regulators for degradation pathways of aromatic compounds. Microbiol. Mol. Biol. Rev. 68:474-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaneechoutte, M., D. M. Young, L. N. Ornston, T. De Baere, A. Nemec, T. Van Der Reijden, E. Carr, I. Tjernberg, and L. Dijkshoorn. 2006. Naturally transformable Acinetobacter sp. strain ADP1 belongs to the newly described species Acinetobacter baylyi. Appl. Environ. Microbiol. 72:932-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, Y. K., and T. R. Hoover. 1997. Alterations within the activation domain of the sigma 54-dependent activator DctD that prevent transcriptional activation. J. Bacteriol. 179:5812-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams, P. A., and L. E. Shaw. 1997. mucK, a gene in Acinetobacter calcoaceticus ADP1 (BD413), encodes the ability to grow on exogenous cis,cis-muconate as the sole carbon source. J. Bacteriol. 179:5935-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 37.Young, D. M., D. Parke, and L. N. Ornston. 2005. Opportunities for genetic investigation afforded by Acinetobacter baylyi, a nutritionally versatile bacterial species that is highly competent for natural transformation. Annu. Rev. Microbiol. 59:519-551. [DOI] [PubMed] [Google Scholar]