Abstract
It was investigated how organic rearing conditions influence the Salmonella enterica infection dynamics in pigs and whether Salmonella persists in the paddock environment. Pigs inoculated with S. enterica serovar Typhimurium were grouped with Salmonella-negative tracer pigs. Bacteriological and serological testing indicated that organic pigs were susceptible to Salmonella infections, as 26 of 46 (56%) tracer pigs turned culture positive. An intermittent and mainly low-level excretion of Salmonella (<100 cells g−1) partly explains why the bacteriological prevalence appeared lower than the seroprevalence. Salmonella persisted in the paddock environment, as Salmonella was isolated from 46% of soil and water samples (n = 294). After removal of pigs, Salmonella was found in soil samples for up to 5 weeks and in shelter huts during the entire test period (7 weeks). Subsequent introduction of Salmonella-negative pigs into four naturally Salmonella-contaminated paddocks caused Salmonella infections of pigs in two paddocks. In one of these paddocks, all tracer pigs (n = 10) became infected, coinciding with a previous high Salmonella infection rate and high Salmonella excretion level. Our results showed that pigs reared under organic conditions were susceptible to Salmonella infections (just like conventional pigs) and that Salmonella persisting in the paddock environment could pose an infection risk. A driving force for these infections seemed to be pigs with a high Salmonella excretion level, which caused substantial contamination of the environment. This suggests that isolation of animals as soon as a Salmonella infection is indicated by clinical symptoms of diarrhea could be a means of reducing and controlling the spread and persistence of Salmonella in outdoor organic pig production environments.
Organic pig production is a growing alternative to conventional and often large-scale pig production and aims at improving animal welfare and providing growth conditions under which the pigs can express natural behaviors. The main differences between organic and conventional production are that the pigs have access to outdoor areas, farrowing takes place outdoors, and piglets live together with the sow for a minimum of 7 weeks before weaning, compared to 3 to 4 weeks in conventional pig production (2).
Outdoor pig production may imply increased exposure to pathogens that persist in the environment or are transmitted through wildlife. The pathogens may not influence the welfare of the pigs, but zoonoses such as Salmonella enterica can subsequently be transferred to humans through consumption of contaminated pork. Several investigations have demonstrated that Salmonella infections in conventional pig farms are able to persist in the herd environment for several months and even years (5, 9, 19, 46). Even though it is difficult to differentiate between the persistence of pathogens in pigs caused by subclinically infected animals and infection originating from the environment, isolations of Salmonella from soil, slurry, manure, and equipment indicate that a contaminated environment may constitute an important source of infection (4, 18).
Organic pig farms often produce less than 200 pigs per year and are therefore not included in the Danish national surveillance program, which monitors the Salmonella seroprevalence in pigs (meat juice) at slaughter (1). Thus, limited data on Salmonella infection rates in organic pig production are available. Nevertheless, a comparison of the Salmonella seroprevalences in Danish pig herds showed that there was a higher risk of meat juice samples from both organic and free-range herds being seropositive than from samples from conventional herds (odds ratio = 1.7; P = 0.0001) (54). The result was significant for the free-range herds (P = 0.001) but not for organic herds, due to a limited number of samples. Similar results were obtained in a Dutch study in which the Salmonella seroprevalence in free-range finishers was significantly higher (44.6%) than in intensive conventional indoor production of finishers (24.5%) (51).
Information about the time of establishment of a Salmonella infection, its duration, and the level of infection in individual animals would help to illuminate the infection dynamics in a herd and thus the potential contamination risk at the time of slaughter. Due to the differences between organic and conventional pig production, the current information on Salmonella dynamics in conventional pigs may not apply to organic and other alternative pig production systems. Little is known about the effect of organic rearing with respect to susceptibility to infections such as salmonellosis. Perhaps the late weaning; the organic feed, including roughage, that potentially alters the microbial composition of the gut; and the lower animal density diminish the levels of Salmonella in organic pigs. Since many of the normal measures taken to prevent and control Salmonella infection in indoor systems do not apply in outdoor systems, obtaining information about potential risk factors is important in limiting the risk of Salmonella infections in these systems.
The aim of the current experimental study was to examine the Salmonella infection dynamics in organic pigs raised outdoors. Noninfected tracer pigs were grouped with pigs inoculated with different concentrations of Salmonella to determine the transmission of Salmonella between animals. Furthermore, the establishment of Salmonella in the pasture environment was studied, including its impact on Salmonella infection of new tracer animals introduced into the pasture.
MATERIALS AND METHODS
Pigs and field sites.
On three occasions 56, 56, and 51 organic pigs with average weights of 16.9 ± 4.0 kg, 12.7 ± 2.4 kg, and 20.6 ± 3.9 kg, respectively, were purchased from a Danish organic farmer at the time of weaning (7 weeks old). Upon arrival at the university research farm in Taastrup, Denmark, rectal fecal and blood samples were collected from the pigs (zero samples) to determine their Salmonella status by microbiological culture and enzyme-linked immunosorbent assay (ELISA) (8). To avoid parasite contamination of the experimental pastures, the pigs were treated with fenbendazole, 10 g active ingredient per 56 animals, administered with the feed for 2 days. The pigs were fed ad libitum with organic pelleted feed and pea/barley silage as roughage.
Six experimental outdoor paddocks, named A, B, C, D, E, and F, were set up on a 3-year-old mixture of grass and clover not previously grazed. The rectangular paddocks (50 m2 per pig) were enclosed with electric fence and placed 2 meters apart to avoid direct contact between the animals. In each paddock, the pigs had free access to an insulated hut with straw bedding, water cups, wallowing area, and a feed dispenser. Before initiation of a new experiment, the old straw bedding in the huts was removed and slaked lime was spread on the surface ground to diminish the survival of Salmonella; however, the efficacy of this procedure was not assessed.
Experimental design.
In three successive experiments, referred to as periods 1 to 3, a total of 163 organic weaning pigs (Salmonella negative) were distributed among the six paddocks. Four paddocks (C to F), each with 10 pigs, were used for Salmonella infection experiments, and two paddocks (A and B), each with 8 pigs, served as Salmonella-negative control paddocks (Table 1). Each of the three experimental periods lasted for 6 weeks and were carried out from late April to the beginning of September 2003.
TABLE 1.
Study design of Salmonella infection in an organic outdoor pig farming environment
| Perioda | No. of pigs in paddockc:
|
|||||
|---|---|---|---|---|---|---|
| Ab | Bb | C | D | E | F | |
| (1) 2 May-10 June | 8 | 8 | 7* | 7* | 7† | 7† |
| (2) 12 June-21 July | 8 | 8 | 10 | 10 | 10 | 10 |
| (3) 23 July-1 Sept | 8 | 7 | 5† | 6† | 7† | 8 |
Three periods that aimed to study (1) transmission of Salmonella from artificially infected pigs to tracer pigs in paddocks C to F, (2) transmission of Salmonella from a contaminated paddock environment to new tracer pigs in paddocks C to F, and (3) repetition of period 2 in paddock F and repetition of period 1 in paddocks C to E.
Salmonella-negative control paddocks and pigs.
*, indicated number of tracer pigs plus three pigs inoculated with 7.4 × 107 Salmonella cells (low dose); †, indicated number of tracer pigs plus three pigs inoculated with 3.2 × 109 Salmonella cells (high dose).
Seven Salmonella-negative pigs, designated tracer pigs, were grouped with three pigs artificially inoculated orally with an S. enterica serovar Typhimurium DT12 test strain to study the transmission of Salmonella from infected pigs to the tracer pigs. Two groups of pigs were inoculated with 7.4 × 107 cells (referred to as low dose) in paddocks C and D (in the first of the three experimental periods), and five groups of pigs were inoculated with 3.2 × 109 cells (referred to as high dose) in paddocks E and F (first experimental period) and paddocks C, D, and E (third experimental period) (Table 1). The bacterial cells were given to each pig via a gastric tube, using a volume of 10 ml physiologic saline (0.9% NaCl) solution. Inoculation experiments were carried out in both the first and the third period, and Salmonella test strains with two different resistance markers, as described below, were used to permit differentiation of Salmonella organisms isolated from the two rounds of inoculation.
The infectivities of Salmonella-contaminated paddock environments were studied in the second experimental period by introducing 10 Salmonella-negative tracer pigs into paddocks C, D, E, and F at 1 day after termination of the first period. In case the pigs became Salmonella infected in the second period, new tracer pigs were introduced into the paddock in the third period to examine whether the contaminated environment continued to be infective. The paddocks in which no or little Salmonella infection occurred in the second period were used for new inoculation experiments in the third period (Table 1).
S. enterica serovar Typhimurium DT12 test strain.
A Danish strain of Salmonella serovar Typhimurium DT12, originally isolated from pigs with clinical signs of salmonellosis, was grown on rifampin- and nalidixic acid-containing nutrient agar (Oxoid Limited, Basingstoke, United Kingdom) to select for single rifampin or double rifampin and nalidixic acid resistance. These strains (Salmonella serovar Typhimurium DT12 rifampinres and Salmonella serovar Typhimurium DT12 rifampin/nalidixic acidres) were cultured as described previously (40), harvested, and diluted to approximately 106 and 108 CFU/ml. The inoculation doses were determined by serial dilutions and growth on brilliant green agar (BGA) (Oxoid).
Sampling of feces, blood, and environmental samples.
Rectal fecal samples (5 g) and blood samples (5 ml) of each pig and seven samples from each paddock environment were collected once per week for 6 weeks in each period. A 50-ml water sample was collected from the water cup (environmental sample 1). Soil samples (>30 g) were collected as pools of five small samples of top-surface soil (1 to 5 cm) from each of the six following locations in the paddock: rear end of the paddock (environmental sample 2), intersection of hut/water cup (environmental sample 3), defecation area (environmental sample 4), feeding area (environmental sample 5), hut (environmental sample 6), and mud hole (environmental sample 7). The first samples were collected 3 days postinoculation or 4 days after introduction of tracer pigs into the contaminated environment (second period). The sampling of environmental samples continued for 7 weeks after the paddocks had been vacated. The samples were transported to the laboratory at ambient temperature (blood and soil) or on ice (feces) and stored at 4°C until testing the next day. To avoid cross-contamination between animals, materials, and samples, only disposable or disinfected equipment was used for collecting samples, as well as good hygiene practices.
Isolation and enumeration of Salmonella organisms.
The detection of Salmonella in fecal samples (5 g) was done as described by Baggesen et al. (8) with minor modifications. A qualitative assessment with a detection limit of 1 CFU per 5 g was used for the control pigs and the samples collected upon arrival at the farm for screening of Salmonella (zero samples), whereas the level of Salmonella excretion was examined by a semiquantitative approach for all other pigs. The fecal materials were diluted 100-fold in buffered peptone water (Merck KGaA, Darmstadt, Germany) supplemented with novobiocin (Sigma-Aldrich Co., St. Louis, MO) (BPW-N) (22 μg/ml) to assess the Salmonella excretion levels in the pigs (30). Dilutions were incubated overnight and spotted onto modified semisolid Rappaport-Vassiliadis agar (Oxoid). After overnight incubation, material from presumptive Salmonella swarming zones was subcultured onto BGA. The test strain was identified by slide agglutination of colonies from BGA plates with polyclonal O5 antiserum and confirmation of the resistance markers by inoculation onto nutrient agar with and without rifampin or rifampin/nalidixic acid (50 mg/ml). Nonresistant Salmonella strains were identified by serotyping by agglutination using polyclonal sera (Statens Seruminstitut, SSI, Copenhagen, Denmark) according to the Kauffmann-White scheme (29, 43).
Survival of Salmonella in the paddock environment was assessed qualitatively by pre-enrichment of 25 g soil in BPW-N (1:9). The water samples were filtered onto a 0.45-μm filter (Millipore, Billerica, MA) by use of vacuum, and filters were pre-enriched in 9 ml of BPW-N. Overnight incubation and further isolation of Salmonella organisms were done as described above.
Postmortem examinations of pigs.
Pigs with health problems were excluded before the end of the trial and subjected to postmortem bacteriological examinations of liver, spleen, intestinal wall, cecum content, and mesenteric and ileocolic lymph nodes, as described by Baggesen et al. (8).
Enumeration of presumptive E. coli in the paddock environment.
Detection of presumptive Escherichia coli was based on standard conventional bacteriological methods and performed as described previously (15). No attempt was made to discriminate between pathogenic and nonpathogenic E. coli organisms. The water and homogenized soil samples (5 g) were 10-fold serially diluted (10−1 to 10−5), and 1 ml from each of the soil dilutions was then mixed with 5 ml of molten tryptone soya agar (BD, Franklin Lakes, NJ) with a temperature of 45°C. Plates were preincubated at room temperature for 1 to 2 h, after which 10 to 15 ml of violet red bile agar (Oxoid) was poured onto the surface. Inoculated plates were incubated overnight at 44°C. The water dilutions were filtered through a 0.45-μm filter (Millipore), and the filters were incubated on membrane lauryl-sulfate agar (Oxoid) overnight at 44°C. Plates with 10 to 100 colonies typical of thermotolerant coliform bacteria were counted, and 5 colonies per plate were confirmed as presumptive E. coli by testing for production of gas in lactose tryptone lauryl-sulfate broth (Oxoid) and indole by addition of Kovács indole reagent (Merck KGaA). The results were expressed as number of presumptive E. coli organisms per gram, and the detection level of the method was 10 CFU g−1.
Serology.
Blood serum from the pigs was tested by ELISA based on O:1,4,5,6,7,12 lipopolysaccharide from different Salmonella types, designated mix-ELISA (40). This test permits detection of the most common serotypes, including the test strain (O:4,5,12). The sample optical density (OD) readings (490 nm) were transformed to calibrated OD%, with a positive cutoff at 10 OD% (40).
Statistical analysis.
Due to the low numbers of pigs at each location, nonparametric methods were used. We used the Wilcoxon rank sum test (32) with a one-sided alternative in all analyses. The effect of the Salmonella dose was investigated by comparing the low-dose group with the high-dose group. This was done for both bacteriological and serological results. The frequencies of tracer pigs becoming infected with Salmonella in the study period were compared, as well as the “waiting time to infection,” i.e., the number of weeks after inoculation until infection was detected in tracer pigs.
The number of times that Salmonella was isolated at the various locations in the paddock was investigated, and we investigated whether one location might be considered an outlier compared to the others with regard to this number by fitting a normal distribution to the others and adjusting for the most extreme case not being included (through simulation). We then calculated the probability that the most extreme case could be as different from the rest as was observed. To investigate whether a different (smaller) number of Salmonella-positive environmental samples at specific locations was related to a different (smaller) load of feces (with E. coli as indicator), we fitted a second-order polynomial to the E. coli numbers for each of the locations as a function of time and compared the results graphically.
We investigated the survival of E. coli in the pasture environment by fitting a standard log-linear regression model to the E. coli numbers as a function of time, from the day on which the paddocks were vacated until decay set in. The regression model was used to estimate decimation time (T90) values both for the average of the locations in the paddock and for the most extreme location (i.e., with the lowest decay rate).
All calculations where carried out with Splus software, version 6.1.
RESULTS
Establishment of Salmonella infection.
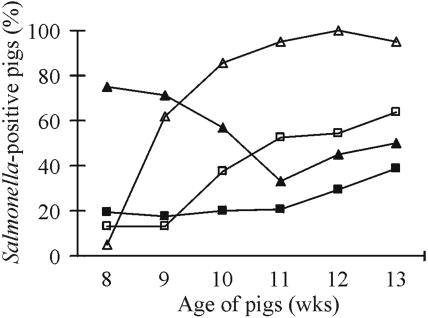
Three pigs in each group (n = 3 × 7) were inoculated with a low or high dose of Salmonella serovar Typhimurium DT12 cells and grouped together with five to seven tracer pigs (n = 46) to study the possible spread of Salmonella between outdoor organic pigs. Two groups of pigs were given a low dose of Salmonella (in the first experimental period), and five groups were given a high dose (in the first or third experimental period). One of the pigs from the third batch was found to be Salmonella positive (serovar Typhimurium, unspecific phage type) when tested after arrival at the research farm (zero samples). This pig was excluded from the experiment but may have contributed to the slight seroresponse (<18 OD%) found in three otherwise culture-negative pigs (data not shown). The frequency of Salmonella-positive animals in the groups and the number of times Salmonella was isolated from each pig during the 6-week period showed large variations (Fig. 1a). The inoculated pigs (n = 21) showed the highest level of Salmonella excretion (75%) immediately after the challenge with Salmonella, whereas the number of Salmonella-positive tracer pigs increased from 20 to 41% over the 6 weeks (Fig. 2).
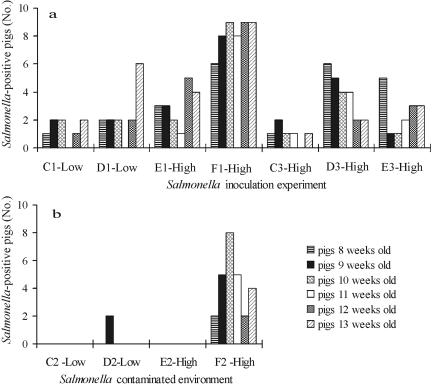
FIG. 1.
(a) Salmonella serovar Typhimurium culture-positive organic pigs found during 6 weeks after inoculation of 3 of 10 pigs with a low dose (paddocks C and D in period 1) or a high dose (paddocks E and F in period 1 and C to E in period 3) of Salmonella serovar Typhimurium cells. (b) Transmission of Salmonella serovar Typhimurium to tracer pigs after introduction into the Salmonella-contaminated environment of paddocks C to F in the second experimental period.
FIG. 2.
Overall percentages of Salmonella-positive pigs over time after artificial inoculation of 3 of 10 pigs (seven groups) based on bacteriological Salmonella serovar Typhimurium status for inoculated pigs (▴) and tracer pigs (▪) and on the antibody level (ELISA serology) for inoculated pigs (▵) and tracer pigs (□).
The three inoculated pigs in each group were Salmonella positive on 5 to 16 out of 18 occasions irrespective of the inoculum dose. The inoculated pigs mostly had intermittent excretion of Salmonella, although three pigs remained Salmonella culture negative and five pigs culture positive throughout the trial (6 weeks). The Salmonella excretion level in the inoculated pigs was generally low, as 72% of the positive samples (n = 68) contained <100 cells g−1. Only two pigs excreted >104 cells; one, an inoculated pig from paddock F, showed clinical symptoms of salmonellosis, with an excretion level of >106 cells g−1 on two occasions before it died (week 4). Salmonella serovar Typhimurium DT12 rifampinres was isolated from this pig in liver, spleen, lung, intestinal wall, cecum content, and mesenteric and ileocolic lymph node samples following postmortem bacteriological examination. In addition, four pigs were excluded before the end of the inoculation trials in the first and third periods due to general poor health (n = 3) and a broken leg (n = 1), and Salmonella spp. were isolated at necropsy in three of these.
Transmission of Salmonella to tracer pigs.
Twenty-six of the 46 tracer pigs (56%) grouped with the inoculated pigs tested Salmonella culture positive (test strain) at least once during the 6 weeks. A pig with at least one positive finding was termed a Salmonella culture-positive pig, and a similar definition was used for ELISA-positive pigs. The numbers of Salmonella culture-positive tracer pigs in the five high-dose groups varied from a single pig (C3) to all seven tracer pigs (F1), while half of the tracer pigs became Salmonella culture positive in the two low-dose groups (Table 2). The mean frequencies of Salmonella culture-positive tracer pigs were 0.50 in the low-dose groups and 0.58 in the high-dose groups. The low-dose and high-dose groups were not found to be significantly different (P = 0.42). In contrast to the results of bacteriological analyses, the mix-ELISA test results indicated that the frequencies of seropositive tracer pigs were significantly different (P = 0.04) between the low- and high-dose groups, with mean frequencies of 0.21 and 0.82, respectively (Table 2).
TABLE 2.
Frequency of tracer pigs testing Salmonella positive based on results by two diagnostic methods
| Diagnostic method | No. positive/total in indicated Salmonella inoculation experimenta
|
||||||
|---|---|---|---|---|---|---|---|
| Low dose
|
High dose
|
||||||
| C-1 | D-1 | E-1 | F-1 | C-3 | D-3 | E-3 | |
| Bacteriology | 2/7 | 5/7 | 3/7 | 7/7 | 1/5 | 5/6 | 3/7 |
| Serologyb | 1/7 | 2/7 | 5/7 | 7/7 | 4/5 | 6/6 | 4/7 |
Number of Salmonella-positive pigs (positive in at least one of six samples) out of the total number of tracer pigs in each group is shown, reflecting transmission of Salmonella from artificially infected pigs (low or high dose of Salmonella cells) to tracer pigs in paddocks C to F in periods 1 and 3 (see Table 1).
ELISA method.
The Salmonella seroprevalence in the pigs was generally higher than their bacteriological status (Fig. 2 and 3). Overall, 22 out of 94 tracer pigs tested seropositive without being Salmonella culture positive. Eight culture-positive tracer pigs were not seroresponding, but half of these were not culture positive until the last week and were therefore unlikely to seroconvert within the time span of the study. Two of the nonresponding pigs excreted <100 CFU g−1 once in the second week, one pig excreted <100 CFU g−1 in weeks 3 and 4, and one pig was positive on three occasions (<104 CFU g−1 the last week) before being killed due to poor health.
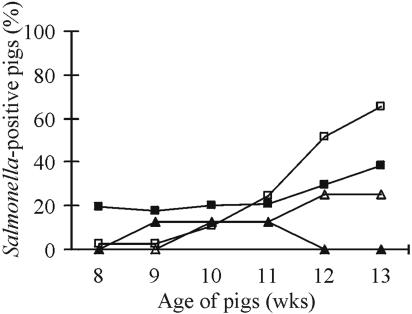
FIG. 3.
Overall percentages of Salmonella-positive pigs after introduction of tracer pigs into a Salmonella-contaminated environment in the second period for paddocks C to F, based on bacteriological (▪) and serological (□) status, and in the third period in paddock F, based on bacteriological (▴) and serological (▵) status.
Measuring the number of weeks (1 to 6) before a tracer pig tested Salmonella culture positive for the first time showed that the seven Salmonella-positive tracer pigs from the low-dose groups were all Salmonella negative until the last week (week 6), except for one (negative until week 5) (mean, 5.9 weeks), whereas most of the high-dose tracer pigs (n = 19) became infected during the first weeks (mean, 2.2 weeks). The number of weeks passed for the high-dose groups was significantly lower than that for the low-dose groups (P = 0.0001). Furthermore, Salmonella was isolated more than twice in 31% of the tracer pigs (n = 32) from the high-dose groups. The infection level seemed to vary under apparently similar conditions, as 33 out of the 65 (50.8%) positive fecal samples (n = 268) came from tracer pigs in paddock F (high dose), while the other four high-dose groups together accounted for 38.5% of the positive samples. The level of Salmonella excretion in the tracer pigs was mainly low, as 93.8% of the positive samples (n = 65) contained <100 cells g−1 feces.
In the third test period, the Salmonella serovar Typhimurium DT12 rifampin/nalidixic acidres test strain was detected once in the environment of Salmonella-negative control paddock A (mud hole) and once in a pig from paddock B. Additionally, a rifampinres (second period) or rifampin/nalidixic acidres (third period) Salmonella serovar Typhimurium organism of phage type 109 (the test strain is DT12) was found in the environment of paddock A once in each of the last two periods and once in paddock B in the third period (Table 3). The doubly resistant strain was isolated from the pig in paddock B that also tested positive for the test strain (two subsequent weeks) (Table 3). In addition, Table 3 shows the detection of serotypes of Salmonella besides the test strain (on 23 occasions in pigs and 40 occasions in environmental samples), which has been described previously (29).
TABLE 3.
Occurrence of the Salmonella serovar Typhimurium DT12 test strain and other Salmonella serotypes in pigs and the environment of paddocks A to F in three periods
| Periodb | Wk | No. of Salmonella-positive samples or other Salmonella serotypes in paddocka:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ac
|
Bc
|
C
|
D
|
E
|
F
|
||||||||
| Pigs | ENV | Pigs | ENV | Pigs | ENV | Pigs | ENV | Pigs | ENV | Pigs | ENV | ||
| 1 | 1 | 1 | 2 | 1 | 3 | 4 | 6 | 5 | |||||
| 2 | 2 | 1 | 2 | 1 | 3 | 6 | 8 | 6 | |||||
| 3 | 2 | 1 | 2 | 1 | 2 | 4 | 9 | 7 | |||||
| 4 | 1 | 2 | 1 | 6 | 8 | 7 | |||||||
| 5 | Gol | 2 | 3 | 5 | 6 | 9 | 6 | ||||||
| 6 | 2 | 3 | 6 | 3 | 4 | 5 | 9 | 6 | |||||
| 2 | 1 | 107 | Ana | 1 | 5 | 2 | 6 | ||||||
| 170 | |||||||||||||
| 2 | Ana | New | Ana | 2 | 2 | 4 | 5 | 6 | |||||
| 107 | 170 | ||||||||||||
| 3 | Rea | 107 | New | 1 | 2 | 8 | 6 | ||||||
| 170 | |||||||||||||
| 4 | Gol | 109 | 107 | 1 | 1 | 3 | 5 | 6 | |||||
| ND (3) | 107 | 170 | |||||||||||
| Sta | Uga | ||||||||||||
| 5 | Der | Liv | 41 | Uga | 3 | 2 | 6 | ||||||
| Der | 41 | 170 | 170 | ||||||||||
| 6 | Der | 41 | Liv | 41 | 2 | 4 | 7 | ||||||
| 3 | 1 | New | New | 1 | 3 | 6 | 6 | 5 | 2 | 5 | |||
| New | 107 | ||||||||||||
| Ohio | |||||||||||||
| 2 | New | 2 | 2 | 5 | 7 | 1 | 3 | 1 | 4 | ||||
| New | New | 107 | |||||||||||
| 3 | New | RDNC | 1 | 2 | 4 | 4 | 1 | 1 | 1 | 4 | |||
| 109 | 109 | 109 | Ind | RDNC | 107 | ||||||||
| 4 | 3.10: | New | 1 | 1 | Liv | 4 | 5 | 2 | 1 | 1 | 5 | ||
| 4.12: | 4.12: | ||||||||||||
| Ago | |||||||||||||
| 5 | 1 | 1 | 2 | 3 | 3 | 4 | |||||||
| 6 | Ohio | 1 | 3 | 2 | 4 | 3 | 2 | 4 | |||||
| NT | 109 | 109 | 109 | ||||||||||
| Uga | |||||||||||||
The numbers refer to the number of times the Salmonella test strain was detected on each sampling occasion. Additional detection of other Salmonella serotypes is shown with the following abbreviations: Ana, Anatum; Ago, Agona; Der, Derby; Gol, Goldcoast; Ind, Indiana; Liv, Livingstone; New, Newport; Rea, Reading; Sta, Stanley; Uga, Uganda; 4.12:, 4.12:d:−; 3.10:, 3.10:−:1.5; NT, not typeable; ND, not serotyped. 41, 107, 109, and 170 are phage type (DT) numbers of serovar Typhimurium; RDNC, unspecific phage type.
Three experimental periods each lasting 6 weeks, running from late April to the beginning of September.
Paddocks A and B are Salmonella-negative control paddocks.
Survival of Salmonella in the pasture environment.
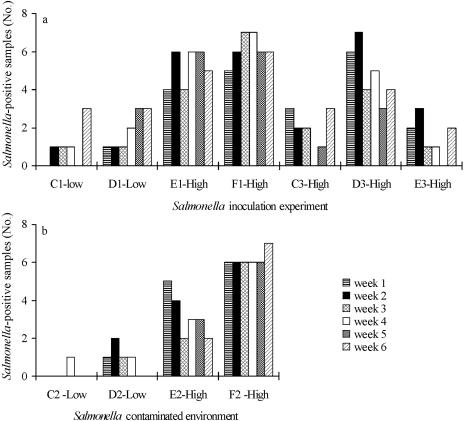
The ability of Salmonella to persist in the nonhost environment was indicated by the detection of Salmonella in 6 to 29 out of the 42 environmental samples from each of the two low- and five high-dose inoculation experiments (Fig. 4a). There was no indication of a relationship between the inoculum dose and number of positive environmental samples.
FIG. 4.
(a) Salmonella serovar Typhimurium culture-positive environmental samples found during 6 weeks after inoculation of pigs with a low or high dose of Salmonella serovar Typhimurium cells in paddocks C to F in period one or three. (b) Survival of Salmonella serovar Typhimurium in the environment of paddocks C to F in the second experimental period.
The overall numbers of times Salmonella was isolated from each of the seven locations (1, water cup; 2, rear end of paddock; 3, intersection of hut/water cup; 4, defecation area; 5, feeding area; 6, hut; 7, mud hole) in the paddocks were 34, 9, 35, 36, 31, 39, and 39, respectively. Location 2, in the rear end of the paddock opposite the hut (9 positive samples), had significantly fewer Salmonella-positive samples (P < 0.0001) than the other locations (mean, 35). This was apparently not due to a lower level of fecal contamination at this more distant location, based on enumeration of presumptive E. coli organisms serving as feces indicator. An approximation to the development over time by a second-order polynomial did not detect abnormal behavior at location 2 (results not shown). No E. coli organisms were detected in the paddock environment before introduction of the pigs. Four weeks after introduction of pigs, the levels of E. coli in the environmental samples had increased to and remained at a range of approximately 3 to 7 log10 CFU g−1 soil, independent of the sample location (data not shown).
After removal of the pigs, the inoculum strain was isolated a total of 11 times (n = 196) from the paddock environment within 5 weeks, and two of the huts were contaminated until the examinations were terminated after 7 weeks. The decline in E. coli numbers within these 7 weeks was found to be an approximate log-linear reduction, with a T90 of 3.4 for all locations and a T90 of 8.4 for the hut (data not shown). Via an extrapolation of this log-linear reduction, a level of 1 CFU was found to be reached after 19 weeks, on average, for the seven locations in the paddock and after 48 weeks in the huts.
Infectivity of the contaminated paddocks.
New tracer pigs (n = 40) were introduced into the paddocks (C to F) at 1 day after the pigs from the first period were removed to ensure the highest possible level of Salmonella organisms remaining in the environment. Salmonella infections were almost limited to tracer pigs in paddock F (high dose), where all 10 pigs excreted Salmonella at least once during the 6 weeks and 46% of the total number of fecal samples (n = 56) were Salmonella positive (Fig. 1b). Two of five pigs (F) with an excretion level of >100 cells g−1 were killed after week 4 because of poor health, and postmortem examinations confirmed symptoms of salmonellosis. An additional seven pigs from the other paddocks were excluded before the end of the second period, and one of these was found to be Salmonella positive.
The high infection rate of the tracer pigs introduced into paddock F was in accordance with a high level of contamination in the environment, since the inoculum strain, Salmonella serovar Typhimurium DT12 rifampinres was detected in 88.1% of the environmental samples (Fig. 4b). In the other high-dose paddock (E), Salmonella was found in 45.2% of the samples, but no Salmonella was isolated from the pigs. Interestingly, the lower environmental contamination level in paddock D (11.9%) resulted in two Salmonella-positive tracer pigs (Fig. 1b and 4b). However, despite the rare isolation of Salmonella, 56% of the tracer pigs in paddocks C to E had seroconverted in the last week (Fig. 3).
When new tracer pigs were introduced into paddock F in the third period, only two of eight pigs excreted Salmonella on three occasions, while three pigs seroconverted, although 61.9% of the environmental samples (n = 42) contained Salmonella.
DISCUSSION
This study aimed to assess the Salmonella infection dynamics in organic outdoor pigs and to determine the level of Salmonella contamination in the paddock environment. Salmonella-negative tracer pigs were grouped with pigs artificially infected with Salmonella or exposed to a Salmonella-contaminated paddock environment to allow natural acquisition of Salmonella infections in the tracer pigs. The artificially infected pigs were inoculated with two different doses of Salmonella in an attempt to resemble low and high infection risk scenarios for the tracer pigs. The study showed that Salmonella infections can spread among 8- to 13-week-old organic pigs reared at low densities in outdoor facilities. Furthermore, Salmonella persisted in the paddock environment, and these contaminated pastures were able to cause infections in introduced tracer pigs.
Imitation of Salmonella infection by artificial inoculation of conventionally reared pigs with moderate doses was previously shown to resemble natural infection with respect to excretion level and antibody response (8). Nevertheless, the Salmonella excretion level in our pigs was low (<100 CFU g−1) in 72% of the Salmonella-positive animals 3 days after inoculation, compared to the higher excretion levels, 2 to 10 log10 CFU g−1, found by Baggesen et al. (8). It is unclear whether this difference was related to an effect of the organic rearing of the pigs. Another possible explanation is a difference in inoculation methods. Baggesen et al. (8) administered the bacteria in small portions of feed, while we gave a Salmonella solution directly to each pig with a gastric tube to control the dose given to individual animals, which may have caused higher killing of Salmonella due to the gastric acid barrier. Furthermore, the additional selection for nalidixic acid resistance might have influenced the infectivity of the test strain, as mutations for resistance are likely to reduce the growth rate and virulence of the bacteria. However, Björkman et al. (13) found that Salmonella serovar Typhimurium resistant to rifampin and nalidixic acid made compensatory mutations that restored fitness without loss of resistance.
The 26 tracer pigs (56%) found to be Salmonella culture positive when grouped with artificially infected pigs were unequally distributed among the seven groups, as the proportion of Salmonella-shedding tracer pigs ranged from 0.14 to 1.0 (Table 2). This is similar to the known within-herd prevalence for Danish conventionally reared pigs, which ranges from 0.1 to 1 with a median of 0.2 (7, 16). When nine conventionally reared pigs were grouped with inoculated pigs shedding 2.7 log10 CFU Salmonella g−1 feces, individual rectal swabs were Salmonella negative 2 days after exposure, while pooled fecal and subsequent necropsy samples were Salmonella positive (21). However, these results were obtained with indoor pigs and therefore are not comparable to the current study. Overall, 29 tracer pigs (63%) seroconverted within the 6-week study period; when serology data were used to determine the proportion of Salmonella-positive tracer pigs, there were significant differences in infection frequencies in the low- and high-risk infection scenarios with different inoculation doses. This is in contrast to the proportion of Salmonella-shedding tracer pigs, which seemed independent of the inoculation dose. However, the inoculation dose seemed to influence the length of time before the pigs tested Salmonella positive in feces (2.1 versus 5.9 weeks), and this indicates the importance of maintaining low exposure to Salmonella in order to limit and control infections.
The observed higher seroprevalence, compared to bacteriological Salmonella prevalence, can be explained partly by the low Salmonella excretion level, intermittent excretion (57, 58), and the low sensitivity of the culture method, previously reported to be 0.5 to 0.6 (6). In this study, a majority of the positive tracer animals (93.7%) excreted low levels of Salmonella (<100 CFU g−1), while an intermittent excretion pattern was observed in 30% of the positive tracer pigs. Another explanation is that serology captures the history of exposure to Salmonella antigens, as pigs may remain seropositive for 10 weeks or more after seroconversion, which has previously been shown to occur within 6 to 37 days after infection (23, 34, 40). This also stresses the importance of obtaining pigs for infection studies with no prior exposure to infections; however, organic rearing of pigs was prioritized in this study, which makes it more difficult to ensure a Salmonella-free status. The delay in seroconversion was seen particularly in the second period, in which the seroprevalence of the tracer pigs continued to increase for 3 weeks after the bacteriological peak (week 3) (Fig. 3), whereas the inoculated pigs exposed directly to high levels of Salmonella started to serorespond within the first week (Fig. 2). The initial delay in seroconversion was probably the major reason why eight Salmonella-shedding tracer pigs (total, 94 tracer pigs) remained seronegative. This concurs with findings showing that the ELISA method is valuable for stating the Salmonella infection risk at the herd level, whereas its capacity for detection of Salmonella in individual animals may be limited (36, 40, 48). The reported number of Salmonella culture-positive pigs was based on isolations of the test strain only, but within the current study there was an unexpectedly high level of detection of Salmonella types other than the test strain, e.g., Salmonella serovars Newport, Livingstone, and Typhimurium DT41 and DT107 (Table 3), which has been reported previously (29). These Salmonella strains may have contributed to the seroresponse; however, Salmonella serovars other than Typhimurium tend to give a moderate serological response in the mix-ELISA (3, 40, 52).
The detection of different Salmonella serovars in both pigs and the environment, including the control paddocks (29), probably reflects the widespread occurrence of Salmonella in nature and the fact that outdoor pigs will be exposed thereto, but it is not clear how their presence influences the introduction of Salmonella in outdoor pigs. The non-test strains were detected in all paddocks except F, in which the infection rate (test strain) was high. A mixed infection was detected on one occasion in paddock C.
The frequent isolation of the Salmonella test strain in the environment (46%; n = 296) indicated the ability of Salmonella to survive outside the host, which has been suggested to be an adaptation to ensure passage to the next host (53). Heavy Salmonella serovar Typhimurium DT120 contamination of soil has also been reported for holding paddocks used to retain sheep prior to slaughter, where Salmonella persisted for 6 months in the soil with no Salmonella-reducing effect of liming, whereas plowing appeared to reduce the level of Salmonella contamination, presumably due to better mixture with other competitive soil bacteria (45).
The Salmonella organisms isolated from the environmental samples must have survived either in the external environment the entire time or via repeated passages through the pigs. Salmonella was detected in soil no longer than 5 weeks after the paddocks were vacated, except in some huts that remained contaminated for all 7 weeks. This underscores the need for good production hygiene to avoid persistence of Salmonella. Salmonella was detected for longer in this study than the reported survival rate of 7 to 14 days in soil amended with naturally contaminated slurry from infected pig herds (9, 15). This further indicates a higher contamination potential when infected pigs in outdoor production systems are allowed to shed directly onto soil, compared to contamination from the spread of slurry. It also suggests that assessment of the Salmonella infection risk associated with contaminated slurry may not apply to outdoor pig production (12). A generally high fecal contamination level was also evident from detection of a high level of E. coli organisms (3 to 7 log10 CFU g−1) in the paddock compared to the levels after normal application of pig slurry (3 log10 CFU g−1 soil) (15). Other studies have reported recovery of Salmonella from soil several months after inoculation, most likely due to different experimental conditions and higher inoculation doses, e.g., 5 log CFU g−1 (22, 28, 38, 39).
E. coli has been reported to a have a survival rate similar to Salmonella, thus providing a good indicator of decimation of Salmonella (38, 41). In contrast, Winfield and Groisman (53) found that Salmonella has better adaptation to nonhost survival than does E. coli. In the current study, the decimation time, T90, of presumptive E. coli after vacation of the paddocks was estimated to be 3.4 weeks. A level of 1 CFU g−1 was estimated to be reached after 19 weeks, on average, for the seven locations and after 48 weeks for the huts. Assuming that Salmonella organisms are equivalent to E. coli with respect to survival, this indicates the possible persistence time of Salmonella in the paddock environment.
The physical and chemical properties of the external environment strongly influence the survival of Salmonella, and it can therefore be difficult to predict the duration of persistence and the associated infection risk for livestock. Alternating temperatures, variable rainfall, and other uncontrollable factors under natural conditions hamper examination of a possible seasonal effect. There have been reports showing no seasonal difference (26), longer survival in winter (41), more rapid decline of Salmonella after exposure to summer temperatures (47), and high summer temperatures increasing the contamination of vegetables fertilized with Salmonella-containing manure (38).
A potential Salmonella infection risk from contaminated soils could perhaps be overcome by soil treatments such as, e.g., plowing. A reduced survival of Salmonella in soil has been reported at depths of 5 to 50 cm, compared to subsurface (42), and after plowing of pastures contaminated by infected sheep (45) or slurry-amended fields (15). However, in other studies, plowing of slurry-amended fields did not prevent detection of Salmonella after 2 weeks (46), and incorporation of contaminated waste to a depth of 10 to 15 cm slowed the decline compared to surface application (26). A higher rate of elimination of bacterial pathogens in surface soil may be due to a more instable environment exposed to extreme temperatures (35, 12), desiccation (45, 59) and UV light (47). Thus, the persistence of Salmonella in the soil environment can be affected by the degree of infiltration into the soil column depending on, e.g., soil texture, water movements, and management (49), and soils with higher clay (38, 42) and organic matter (17) content may protect Salmonella. In the current study, the different contamination levels obtained in the paddocks did not permit a comparable examination of the pathogen-reducing effect of plowing.
The Salmonella infection risk associated with contaminated pastures was assessed by introduction of new tracer pigs in the second study period, where the first pig to contract infection could be assigned solely to the contamination carried over from the first period. A total of 12 pigs (30%) shed Salmonella at least once, while 24 (60%) pigs seroconverted. The presence of Salmonella was not necessarily predictive of the occurrence of Salmonella infection in pigs, as very low Salmonella levels gave rise to Salmonella-shedding pigs (paddock D; Fig. 1b and 4b) and rather high Salmonella levels did not (paddock E; Fig. 1b and 4b). Exposure to low levels of Salmonella (103 CFU g−1 material) in a preslaughter environment has previously been shown to promote Salmonella infection in pigs (24). Again, serology proved better for detection of the low-level infections with the test strain, as a minimum of 40% of the pigs in each group seroconverted, indicating that the pigs' immune systems were stimulated even at low contamination levels. The use of a 10 OD% cutoff in the mix-ELISA should avoid false-positive reactions, as evaluated in Danish pigs (38). However, this did not apply to Swedish pigs in a region of non-Salmonella endemicity (56). A dose-dependent protection against rechallenge with Salmonella has been found in conventionally reared pigs (55), although another study showed that pigs were receptive to reinfection when reexposed to Salmonella (31).
To our knowledge, the infection risk associated with Salmonella-contaminated pastures for pigs has not been assessed, but the infectivity of Salmonella-contaminated pastures has previously been assessed for calves grazing pastures spread with Salmonella serovar Dublin-containing slurry, leading to isolation of Salmonella in 1 of 12 calves (50). The different infection rates of Salmonella among the groups of organic pigs in this study probably reflects a variable susceptibility to Salmonella infections caused by a number of factors, including the general health status of the pigs and previous exposure. These variations in Salmonella infection rates are similar to those seen in infections of conventionally reared pigs (31). Furthermore, Salmonella organisms recovered from the paddock environment may have lost or attenuated virulence factors important for the ability to infect pigs (33, 37).
The results of our study showed that profound contamination of the environment with Salmonella, as in paddock F (88% positive environmental samples), e.g., due to pigs with symptoms of salmonellosis, tended to increase the risk of a high infection rate. However, even a low-grade Salmonella contamination of the environment confers a risk of infection in pigs, as was seen in paddock D in the second period. Organically reared pigs presumably benefit from a long suckling period, which is approximately 3 weeks longer than in conventional pig production. This allows the establishment of a more robust intestinal flora that renders the pig less susceptible to infection during the changes in the gut ecosystem following the shift from milk to solid feed at weaning (14). However, the organic rearing conditions and the low stocking density in the outdoor facilities do not prevent infections, probably because there is still close contact between the pigs, e.g., in the hut, and their rooting behavior is likely to pose a high risk of ingestion of Salmonella from the contaminated environment (20, 44). The high infection rate in paddock F points to the importance of removing pigs with clinical symptoms of salmonellosis as soon as possible, because these animals contribute significantly to the persistence of Salmonella and may in addition serve as vehicles for spread to other animals and the environment, though no Salmonella was detected when a total of 22 rodents and 22 birds were examined for the occurrence of Salmonella in a small wildlife study performed in connection with this study, as described elsewhere (29). Finding of the test strain in the control paddocks and pigs on a few occasions indicated that dissemination occurs despite the preventive measures taken to avoid cross-contamination between paddocks and samples. Such reservoirs of Salmonella should be limited, to reduce long-term persistence (10, 46) and potential reentry into an active shedding status triggered by some favorable condition or stress, e.g., from transport to the slaughterhouse (25, 27). An active Salmonella-shedding status should be avoided because in particular it challenges food safety at slaughter due to fecal contamination of the carcasses (11).
It cannot be concluded from this study that organic rearing conditions serve as protection against Salmonella infections, as a high infection rate did occur under some circumstances. In addition, Salmonella organisms were able to survive in the paddock environment for several weeks, and even an estimated low level of Salmonella contamination was able to pose an infection risk to newly introduced animals. However, the longer- term persistence/infection risk was not assessed.
Acknowledgments
We thank the Salmonella group at the Danish Institute for Food and Veterinary Research and the animal assistants at the research farm, Rørrendegård, for sampling of the pigs.
This work was supported by the Danish Research Centre of Organic Farming (DARCOF, II.10).
REFERENCES
- 1.Alban, L., H. Stege, and J. Dahl. 2002. The new classification system for slaughter-pig herds in the Danish Salmonella surveillance-and-control program. Prev. Vet. Med. 53:133-146. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 24. August 1999, posting date. Council regulation (EC) no. 1804/1999. Official J. Eur. Communities. 27(6):2005. [Online.] http://europa.eu.int/eur-lex/pri/en/oj/dat/1999/l_222/l_22219990824en00010028.pdf. [Google Scholar]
- 3.Baggesen, D. L., and J. Christensen. 1997. Distribution of Salmonella enterica serotypes and phage types in Danish pig herds, p. 107-109. In S. Bech-Nielsen and J. P. Nielsen (ed.), Proceedings of the Second International Symposium on Epidemiology and Control of Salmonella in Pork. Copenhagen, Denmark.
- 4.Baggesen, D. L., J. Dahl, A. Wingstrand, and B. Nielsen. 1997. Detection of Salmonella enterica in different materials from the environment of pig herds, p. 173-175. In S. Bech-Nielsen and J. P. Nielsen (ed.), Proceedings of the Second International Symposium on Epidemiology and Control of Salmonella in Pork. Copenhagen, Denmark.
- 5.Baggesen, D. L., D. Sandvang, and F. M. Aarestrup. 2000. Characterization of Salmonella enterica serovar Typhimurium DT104 isolated from Denmark and comparison with isolates from Europe and the United States. J. Clin. Microbiol. 38:1581-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggesen, D. L., and H. C. Wegener. 1993. Påvisning af Salmonella hos svin. [Detection of Salmonella in pigs.] Dan. Vet. Tidsskr. 76:41-47. [Google Scholar]
- 7.Baggesen, D. L., H. C. Wegener, F. Bager, H. Stege, and J. Christensen. 1996. Herd prevalence of Salmonella enterica infections in Danish slaughter pigs determined by microbiological testing. Prev. Vet. Med. 26:201-213. [Google Scholar]
- 8.Baggesen, D. L., A. Wingstrand, B. Carstensen, B. Nielsen, and F. M. Aarestrup. 1999. Effects of the antimicrobial growth promoter tylosin on subclinical infection of pigs with Salmonella enterica serotype Typhimurium. Am. J. Vet. Res. 60:1201-1206. [PubMed] [Google Scholar]
- 9.Baloda, S. B., L. Christensen, and S. Trajcevska. 2001. Persistence of a Salmonella enterica serovar Typhimurium DT12 clone in a piggery and in agricultural soil amended with Salmonella-contaminated slurry. Appl. Environ. Microbiol. 67:2859-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber, D. A., P. B. Bahnson, R. Isaacson, C. J. Jones, and R. M. Weigel. 2002. Distribution of Salmonella in swine production ecosystems. J. Food Prot. 65:1861-1868. [DOI] [PubMed] [Google Scholar]
- 11.Berends, B. R., F. van Knapen, J. M. Snijders, and D. A. Mossel. 1997. Identification and quantification of risk factors regarding Salmonella spp. on pork carcasses. Int. J. Food Microbiol. 36:199-206. [DOI] [PubMed] [Google Scholar]
- 12.Bicudo, J. R., and S. M. Goyal. 2003. Pathogens and manure management systems: a review. Environ. Technol. 24:115-130. [DOI] [PubMed] [Google Scholar]
- 13.Bjorkman, J., D. Hughes, and D. I. Andersson. 1998. Virulence of antibiotic-resistant Salmonella Typhimurium. Proc. Natl. Acad. Sci. USA 95:3949-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blecha, F., D. S. Pollmann, and D. A. Nichols. 1983. Weaning pigs at an early age decreases cellular immunity. J. Anim. Sci. 56:396-400. [DOI] [PubMed] [Google Scholar]
- 15.Boes, J., L. Alban, J. Bagger, V. Mogelmose, D. L. Baggesen, and J. E. Olsen. 2005. Survival of Escherichia coli and Salmonella Typhimurium in slurry applied to clay soil on a Danish swine farm. Prev. Vet. Med. 69:213-228. [DOI] [PubMed] [Google Scholar]
- 16.Christensen, J., D. L. Baggesen, B. Nielsen, and H. Stryhn. 2002. Herd prevalence of Salmonella spp. in Danish pig herds after implementation of the Danish Salmonella Control Program with reference to a pre-implementation study. Vet. Microbiol. 88:175-188. [DOI] [PubMed] [Google Scholar]
- 17.Cools, D., R. Merckx, K. Vlassak, and J. Verhaegen. 2005. Survival of E. coli and Enterococcus spp. derived from pig slurry in soils of different texture. Appl. Soil Ecol. 17:53-62. [Google Scholar]
- 18.Dahl, J., A. Wingstrand, B. Nielsen, and D. L. Baggesen. 1997. Elimination of Salmonella Typhimurium infection by the strategic movement of pigs. Vet. Rec. 140:679-681. [DOI] [PubMed] [Google Scholar]
- 19.Davies, R. H., and I. M. McLaren. 2001. A six year study of the persistence of Salmonella Typhimurium DT104 on a farrow to finish pig farm, p. 265-273. In P. J. van der Wolf (ed.), Proceedings of the 4th International Symposium on the Epidemiology and Control of Salmonella and Other Food Borne Pathogens in Pork. ADDIX, Wijk bij Duurstede, The Netherlands.
- 20.Fedorka-Cray, P. J., J. T. Gray, and C. Wray. 2000. Salmonella infections in pigs, p. 191-207. In C. Wray and A. Wray (ed.), Salmonella in domestic animals. CABI Publishing, Oxon, United Kingdom.
- 21.Fedorka-Cray, P. J., S. C. Whipp, R. E. Isaacson, N. Nord, and K. Lager. 1994. Transmission of Salmonella Typhimurium to swine. Vet. Microbiol. 41:333-344. [DOI] [PubMed] [Google Scholar]
- 22.Guo, X., J. Chen, R. E. Brackett, and L. R. Beuchat. 2002. Survival of Salmonella on tomatoes stored at high relative humidity, in soil, and on tomatoes in contact with soil. J. Food Prot. 65:274-279. [DOI] [PubMed] [Google Scholar]
- 23.Holt, P. S. 2000. Host susceptibility, resistance and immunity to Salmonella in animals, p. 73-87. In C. Wray and A. Wray (ed.), Salmonella in domestic animals. CABI Publishing, Oxon, United Kingdom.
- 24.Hurd, H. S., J. K. Gailey, J. D. McKean, and M. H. Rostagno. 2001. Rapid infection in market-weight swine following exposure to a Salmonella Typhimurium-contaminated environment. Am. J. Vet. Res. 62:1194-1197. [DOI] [PubMed] [Google Scholar]
- 25.Hurd, H. S., J. D. McKean, R. W. Griffith, I. V. Wesley, and M. H. Rostagno. 2002. Salmonella enterica infections in market swine with and without transport and holding. Appl. Environ. Microbiol. 68:2376-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchison, M. L., L. D. Walters, A. Moore, K. M. Crookes, and S. M. Avery. 2004. Effect of length of time before incorporation on survival of pathogenic bacteria present in livestock wastes applied to agricultural soil. Appl. Environ. Microbiol. 70:5111-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isaacson, R. E., L. D. Firkins, R. M. Weigel, F. A. Zuckermann, and J. A. DiPietro. 1999. Effect of transportation and feed withdrawal on shedding of Salmonella Typhimurium among experimentally infected pigs. Am. J. Vet. Res. 60:1155-1158. [PubMed] [Google Scholar]
- 28.Islam, M., J. Morgan, M. P. Doyle, S. C. Phatak, P. Millner, and X. Jiang. 2004. Fate of Salmonella enterica serovar Typhimurium on carrots and radishes grown in fields treated with contaminated manure composts or irrigation water. Appl. Environ. Microbiol. 70:2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen, A. N., J. Lodal, and D. L. Baggesen. 2004. High diversity of Salmonella serotypes found in an experiment with outdoor pigs. Wageningen J. Life Sci. 52:109-117. [Google Scholar]
- 30.Jensen, A. N., G. Sorensen, D. L. Baggesen, R. Bødker, and J. Hoorfar. 2003. Addition of novobiocin in pre-enrichment step can improve Salmonella culture protocol of modified semisolid Rappaport-Vassiliadis. J. Microbiol. Methods 55:249-255. [DOI] [PubMed] [Google Scholar]
- 31.Kranker, S., L. Alban, J. Boes, and J. Dahl. 2003. Longitudinal study of Salmonella enterica serotype Typhimurium infection in three Danish farrow-to-finish swine herds. J. Clin. Microbiol. 41:2282-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann, E. L. 1975. Nonparametrics: statistical methods based on ranks. Holden and Day, San Francisco, Calif.
- 33.Lesne, J., S. Berthet, S. Binard, A. Rouxel, and F. Humbert. 2000. Changes in culturability and virulence of Salmonella Typhimurium during long-term starvation under desiccating conditions. Int. J. Food Microbiol. 60:195-203. [DOI] [PubMed] [Google Scholar]
- 34.Lo Fo Wong, D. M., J. Dahl, P. J. van der Wolf, A. Wingstrand, L. Leontides, and A. von Altrock. 2003. Recovery of Salmonella enterica from seropositive finishing pig herds. Vet. Microbiol. 97:201-214. [DOI] [PubMed] [Google Scholar]
- 35.Mitscherlich, E., and E. H. Marth. 1984. Microbial survival in the environment. Springer-Verlag, Berlin, Germany.
- 36.Mousing, J., P. T. Jensen, C. Halgaard, F. Bager, N. Feld, B. Nielsen, J. P. Nielsen, and S. Bech-Nielsen. 1997. Nation-wide Salmonella enterica surveillance and control in Danish slaughter swine herds. Prev. Vet. Med. 29:247-261. [DOI] [PubMed] [Google Scholar]
- 37.Mouslim, C., F. Hilbert, H. Huang, and E. A. Groisman. 2002. Conflicting needs for a Salmonella hypervirulence gene in host and non-host environments. Mol. Microbiol. 45:1019-1027. [DOI] [PubMed] [Google Scholar]
- 38.Natvig, E. E., S. C. Ingham, B. H. Ingham, L. R. Cooperband, and T. R. Roper. 2002. Salmonella enterica serovar Typhimurium and Escherichia coli contamination of root and leaf vegetables grown in soils with incorporated bovine manure. Appl. Environ. Microbiol. 68:2737-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson, F. A., S. J. Groves, and B. J. Chambers. 2005. Pathogen survival during livestock manure storage and following land application. Bioresour. Technol. 96:135-143. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen, B., D. Baggesen, F. Bager, J. Haugegaard, and P. Lind. 1995. The serological response to Salmonella serovars Typhimurium and Infantis in experimentally infected pigs. The time course followed with an indirect anti-LPS ELISA and bacteriological examinations. Vet. Microbiol. 47:205-218. [DOI] [PubMed] [Google Scholar]
- 41.Placha, I., J. Venglovsky, N. Sasakova, and I. F. Svoboda. 2001. The effect of summer and winter seasons on the survival of Salmonella Typhimurium and indicator micro-organisms during the storage of solid fraction of pig slurry. J. Appl. Microbiol. 91:1036-1043. [DOI] [PubMed] [Google Scholar]
- 42.Platz, S. 1980. Studies on survival of Salmonella Typhimurium in different types of soils under outdoor climatic conditions. Zentralbl. Bakteriol. Mikrobiol. Hyg. B 171:256-268. [PubMed] [Google Scholar]
- 43.Popoff, M. Y. 2001. Antigenic formulas of the Salmonella serovars. WHO Collaborating Centre for Reference and Research on Salmonella. Institute Pasteur, Paris, France.
- 44.Proux, K., R. Cariolet, P. Fravalo, C. Houdayer, A. Keranflech, and F. Madec. 2001. Contamination of pigs by nose-to-nose contact or airborne transmission of Salmonella Typhimurium. Vet. Res. 32:591-600. [DOI] [PubMed] [Google Scholar]
- 45.Purvis, G. M., S. J. Evans, S. J. S. Pascoe, R. H. Davies, and K. Hullah. 2002. Environmental persistence of Salmonella Typhimurium DT 120, p. 269-274. In P. Colin and G. Clément (ed.), Proceedings of the International Symposium on Salmonella and Salmonellosis. ZOOPOLE Developpement, Ploufragan, France.
- 46.Sandvang, D., L. B. Jensen, D. L. Baggesen, and S. B. Baloda. 2000. Persistence of a Salmonella enterica serotype Typhimurium clone in Danish pig production units and farmhouse environment studied by pulsed field gel electrophoresis (PFGE). FEMS Microbiol. Lett. 187:21-25. [DOI] [PubMed] [Google Scholar]
- 47.Schlundt, J. 1984. Survival of pathogenic enteric bacteria in anaerobic digestion and on slurry-treated land. Diss. Abstr. Int. C 45:1025. [Google Scholar]
- 48.Sorensen, L. L., L. Alban, B. Nielsen, and J. Dahl. 2004. The correlation between Salmonella serology and isolation of Salmonella in Danish pigs at slaughter. Vet. Microbiol. 101:131-141. [DOI] [PubMed] [Google Scholar]
- 49.Stoddard, C. S., M. S. Coyne, and J. H. Grove. 1998. Fecal bacteria survival and infiltration through a shallow agricultural soil: timing and tillage effects. J. Environ. Qual. 27:1516-1523. [Google Scholar]
- 50.Taylor, R. J. 1973. A further assessment of the potential hazard for calves allowed to graze pasture contaminated with Salmonella Dublin in slurry. Br. Vet. J. 129:354-358. [DOI] [PubMed] [Google Scholar]
- 51.van der Wolf, P. J., A. R. Elbers, H. M. van der Heijden, F. W. van Schie, W. A. Hunneman, and M. J. Tielen. 2001. Salmonella seroprevalence at the population and herd level in pigs in The Netherlands. Vet. Microbiol. 80:171-184. [DOI] [PubMed] [Google Scholar]
- 52.van Winsen, R. L., A. van Nes, D. Keuzenkamp, H. A. Urlings, L. J. Lipman, S. Biesterveld, J. M. Snijders, J. H. Verheijden, and F. van Knapen. 2001. Monitoring of transmission of Salmonella enterica serovars in pigs using bacteriological and serological detection methods. Vet. Microbiol. 80:267-274. [DOI] [PubMed] [Google Scholar]
- 53.Winfield, M. D., and E. A. Groisman. 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 69:3687-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wingstrand, A., J. Dahl, and D. M. A. Lo Fo Wong. 1999. Salmonella-prevalence in Danish organic, free-range, conventional and breeding herds, p. 186-189. In P. Bahnson and T. Blaha (ed.), Proceedings of the 3rd International Symposium on the Epidemiology and Control of Salmonella in Pork. Washington, D.C.
- 55.Wingstrand, A., J. P. Nielsen, C. Heisel, D. L. Baggesen, and J. Dahl. 1997. Dose dependent establishment of subclinical Salmonella Typhimurium infection in pigs and protection against homologous re-challenge, p. 85-87. In S. Bech-Nielsen and J. P. Nielsen (ed.), Proceedings of the 2nd International Symposium on Epidemiology and Control of Salmonella in Pork. Copenhagen, Denmark.
- 56.Wiuff, C., B. M. Thorberg, A. Engvall, and P. Lind. 2002. Immunochemical analyses of serum antibodies from pig herds in a Salmonella non-endemic region. Vet. Microbiol. 85:69-82. [DOI] [PubMed] [Google Scholar]
- 57.Wood, R. L., A. Pospischil, and R. Rose. 1989. Distribution of persistent Salmonella Typhimurium infection in internal organs of swine. Am. J. Vet. Res. 50:1015-1021. [PubMed] [Google Scholar]
- 58.Wood, R. L., and R. Rose. 1992. Populations of Salmonella Typhimurium in internal organs of experimentally infected carrier swine. Am. J. Vet. Res. 53:653-658. [PubMed] [Google Scholar]
- 59.Zibilske, L. M., and R. W. Weaver. 1978. Effect of environmental factors on survival of Salmonella Typhimurium in soil. J. Environ. Qual. 7:593-597. [Google Scholar]