Abstract
The gene cassette (camA+ camB+ camC) encoding a cytochrome P-450cam variant was integrated into the nonessential gene pcpM of the pentachlorophenol degrader Sphingobium chlorophenolicum ATCC 39723 by homologous recombination. The recombinant strain could degrade hexachlorobenzene at a rate of 0.67 nmol · mg (dry weight)−1 · h−1, and intermediate pentachlorophenol was also identified.
Hexachlorobenzene (C6Cl6; HCB) was listed as 1 of the 12 persistent organic pollutants in the Stockholm Convention for its tendency to accumulate along the food chain and its recalcitrance to degradation, together with its harmful effects on human beings and the environment (1). Microbial degradation is a promising effective way to bioremediate environmental pollutants, including persistent organic pollutants. However, a pure culture capable of completely catabolizing HCB has not yet been isolated. Therefore, constructing an HCB-degrading strain via metabolic engineering may be a practical alternative to eliminate HCB in the environment.
It had been demonstrated that the F87W/Y96F/L244A/V247L mutant of cytochrome P-450cam (CYP101) can oxidize HCB to pentachlorophenol (PCP) (4), which can be completely degraded by many microorganisms in the environment. In particular, the catabolic pathway of PCP in Sphingobium chlorophenolicum ATCC 39723 has been characterized thoroughly both biochemically and genetically (2).
In this study, we report the conversion of the PCP utilizer ATCC 39723 to an HCB degrader by introducing a genetic segment (camA+ camB+ camC), which codes for the mutant of CYP101, into the target gene pcpM through homologous recombination.
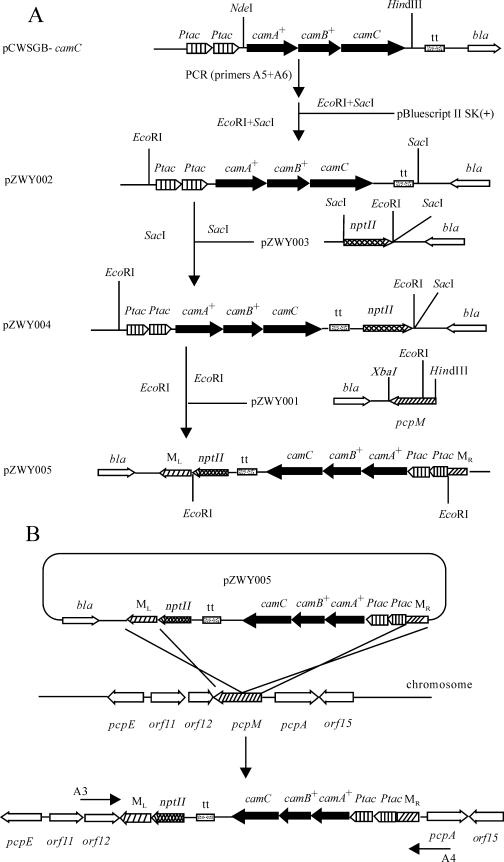
Construction of recombinant plasmids and strains.
The plasmids, strains, and the primers used are listed in Table 1. DNA isolations and manipulations were performed according to established methods (11). The construction of the targeting vector for disruption of the genomic pcpM gene is illustrated in Fig. 1A. pcpM was amplified from strain ATCC 39723 with primers MF-1 and MR-1. The resulting 916-bp PCR product was then digested with XbaI and HindIII before being ligated into pBluescript II SK(+) to create pZWY001. The functional expression cassette, including the Ptac-tac-camA+B+C transcriptional terminator, was PCR amplified from plasmid pCWSGB-camC using primers A5 and A6. The amplified 3,386-bp fragment was then digested with EcoRI and SacI before being ligated into pBluescript II SK(+) to generate pZWY002. The neomycin phosphotransferase II gene (nptII) was PCR amplified from pTnMod-OKm (5) with primers KF-1 (containing SacI and EcoRI sites in tandem) and KR-1 (containing a SacI site). The amplified 1,179-bp fragment was digested with SacI before being ligated into pBluescript II SK(+) to generate pZWY003. The nptII gene cassette was then excised from pZWY003 with SacI and cloned into pZWY002 to generate pZWY004. The insert from pZWY004 was released with EcoRI and cloned into pZWY001 to generate pZWY005. All constructs were confirmed by DNA sequencing (GiKang, Shanghai, China).
TABLE 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description or sequence | Source or reference |
|---|---|---|
| Strains | ||
| S. chlorophenolicum ATCC 39723 | Pentachlorophenol utilizer | Luying Xun |
| Escherichia coli DH5α | supE44 ΔlacY169 (φ80 lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Gibco, BRL |
| S. chlorophenolicum ZWY005 | ATCC 39723 with insertion of camA+B+C-nptII into pcpM in its chromosomal DNA | This work |
| Plasmids | ||
| pCWSGB-camC | pCWori+ derivative containing camA+B+C expression cassette, which encodes putidaredoxin reductase (PdR), putidaredoxin (Pd), and the F87W/Y96F/L244A/V247L mutant of cytochrome P-450cam, respectively; Apr | Luet-Lok Wong |
| pBluescript II SK(+) | Cloning vector, multiple cloning site in lacZα, Apr | Stratagene (La Jolla, Calif.) |
| pZWY001 | pcpM cloned into pBluescript II SK(+) | This work |
| pZWY002 | Ptac-tac-camA+B+C gene block cloned into pBluescript II SK(+) | This work |
| pZWY003 | Neomycin phosphotransferase II gene (nptII) cloned into pBluescript II SK(+) | This work |
| pZWY004 | Ptac-tac-camA+B+C-nptII cloned into pBluescript II SK(+) | This work |
| pZWY005 | pcpM::camA+B+C-nptII in pBluescript II SK(+) | This work |
| Plasposon pTnMod-OKm | pMB1 replicon, Kmr | 5 |
| Primers | ||
| MF-1 | 5′-AATTCTAGATTTCCGCTCAATAACTTAGT-3′ | |
| MR-1 | 5′-TAAAAGCTTGATATCCTTCATGCATCTGA-3′ | |
| KF-1 | 5′-ATAGAGCTCGAATTCTCGTGAAGAAGGTGTT-3′ | |
| KR-1 | 5′-GCCGAGCTCTGTCTCAAAATCTCTGAT-3′ | |
| A3 | 5′-GGCGCAGAGCGTGGTGTT-3′ | |
| A4 | 5′-TTCGCGAATATCCCGCATT-3′ | |
| A5 | 5′-TCAGAATTCACCCCAGGCTTTACACT-3′ | |
| A6 | 5′-TATGAGCTCGAGGCCCTTTCGTCTT-3′ |
FIG. 1.
Illustration of construction of plasmids and the engineered strain. (A) Construction of the gene targeting vector pZWY005 for allelic exchange in Sphingobium chlorophenolicum ATCC 39723. (B) Homologous recombination between the flanking sequences of pcpM in the targeting vector pZWY005 and the pcpM sequence in chromosomal DNA in Sphingobium chlorophenolicum ATCC 39723. camA+, camB+, and camC denote the genes encoding putidaredoxin reductase (PdR), putidaredoxin (Pd), and mutant cytochrome P450cam, respectively. nptII, neomycin phosphotransferase II gene; Apr, ampicillin resistance gene (bla); Ptac, tac promoter; tt, transcriptional terminator; ML and MR, the left part and the right part of pcpM, respectively. Arrows indicate the direction of transcription for the respective genes. Relevant restriction sites are indicated.
The plasmid pZWY005 was introduced into strain ATCC 39723 by electroporation as previously described (8). Positive clones containing a pcpM-disrupted allele were screened by their kanamycin resistance (15 μg/ml) and ampicillin sensitivity (15 μg/ml). One hundred kanamycin-resistant colonies were identified, of which 10 were ampicillin sensitive, implying that the disruptions resulted from a double-crossover recombination rather than delivery plasmid cointegration (single crossover). The homologous recombination (double crossover) is illustrated in Fig. 1B.
Further identification of the recombinants was performed by PCR with primers A3 and A4 designed from the sequences flanking pcpM. A 5.8-kb fragment was amplified using genomic DNA of the recombinants as template. This clearly indicated that allelic exchange had occurred via homologous recombination between the flanking sequences of pcpM in the targeting vector and the pcpM sequence in the host genome, resulting in an interrupted pcpM allele in the genome. In contrast, a 1.3-kb fragment was produced in a control experiment using wild-type ATCC 39723 genomic DNA as template. One such recombinant was named ZWY005, which still grows on PCP as well as the wild type.
HCB degradation by whole cells of engineered strain ZWY005.
Strains ZWY005 and ATCC 39723 were grown in glutamate mineral salts medium (10) at 30°C with shaking. When the A600 of a cell culture reached 0.3 to 0.4 (early exponential phase), 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added and the culture was incubated for an additional 8 h. Cells were harvested by centrifugation at 3,500 × g for 8 min and washed twice with MS (glutamate mineral salts medium without monosodium l-glutamate). The cells were then resuspended in 300 ml MS with an A600 of 3 (1 g [dry weight] of cells per liter) and were shaken at 30°C for HCB degradation. HCB was added as a 1 mM stock in ethanol to a final concentration of 4.3 μM. For the purpose of analysis of the degradation of HCB and production of PCP, two 20-ml aliquots of the cultures were removed periodically to 50-ml centrifuge tubes.
An internal standard, 1,3,5-tribromobenzene, was added to the samples for quantitative analysis of HCB to standardize both the extraction and analysis steps. The sample was centrifuged at 6,000 × g for 10 min. Organics in the supernatant collected were adsorbed onto an Argonaut/IST ISOLUTE C18 (EC) column, and the bound organics were eluted with n-hexane. On the other hand, organics adsorbed on the cell pellet were extracted according to methods described previously (9, 12). Control experiments showed that the efficiency of this solid-phase extraction combined with vortex mixer extraction method was pretty high (>96%), and the results were highly reproducible. The n-hexane layers from all above extractions were pooled and dried over anhydrous sodium sulfate for quantitation by gas chromatography (GC).
GC analysis was carried out on an Agilent 6820 series instrument equipped with a 63Ni electron capture detector on a DB-5 fused silica capillary column (0.32-mm inner diameter by 30 m by 0.25 μm). The column temperature was held at 40°C for 1 min and then increased to 100°C at 15°C/min, from 100°C to 240°C at 20°C/min, and from 240°C to 280°C at 10°C/min. The injector and detector temperatures were 280°C and 300°C, respectively. Nitrogen at a flow rate of 40 ml/min was used as the carrier gas. The injection volume was 1 μl, and splitless injection was used. The retention times of HCB and 1,3,5-tribromobenzene were 12.43 min and 10.36 min, respectively.
Degradation studies demonstrated that the recombinant cells could degrade 4 μM HCB within 6 h with 1 g (dry weight) of cells per liter (or at a rate of 0.67 nmol · mg [dry weight]−1 · h−1). No degradation of HCB was observed in the control with ATCC 39723.
Identification of PCP as an intermediate metabolite produced by oxidation of HCB.
In the wild-type ATCC 39723, transcription of a gene cluster (pcpB pcpA pcpE) encoding PCP-degrading enzymes has been shown to be inducible by PCP, which takes about 1 to 2 h (13). PCP transformed from HCB by the engineered strain should then be detected in the recombinant culture within the early hours after the addition of HCB, since it is not further degraded within this period.
For qualitative and quantitative analysis of PCP, an internal standard, 2,4,6-tribromophenol, was added to the samples after being acidified to pH 2.0 with concentrated sulfuric acid. The extraction procedures were the same as described above, except that the samples were extracted with n-hexane-acetone (1:1) instead of n-hexane only (9). Assays of the trapped intermediate PCP were performed by derivatization with acetic anhydride to pentachlorophenyl acetate prior to measurements (9).
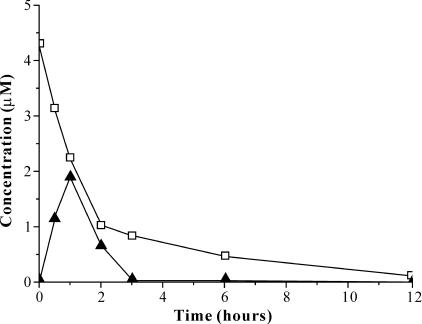
The results of GC analysis indicated that PCP had been indeed produced during the HCB degradation by the recombinants. The retention time of the derivative from the samples corresponded to that of the derivative of authentic PCP, while the samples from the wild-type ATCC 39723 control or bacteria-free control did not have the corresponding peak of the PCP derivative. The retention times were as follows: pentachlorophenyl acetate, 13.06 min; 2,4,6-tribromophenyl acetate, 12.49 min. The time course analysis showed that the stoichiometric amount of PCP was formed concomitantly within the first hour of incubation during the degradation of HCB, apparently before the PCP degradation pathway was induced (Fig. 2).
FIG. 2.
HCB degradation by whole cells of engineered strain ZWY005. □, HCB concentration; ▴, PCP concentration. Extracted samples were analyzed with a gas chromatograph equipped with an electron capture detector.
The identity of intermediate PCP was further confirmed by GC-mass spectrometry (GC-MS), in which the scanning model was adopted. GC-MS analysis was carried out on an Agilent 6890N/5973 Inert quadrupole instrument. An HP-5 MS column (0.25-mm inner diameter by 30 m by 0.25 μm) was used with temperature programming from 40°C to 280°C at 20°C/min and held at this temperature for 5 min. Injection volume was 1 μl, and splitless injection was used. Helium was used as the carrier gas at a flow rate of 1 ml/min. The MS ion source temperature was 230°C, and the quadrupole temperature was 150°C. Electron energy was 70 eV. Scan range was from 50 to 400 atomic mass units. The possibility that the metabolic intermediate of HCB is PCP was confirmed by comparison of the mass fragmentation patterns of intermediate and authentic PCP in the NIST02 mass spectral database. Molecular ion peaks were observed at m/z 312 (M + 6), 310 (M + 4), 308 (M + 2), and 306 (M+), whose masses and relative intensities are characteristic of a molecule containing five chlorine atoms. The relative intensities are consistent with a natural abundance of 76% for 35Cl and 24% for 37Cl. Major fragments were observed at m/z 270, 268, 266 (base peak), and 264 (loss of CH2=C=O from 312, 310, 308, and 306) and m/z 241, 239, 237, and 235 (loss of HCO from 270, 268, 266, and 264).
As a leaky tac-tac promoter existed in the expression cassette within the engineered strain, HCB degradation and PCP formation were also observed in the resting cells without induction of IPTG, but at a lower rate.
In conclusion, an engineered strain with the ability to completely degrade HCB has been successfully constructed by metabolic engineering for the first time, albeit with a low degradation activity. This is in contrast to previous studies on the reductive dechlorination of HCB, which led to accumulation of toxic intermediates (3, 6, 7, 14).
.
Acknowledgments
This work was supported by Knowledge Innovation Project grants from the Chinese Academy of Sciences (KSCX2-SW-128) and Wuhan Institute of Virology, Chinese Academy of Sciences (LYQY-020405).
We thank Luet-Lok Wong for the gift of plasmid pCWSGB-camC and Luying Xun for providing Sphingobium chlorophenolicum ATCC 39723.
REFERENCES
- 1.Barber, J. L., A. J. Sweetman, D. van Wijk, and K. C. Jones. 2005. Hexachlorobenzene in the global environment: emissions, levels, distribution, trends and processes. Sci. Total Environ. 349:1-44. [DOI] [PubMed] [Google Scholar]
- 2.Cai, M., and L. Xun. 2002. Organization and regulation of pentachlorophenol-degrading genes in Sphingobium chlorophenolicum ATCC 39723. J. Bacteriol. 184:4672-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, B. V., C. J. Su, and S. Y. Yuan. 1998. Microbial hexachlorobenzene dechlorination under three reducing conditions. Chemosphere 36:2721-2730. [DOI] [PubMed] [Google Scholar]
- 4.Chen, X., A. Christopher, L. P. Jones, S. G. Bell, Q. Guo, F. Xu, Z. Rao, and L.-L. Wong. 2002. Crystal structure of the F87W/Y96F/V247L mutant of cytochrome P-450cam with 1,3,5-trichlorobenzene bound and further protein engineering for the oxidation of pentachlorobenzene and hexachlorobenzene. J. Biol. Chem. 277:37519-37526. [DOI] [PubMed] [Google Scholar]
- 5.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fathepure, B. Z., J. M. Tiedje, and S. A. Boyd. 1988. Reductive dechlorination of hexachlorobenzene to tri- and di-chlorobenzenes in anaerobic sewage sludge. Appl. Environ. Microbiol. 54:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayachandran, G., H. Görish, and L. Adrian. 2003. Dehalorespiration with hexachlorobenzene and pentachlorobenzene by Dehalococcoides sp. strain CBDB1. Arch. Microbiol. 180:411-416. [DOI] [PubMed] [Google Scholar]
- 8.Lange, C. C., B. J. Schneider, and C. S. Orser. 1996. Verification of the role of PCP 4-monooxygenase in chlorine elimination from pentachlorophenol by Flavobacterium sp. strain ATCC 39723. Biochem. Biophys. Res. Commun. 219:146-149. [DOI] [PubMed] [Google Scholar]
- 9.Polese, L., and M. L. Ribeiro. 1998. Methods for determination of hexachlorobenzene and pentachlorophenol in soil samples. Talanta 46:915-920. [DOI] [PubMed] [Google Scholar]
- 10.Saber, D. L., and R. L. Crawford. 1985. Isolation and characterization of Flavobacterium strains that degrade pentachlorophenol. Appl. Environ. Microbiol. 50:1512-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 12.Wall, A. J., and G. W. Stratton. 1991. Comparison of methods for the extraction of pentachlorophenol from aqueous and soil systems. Chemosphere 22:99-106. [Google Scholar]
- 13.Xun, L., and C. S. Orser. 1991. Purification of a Flavobacterium pentachlorophenol-induced periplasmic protein (PcpA) and nucleotide sequence of the corresponding gene (pcpA). J. Bacteriol. 173:2920-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh, D. H., and S. G. Pavlostathis. 2001. Development of hexachlorobenzene-dechlorinating mixed cultures using polysorbate surfactants as a carbon source. Water Sci. Technol. 43:43-50. [PubMed] [Google Scholar]