Abstract
We isolated Comamonas sp. strain E6, which utilizes terephthalate (TPA) as the sole carbon and energy source via the protocatechuate (PCA) 4,5-cleavage pathway. Two almost identical TPA degradation gene clusters, tphRICIA2IA3IBIA1I and tphRIICIIA2IIA3IIBIIA1II, were isolated from this strain. Based on amino acid sequence similarity, the genes tphR, tphC, tphA2, tphA3, tphB, and tphA1 were predicted to code, respectively, for an IclR-type transcriptional regulator, a periplasmic TPA binding receptor, the large subunit of the oxygenase component of TPA 1,2-dioxygenase (TPADO), the small subunit of the oxygenase component of TPADO, a 1,2-dihydroxy-3,5-cyclohexadiene-1,4-dicarboxylate (DCD) dehydrogenase, and a reductase component of TPADO. The growth of E6 on TPA was not affected by disruption of either tphA2I or tphA2II singly; however, the tphA2I tphA2II double mutant no longer grew on TPA, suggesting that both TPADO genes are involved in TPA degradation. Introduction of a plasmid carrying tphRIICIIA2IIA3IIBIIA1II conferred the TPA utilization phenotype on Comamonas testosteroni IAM 1152, which is able to grow on PCA but not on TPA. Disruption of either tphRII or tphCII on this plasmid resulted in the loss of the growth of IAM 1152 on TPA, suggesting that these genes are essential to convert TPA to PCA in E6. The genes tphA1II, tphA2II, tphA3II, and tphBII were expressed in Escherichia coli, and the resultant cell extracts containing TphA1II, TphA2II, and TphA3II converted TPA in the presence of NADPH into a product which was transformed to PCA by TphBII. On the basis of these results, TPADO was strongly suggested to be a two-component dioxygenase which consists of the terminal oxygenase component (TphA2 and TphA3) and the reductase (TphA1), and tphB codes for the DCD dehydrogenase.
Protocatechuate (PCA) is an important intermediate metabolite in the bacterial degradation of various aromatic compounds, including vanillate, hydroxybenzoates, and phthalates. There are three recognized catabolic pathways for the degradation of PCA: the PCA 2,3-cleavage (9), PCA 3,4-cleavage (16), and PCA 4,5-cleavage (19, 20) pathways. Previously, we characterized the genes and enzymes involved in the PCA 4,5-cleavage pathway of Sphingomonas paucimobilis SYK-6, a well-characterized degrader of lignin-derived compounds (14, 15, 21, 22). During the course of the study on the PCA 4,5-cleavage pathway genes, one of the intermediate metabolites, 2-pyrone-4,6-dicarboxylate (PDC), was found to be useful as a starting material for the production of biodegradable PDC polyamides and polyesters (29, 30). PDC production from lignin is expected to serve as a new process to exploit lignin as an abundant aromatic bioresource. In addition, PDC production via PCA from various petrochemical aromatic compounds, including phthalate isomers, may also be valuable for the production of biodegradable polymers from petrochemical materials. Terephthalate (TPA) is largely used for the production of polyethylene terephthalate and may be a good starting compound for PDC production. To develop the conversion of TPA to PDC, we need to understand the enzymatic systems and the corresponding genes for TPA degradation. However, little is known about the TPA degradation genes.
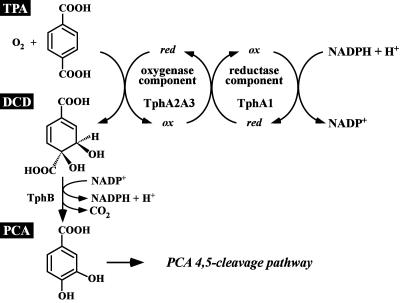
Comamonas testosteroni T-2 is reported to degrade TPA to 1,2-dihydroxy-3,5-cyclohexadiene-1,4-dicarboxylic acid (DCD) by using TPA 1,2-dioxygenase (TPADO), and the resulting DCD is converted to PCA (28) (Fig. 1). Schläfli et al. first reported the purification of the oxygenase component of TPADO from strain T-2, which consists of large and small subunits of 49,000 and 18,000 Da, respectively; however, the reductase component was not identified (28). Shigematsu et al. purified the oxygenase component of TPADO from Delftia tsuruhatensis T7, and the nucleotide sequences of the oxygenase terZα large-subunit gene and the partial sequence of the oxygenase small-subunit gene were determined (31). The characterization of the TPA degradation tph gene clusters of C. testosteroni YZW-D (36) and Rhodococcus sp. strain DK17 (7) were reported; however, detailed results on the characterization of the tph genes of these strains were not presented.
FIG. 1.
Conversion of TPA to PCA by Comamonas sp. strain E6. The following gene products are involved in the conversion of TPA to PCA: TphA2I and TphA2II, oxygenase large subunit of TPADO; TphA3I and TphA3II, oxygenase small subunit of TPADO; TphA1I and TphA1II, reductase component of TPADO; and TphBI and TphBII, DCD dehydrogenase.
In this study, we isolated a TPA degrader, Comamonas sp. strain E6, and found two DNA regions in E6 containing DNA segments which are similar to the terZα gene; they were isolated, sequenced, and characterized. Disruption of the cloned genes in E6 and their expression in Escherichia coli and Comamonas hosts revealed the function of the genes for TPA degradation.
MATERIALS AND METHODS
Isolation of bacteria.
Strain E6 was isolated from soil in Nagaoka, Niigata, Japan. Two grams of soil was inoculated in 10 ml of W minimal salt medium (24) containing 10 mM TPA as the sole carbon and energy source, and the cultures were incubated at 28°C with shaking at 150 rpm. After growth was observed, 400 μl of culture was transferred to 10 ml of fresh medium. After nine serial subcultures, a sample was taken from the final enrichment tube, serially diluted, and plated on W medium agar plates containing 10 mM TPA. Single colonies were obtained after incubation for 1 day at 30°C. After three serial single-colony isolations on W medium plates containing 10 mM TPA, a single colony was designated strain E6.
Strains and plasmids.
The strains and plasmids used for this study are listed in Table 1. Comamonas sp. strain E6 was grown in W minimal salt medium containing 10 mM TPA or in Luria-Bertani (LB) medium at 30°C. The E6 mutants were grown in LB medium. If necessary, 50 mg of kanamycin/liter and 30 mg chloramphenicol/liter were added to the cultures. C. testosteroni IAM 1152 transformants harboring plasmids carrying the tphII genes were grown in W medium containing 10 mM TPA or in LB medium. Escherichia coli strains were grown on LB medium at 37°C. For cultures of cells carrying antibiotic resistance markers, the medium for E. coli transformants was supplemented with 100 mg of ampicillin/liter or 25 mg of kanamycin/liter, and the media for IAM 1152 transformants was supplemented with 50 mg of tetracycline/liter or 100 mg of kanamycin/liter.
TABLE 1.
Strains and plasmids used in this study
| Species and strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Comamonas sp. | ||
| E6 | Wild type; TPA+ | This study |
| DE61 | E6 derivative, tphA2I::kan Kmr | This study |
| DE62 | E6 derivative, tphA2II::cat Cmr | This study |
| DE612 | DE61 derivative, tphA2I::kan tphA2II::cat Kmr Cmr | This study |
| C. testosteroni IAM 1152 | TPA− PCA+ | 34 |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 13 |
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) F′[traD36 proAB+lacIqlacZΔM15] | 38 |
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3); T7 RNA polymerase gene under the control of the lacUV promoter | 33 |
| Plasmids | ||
| pBluescript II SK(+) | Cloning vector; Apr | 32 |
| charomid 9-36 | pTBE-PL9 derivative, Apr | 26 |
| pUC4K | Apr Kmr | 35 |
| pUC6C | pUC1918 carrying cat; Apr Cmr | T. Iwasaki |
| pT7Blue | PCR fragment cloning vector; Apr | Novagen |
| pGEM-T | PCR fragment cloning vector; Apr | Invitrogen |
| pK19mobsacB | oriT sacB Kmr | 27 |
| pKT230 | IncQ broad-host-range cloning vector, Kmr | 3 |
| pKT230MC | pKT230 with multicloning sites of pUC119 | 23 |
| pJB866 | RK2 broad-host-range expression vector; Tcr PmxylS | 5 |
| pETDuet-1 | Expression vector; ColE1 replicon, T7 promoters, Apr | Novagen |
| pRSFDuet-1 | Expression vector; RSF1030-derived replicon, T7 promoters, Kmr | Novagen |
| pVAS4 | Charomid 9-36 with a 4.3-kb HindIII fragment carrying tphA2IA3IBIA1I | This study |
| pVAS8 | Charomid 9-36 with a 6.3-kb HindIII fragment carrying tphA2IIA3IIBIIA1II | This study |
| pSCH1 | Charomid 9-36 with a 9.2-kb SacI fragment carrying tphRICIA2IA3IBIA1I | This study |
| pSCH14 | Charomid 9-36 with a 7.8-kb SacI fragment carrying tphRIICIIA2IIA3IIBIIA1II | This study |
| pSK43 | SK(+) with a 4.3-kb HindIII fragment carrying tphA2IA3IBIA1I | This study |
| pSK63 | SK(+) with a 6.3-kb HindIII fragment carrying tphA2IIA3IIBIIA1II | This study |
| pETtpa23 | pETDuet-1 with a 1.8-kb NdeI-XhoI fragment carrying tphA2IIA3II | This study |
| pRSFtpa1 | pRSFDuet-1 with a 1.1-kb NdeI-XhoI fragment carrying tphA1II | This study |
| pT7tpbc | pT7Blue with a 1.2-kb NdeI-SacI fragment carrying tphBII | This study |
| pETtpb | pETDuet-1 with a 1.2-kb NdeI-SacI fragment carrying tphBII | This study |
| pDSR431 | SK(+) with a 2.0-kb HindIII-Eco47III fragment carrying tphA2I of pSK43 | This study |
| pDSR432 | pDSR431 with an insertion of the 1.2-kb PstI fragment carrying kan from pUC4K replacing the 0.19-kb PstI fragment | This study |
| pDSR43 | pK19mobsacB with a 3.1-kb SacI-KpnI fragment of pDSR432 | This study |
| pDSR631 | SK(+) with a 1.7-kb HindIII-Eco47III fragment carrying tphA2II of pSK63 | This study |
| pDSR632 | pDSR631 with an insertion of the 1.0-kb SmaI-PstI fragment carrying cat from pUC6C replacing the 0.5-kb SphI-PstI fragment | This study |
| pDSR63 | pK19mobsacB with a 2.3-kb SacI-XhoI fragment of pDSR632 | This study |
| pJSK432 | pJB866 with a 2.0-kb HindIII-Eco47III fragment carrying tphA2IA3I of pSK43 | This study |
| pJSK63 | pJB866 with a 6.3-kb HindIII fragment carrying tphA2IIA3IIBIIA1II of pSK63 | This study |
| pEJ89 | pJB866 with a 8.9-kb EcoRI fragment carrying tphRIICIIA2IIA3IIBIIA1II | This study |
| pEJ80 | pEJ89 with a deletion of a 0.5-kb NheI-HindIII fragment carrying part of tphCII | This study |
| pEJ84 | pEJ89 with a deletion of a 0.6-kb MunI-Tth111I fragment carrying part of tphRII | This study |
| pKTC | pKT230 with a 1.6-kb XbaI-BglII fragment carrying tphCII | This study |
| pKTR | pKT230MC with a 1.8-kb KpnI fragment carrying tphRII | This study |
Kmr, Cmr, Apr, and Tcr, resistance to kanamycin, chloramphenicol, ampicillin, and tetracycline, respectively.
DNA manipulations and DNA sequencing.
DNA manipulations, including total DNA isolation, construction of deletion derivatives, and DNA sequencing, were performed as described in a previous study (1). The 16S rRNA gene was amplified by PCR with the universal primers 8F and 1492R (Table 2). The PCR products were purified from a 0.8% agarose gel and were sequenced using primers r1L, r2L, r3L, r4L, and f3L. Sequence analysis was performed using the GeneWorks program (Intelligenetics, Inc., Mountain View, CA.). Homology searches were performed using the DDBJ database with the BLAST program. A pairwise alignment was performed with the EMBOSS alignment tool (http://www.ebi.ac.uk/emboss/align).
TABLE 2.
PCR primers used in this study
| Primer | Sequence |
|---|---|
| 8F | 5′-AGAGTTTGATCCTGGCTCAG-3′ |
| 1492R | 5′-CGGTTACCTTGTTACGACTT-3′ |
| r1L | 5′-GTATTACCGCGGCTGCTGG-3′ |
| r2L | 5′-CATCGTTTACGGCGTGGAC-3′ |
| r3L | 5′-TTGCGCTCGTTGCGGGACT-3′ |
| r4L | 5′-ACGGGCGGTGTGTACAAG-3′ |
| f3L | 5′-GTCCCGCAACGAGCGCAAC-3′ |
| TPA-F | 5′-TGCGTCTAYCACSMVTGG-3′ |
| TPA-R | 5′-TCSRMRTAGAGCTTCCAGTT-3′ |
| TphA2-F | 5′-GGAACGCATATGCAAGAATCC-3′ |
| TphA2-R | 5′-TTGACACCTATCTCGAAGG-3′ |
| TphA1-F | 5′-GAGGACTCCATATGAACCACC-3′ |
| TphA1-R | 5′-TATTACTGCCTCGAGTCCGC-3′ |
| TphB-F | 5′-CCATATGACAATAGTGCACC-3′ |
| TphB-R | 5′-TGACAGAACCATGTCATCTC-3′ |
Cloning of tph genes.
The TPA-F and TPA-R primer set (Table 2) was designed based on the conserved sequence in the terZα gene from Delftia tsuruhatensis T7 and the bphA1c and bphA1d genes from Novosphingobium aromaticivorans DSM 12444 and were used to amplify the tphA2 gene sequence in strain E6. A 305-bp PCR-amplified fragment was used for colony hybridization as a probe to isolate the TPA degradation genes from E6 gene libraries which were constructed using charomid 9-36 (26) with HindIII or SacI digests of the E6 total DNA. Colony and Southern hybridization analyses were performed using the digoxigenin system (Roche, Mannheim, Germany).
Expression of tphA1II, tphA2II, tphA3II, and tphBII in E. coli and preparation of the cell extract.
The 0.4-kb DNA fragment carrying the 5′ terminus of the tphA2II gene was amplified by PCR using ExTaq polymerase (Takara Bio Inc., Kyoto, Japan) with pSK63 as the template and the TphA2-F and TphA2-R primer set. The 0.4-kb PCR product was cloned into pT7Blue and sequenced. The 0.3-kb NdeI-BglII fragment of the resultant plasmid was inserted into pETDuet-1, and the 1.5-kb BglII-XhoI fragment carrying the 3′ terminus of tphA2II and tphA3II was inserted into the same restriction sites to generate pETtpa23. The coding sequence of tphA1II was amplified by PCR using pSK63 as the template with the TphA1-F and TphA1-R primer set. The 1.1-kb PCR product was cloned into pGEM-T and sequenced. The 1.1-kb NdeI-XhoI fragment of the resulting plasmid was inserted into pRSFDuet-1 to generate pRSFtpa1. The 0.7-kb DNA fragment carrying the 5′ terminus of the tphBII gene was amplified by PCR using pSK63 as the template with the TphB-F and TphB-R primer set. The 0.7-kb PCR product was cloned into pT7Blue and sequenced. The 1.1-kb XhoI-SacI fragment of pSK63 was inserted into the same sites in the resultant plasmid to generate pT7tpbc. The 1.2-kb NdeI-SacI fragment of pT7tpbc was cloned into pETDuet-1 to construct pETtpb. E. coli BL21(DE3) cells harboring pETtpa23, pRSFtpa1, both pETtpa23 and pRSFtpa1, or pETtpb were grown in LB medium containing ampicillin, kanamycin, ampicillin and kanamycin, or ampicillin, respectively, at 30°C. Expression of the genes was induced for 3 h by adding 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) when the optical density of the culture at 600 nm reached 0.5. The cells were harvested by centrifugation and suspended in FE2 buffer (50 mM Tris-HCl buffer [pH 7.0], 10% glycerol, 0.1 mM ferrous ammonium sulfate, and 2 mM l-cysteine hydrochloride) for the BL21(DE3) cells harboring pETtpa23 and pRSFtpa1 and in 50 mM Tris-HCl (pH 7.0) for the BL21(DE3) cells harboring pETtpb. Cells suspended in the buffer were sonicated, and the cell lysate was centrifuged at 15,000 × g for 10 min. The resulting supernatant was used as the cell extract. The protein concentration was determined using the method of Bradford (6). The expression of the gene was confirmed using sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis. The gels were stained with Coomassie brilliant blue.
Enzyme assays.
The activities of transformation of the crude TPADO to TPA and other substrates were determined by measuring the decrease in the amount of the substrates by high-pressure liquid chromatography (HPLC) (Alliance 2690 separations module; Waters, Milford, MA) with a TSKgel ODS-80 column (6 by 150 mm; Tosoh, Tokyo, Japan). The 1-ml assay mixture contained FE2 buffer, 100 μM substrate, 1 mM NADPH, and either a mixture of cell extracts of E. coli BL21(DE3) harboring pETtpa23 and BL21(DE3) harboring pRSFtpa1 (100 μg of protein each) or the cell extract of BL21(DE3) harboring both pETtpa23 and pRSFtpa1 (100 μg of protein). A portion of the reaction mixture was removed at various sampling times and analyzed using HPLC. For analysis of the conversion of substrates, the mobile phase was a mixture of water (74.5%), acetonitrile (24.5%), and phosphoric acid (1%) at a flow rate of 1 ml/min. Compounds were detected using the following wavelengths: for TPA, benzoate, and p-hydroxybenzoate, 280 nm; and for phthalate and isophthalate, 240 nm. The retention times of TPA, benzoate, p-hydroxybenzoate, phthalate, and isophthalate were 6.2, 14.1, 6.2, 6.3, and 6.8 min, respectively.
Identification of the reaction product.
To identify the reaction product of TPA catalyzed by TPADO, 1 mM TPA was incubated for 30 min at 30°C with the cell extract of E. coli BL21(DE3) harboring pETtpa23 and pRSFtpa1 (1 mg protein) in a 1-ml reaction mixture containing FE2 buffer and 5 mM NADPH. The reaction was stopped by adding methanol (25% final concentration). The reaction products were analyzed using HPLC-mass spectrometry (HP1100 series LC-MSD; Agilent Technologies Co., Palo Alto, CA). The mobile phase was a mixture of water (74.5%), acetonitrile (24.5%), and acetic acid (1%), using a flow rate of 1 ml/min. The compounds were detected at 280 nm, where the mass spectra were obtained using the method described previously (1). The 100-μl reaction mixture containing the product from TPA catalyzed by TPADO was prepared as described above and incubated for 60 min at 30°C with the cell extract of E. coli BL21(DE3) harboring pETtpb (100 μg protein) in a 1-ml reaction mixture containing 50 mM Tris-HCl buffer (pH 7.0) and 100 μM NADP+. The reaction mixture was analyzed using HPLC as described for the enzyme assay. The compounds were detected at 280 nm.
Construction and analysis of mutants of Comamonas sp. strain E6.
A 2.0-kb HindIII-Eco47III fragment carrying tphA2I was cloned into the HindIII-SmaI site of pBluescript II SK(+) to generate pDSR431, and a 0.19-kb PstI fragment was deleted for tphA2I disruption. A 1.2-kb PstI fragment carrying the kanamycin resistance gene (kan) from pUC4K was inserted into the PstI site to construct pDSR432. A 3.1-kb SacI-KpnI fragment of pDSR432 was blunt ended and cloned into the SmaI site of pK19mobsacB to generate pDSR43. A 1.7-kb HindIII-Eco47III fragment carrying tphA2II of pSK63 was cloned into SK(+) to generate pDSR631, and a 0.5-kb SphI-PstI fragment was deleted for tphA2II disruption. A 1.0-kb SmaI-PstI fragment carrying the chloramphenicol resistance gene (cat) from pUC6C was inserted into the blunt-ended SphI-PstI sites to construct pDSR632. A 2.3-kb SacI-XhoI fragment was cloned into the SmaI site of pK19mobsacB to generate pDSR63.
pDSR43 and pDSR63 were introduced into E6 cells by electroporation, and the candidates for the tphA2I and tphA2II mutants were screened using the method described previously (1). The tphA2I tphA2II double mutant was obtained by the introduction of pDSR63 into the tphA2I mutant cells. Southern hybridization analysis was performed to examine the disruption of tphA2I and tphA2II, using a digoxigenin system. The total DNA of the tphA2I mutant was digested with SphI, and the 0.7-kb SalI fragment carrying tphA2I and the 1.2-kb PstI fragment carrying kan were used as the probes. The total DNAs of the tphA2II mutant and the tphA2I tphA2II double mutant were digested using SmaI-XhoI and EcoRI-XhoI, and the 1.0-kb PstI fragment carrying cat was used as the probe. The resulting strains, i.e., the tphA2I mutant, the tphA2II mutant, and the tphA2I tphA2II mutant, were designated DE61, DE62, and DE612, respectively. A complementary plasmid, pJSK432, carrying tphA2IA3I was introduced into DE612 cells by electroporation.
Expression of tphII genes in C. testosteroni IAM 1152.
pEJ89 carrying tphRIICIIA2IIA3IIBIIA1II was introduced into C. testosteroni IAM 1152 by electroporation. The deletion derivatives of pEJ89 were constructed as shown in Table 1 and Fig. 2. The IAM 1152 transformants harboring the pEJ89 derivatives were preincubated in LB medium containing tetracycline, washed with W medium, and then transferred to 10 ml W medium containing 10 mM TPA and tetracycline at 30°C. The growth of the transformants on TPA was examined by monitoring the optical density at 600 nm of the cultures. Complementary plasmids, pKTC and pKTR, were introduced into the IAM 1152 cells harboring pEJ80 and pEJ84, respectively, by electroporation. These cells were cultured in W medium containing 10 mM TPA, tetracycline, and kanamycin.
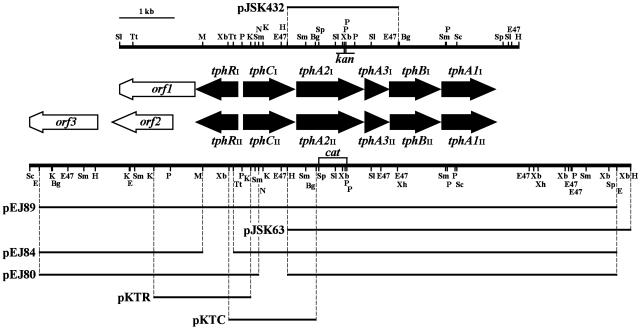
FIG. 2.
Organization of the two TPA degradation gene clusters in Comamonas sp. strain E6 and deletion analysis. Restriction maps of the 7.3-kb SalI-HindIII fragment carrying the tphI genes and the 11-kb SacI-HindIII fragment carrying the tphII genes are shown. The lines above and below the maps indicate the region included in each plasmid. These plasmids (except pJSK432, which was used as a complementary plasmid for the tphA2I tphA2II double mutant) were introduced into C. testosteroni IAM 1152 to examine role of each tph gene. kan and cat indicate the positions of the kan and cat gene insertions into the tphA2I mutant, the tphA2II mutant, and the tphA2I tphA2II double mutant. Abbreviations for restriction enzymes: Bg, BglII; E, EcoRI; E47, Eco47III; H, HindIII; K, KpnI; M, MunI; N, NheI; P, PstI; Sc, SacI; Sl, SalI; Sm, SmaI; Sp, SphI; Tt, Tth111I; Xb, XbaI; Xh, XhoI.
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this paper were deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession numbers AB238678, AB238679, and AB238680.
RESULTS AND DISCUSSION
Isolation of a TPA degrader, Comamonas sp. strain E6.
The TPA degrader E6 was isolated from the enrichment culture by using selection on W medium containing 10 mM TPA as the sole source of carbon and energy. The nucleotide sequence of the 1,481 bp carrying the 16S rRNA gene of strain E6 showed >99% identity with the 16S rRNA gene of the type strain of Comamonas testosteroni ATCC 11996. E6 was therefore classified as a Comamonas sp.
E6 grew on phthalate, isophthalate, vanillate, syringate, and PCA as well as TPA. When E6 was grown on TPA, PCA 4,5-dioxygenase and 2-pyrone-4,6-dicarboxylate hydrolase activities were detected (data not shown), suggesting that TPA is degraded through the PCA 4,5-cleavage pathway in E6.
Cloning and nucleotide sequences of the two TPA degradation gene clusters.
The deduced amino acid sequence of the product of the terZα gene from D. tsuruhatensis T7, coding for the oxygenase large subunit of TPADO (31), showed 42 to 46% identity with those of the products of putative oxygenase large-subunit genes, bphA1c and bphA1d, from Novosphingobium aromaticivorans DSM 12444 (25). Based on the amino acid sequence similarity of these enzymes, a degenerate primer set for the amplification of DNA containing a motif for a Rieske-type [2Fe-2S] iron-sulfur cluster was designed and used to generate a 305-bp fragment from E6. The nucleotide sequence of this fragment showed 99% identity with the T7 terZα sequence. Southern hybridization analysis of the E6 total DNA using the 305-bp amplified product as a probe showed two band patterns (data not shown), suggesting the presence of the two TPADO genes. Using colony hybridization with the amplified 305-bp fragment as the probe, pVAS4 carrying the 4.3-kb HindIII fragment and pVAS8 carrying the 6.3-kb HindIII fragment were isolated from the E6 charomid libraries, and pSCH1 carrying the 9.2-kb SacI fragment and pSCH14 carrying the 7.8-kb SacI fragment that overlaps the 4.3-kb HindIII fragment and 6.3-kb HindIII fragment were also isolated. The nucleotide sequences of these fragments showed two sets of six open reading frames (ORFs). Each of these ORFs were more than 90% similar to the TPA degradation genes (tph genes) of C. testosteroni YZW-D, whose nucleotide sequences have been recently deposited in the database (accession number AY923836). The E6 genes were designated tphRI and tphRII, tphCI and tphCII, tphA2I and tphA2II, tphA3I and tphA3II, tphBI and tphBII, and tphA1I and tphA1II, as shown in Fig. 2. Similarities between E6 tph genes and representative homologs are summarized in Table 3.
TABLE 3.
Comamonas sp. strain E6 tph genes and predicted products
| Genea | Representative homolog | Identity (%)b
|
Organism | Accession no. | |
|---|---|---|---|---|---|
| tphI | tphII | ||||
| tphR (99.2) | Transcriptional regulator (tphR) | 97.6 | 97.3 | C. testosteroni YZW-D | AAX18939 |
| IclR-type transcriptional regulator (pcaR) | 38.9 | 38.9 | R. opacus 1CP | AAC38247 | |
| tphC (100) | Terephthalate permease (tphC) | 96.3 | 96.3 | C. testosteroni YZW-D | AAX18940 |
| Extracytoplasmic solute receptor (bugT) | 34.7 | 34.7 | B. pertussis | AAO61652 | |
| tphA2 (99.5) | Terephthalate 1,2-dioxygenase oxygenase component large subunit (terZα) | 96.6 | 96.4 | D. tsuruhatensis T7 | BAC15591 |
| Terephthalate 1,2-dioxygenase oxygenase component large subunit (tphA2) | 99.5 | 99.5 | C. testosteroni YZW-D | AAX18941 | |
| Terephthalate 1,2-dioxygenase oxygenase component large subunit (tphA1) | 65.4 | 65.4 | Rhodococcus sp. DK17 | AAR90187 | |
| Large-subunit aromatic oxygenase (bphA1c) | 45.3 | 45.5 | N. aromaticivorans DSM 12444 | AAD04009 | |
| Large-subunit aromatic oxygenase (bphA1d) | 41.6 | 41.4 | N. aromaticivorans DSM 12444 | AAD04002 | |
| Naphthalene 1,2-dioxygenase iron sulfur protein component large subunit (nahAc) | 24.2 | 24.4 | P. putida NCIB 9816-4 | AAO64274 | |
| tphA3 (98.1) | Terephthalate 1,2-dioxygenase oxygenase component small subunit (tphA3) | 95.5 | 97.4 | C. testosteroni YZW-D | AAX18942 |
| Terephthalate 1,2-dioxygenase oxygenase component small subunit (tphA2) | 50.0 | 50.0 | Rhodococcus sp. DK17 | AAR90188 | |
| Small-subunit aromatic oxygenase (bphA2c) | 33.3 | 34.0 | N. aromaticivorans DSM 12444 | AAD04008 | |
| Small-subunit aromatic oxygenase (bphA2d) | 37.0 | 36.4 | N. aromaticivorans DSM 12444 | AAD04001 | |
| Naphthalene 1,2-dioxygenase iron sulfur protein component small subunit (nahAd) | 19.2 | 19.1 | P. putida NCIB 9816-4 | AAO64275 | |
| tphB (94.3) | Terephthalate dihydrodiol dehydrogenase (tphB) | 98.4 | 94.0 | C. testosteroni YZW-D | AAX18943 |
| Terephthalate dihydrodiol dehydrogenase (tphB) | 40.1 | 40.7 | Rhodococcus sp. DK17 | AAR90189 | |
| 4-Hydroxythreonine-4-phosphate dehydrogenase (pdxA) | 35.8 | 35.2 | Rhizobium sp. NGR234 | AAQ87151 | |
| tphA1 (95.8) | Terephthalate 1,2-dioxygenase reductase (tphA1) | 94.9 | 93.2 | C. testosteroni YZW-D | AAX18944 |
| Terephthalate 1,2-dioxygenase reductase (tphA4) | 42.9 | 42.6 | Rhodococcus sp. DK17 | AAR90190 | |
| Naphthalene 1,2-dioxygenase reductase component (nahAa) | 26.6 | 26.0 | P. putida NCIB 9816-4 | AAA25904 | |
The numbers in parentheses indicate the percent identity between the deduced amino acid sequences of tphI and tphII of E6.
Percentages of identity were obtained by aligning the deduced amino acid sequence by use of EMBOSS alignment tool.
Based on the sequence similarities, TphA2I/II and TphA3I/II appear to be the oxygenase large and small subunits, respectively, of TPADO. TphA2I and TphA2II have Cys-X1-His-X17-Cys-X2-His, in agreement with the consensus sequence for the Rieske-type [2Fe-2S] cluster. The amino acid sequence alignment between TphA2I/II and the oxygenase large subunit of naphthalene dioxygenase (NahAc) of Pseudomonas putida NCIB 9816-4 shows the conserved residues His210, His215, and Asp356, which may be involved in the binding of mononuclear iron (18).
The NCBI conserved-domain search revealed that TphA1I and TphA1II contain the motifs for a plant-type [2Fe-2S] iron-sulfur cluster (cd00207), a FAD binding domain (pfam00970), and a NAD binding domain (pfam00175). The presence of a motif for a [2Fe-2S] cluster in TphA1I and TphA1II and the fact that the ferredoxin gene was not found in the cloned regions may suggest that TPADO is a two-component dioxygenase classified as a class I dioxygenase (4). However, with naphthalene dioxygenase, ferredoxin is required for activity, although the ferredoxin reductase contains the plant-type [2Fe-2S] cluster (11, 12).
TphBI and TphBII showed 35 to 36% similarity with the putative 4-hydroxythreonine-4-phosphate dehydrogenase (PdxA) from Rhizobium sp. strain NGR234.
TphCI and TphCII showed 35% similarity to BugT from Bordetella pertussis, which is a periplasmic solute binding receptor of the tripartite tricarboxylate transporter family (2). The tripartite tricarboxylate transporter system consists of three proteins: a periplasmic solute binding receptor, a membrane protein with 12 putative transmembrane α-helical spanners, and a small poorly conserved membrane protein with four putative transmembrane α-helical spanners (37). The similarity between TphCI/II and a periplasmic solute binding receptor suggests that tphCI and tphCII each code for a periplasmic TPA binding receptor.
The tphRI and tphRII genes are carried on opposite strands in other tph genes and were located upstream of tphCI and tphCII, respectively. TphRI and TphRII showed 39% similarity to the PcaR from Rhodococcus opacus 1CP (10), which is a member of the IclR-type transcriptional regulators.
Downstream of tphRI, an ORF (orf1) showed 31% identity with a serine protease, DegP, from Arabidopsis thaliana (17); however, this ORF may not be involved in TPA degradation. orf2 and orf3, which are located downstream of tphRII, showed 55% and 40% identity, respectively, to a putative transposase from Ralstonia eutropha H16 (Q7WWT0) and an IS1353 putative transposase from Klebsiella pneumoniae (Q8GEE9). These putative transposase genes may be involved in the duplication of the tph genes in Comamonas sp. strain E6.
Disruption of tphA2I and tphA2II in Comamonas sp. strain E6.
To examine the function of tphA2I and tphA2II in TPA degradation by Comamonas sp. strain E6, tphA2I and tphA2II were disrupted using a gene replacement technique with pDSR43 and pDSR63, where tphA2I and tphA2II were inactivated using the insertion of kan and cat, respectively (Fig. 2). The disruption of tphA2I and tphA2II in the mutant candidates was confirmed using Southern hybridization analysis with the 0.7-kb SalI fragment carrying tphA2I, the 1.2-kb PstI fragment carrying kan, and the 1.0-kb PstI fragment carrying cat. The resulting tphA2I mutant (strain DE61) and tphA2II mutant (strain DE62) were cultured in W medium containing 10 mM TPA; however, the growth rates of both mutants were not affected (Fig. 3A). Therefore, the tphA2II gene in DE61 was disrupted by introducing pDSR63 into DE61 cells to obtain the tphA2I tphA2II double mutant (strain DE612). As a result, DE612 no longer grew on TPA (Fig. 3A). To determine if this growth deficiency was caused by the disruption of tphA2I and tphA2II, pJSK432 containing the 2.0-kb HindIII-Eco47III fragment carrying tphA2IA3I in pJB866 was introduced into DE612. The tphA2IA3I genes were expected to be expressed by using the Pm promoter regulated by XylS in the presence of m-toluate. The DE612 cells harboring pJSK432 grew on TPA in the presence of m-toluate, which is not a growth substrate of E6 (Fig. 3B). Although it took approximately 3.5 times longer for DE612 cells harboring pJSK432 than for the E6 cells harboring pJB866 to enter stationary phase, this indicates that both tphA2I and tphA2II are functional in E6 and that one of these genes is essential for the growth of E6 on TPA.
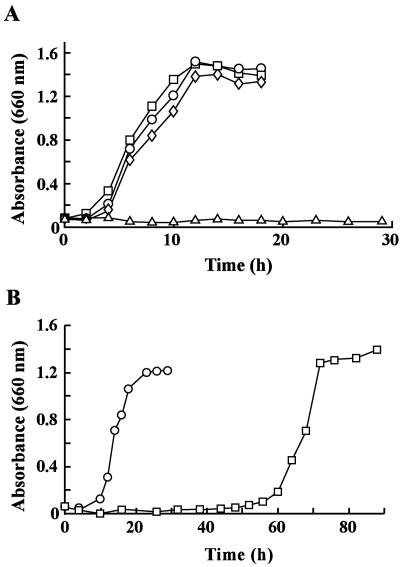
FIG. 3.
Disruption of tphA2I and tphA2II in Comamonas sp. strain E6. (A) Growth on TPA of E6 (circles), the tphA2I mutant (DE61) (squares), the tphA2II mutant (DE62) (diamonds), and the tphA2I tphA2II mutant (DE612) (triangles). These mutants were incubated in 10 ml W medium containing 10 mM TPA. (B) Complementation of tphA2IA3I in the DE612 mutant. Growth of E6 cells harboring pJB866 (circles) and of DE612 cells harboring pJSK432 carrying tphA2IA3I (squares) is shown. The cells were incubated in W medium containing 10 mM TPA, tetracycline, and m-toluate.
Expression of tphII genes in C. testosteroni IAM 1152.
To examine the role of the tph genes, pJSK63, containing the 6.3-kb HindIII fragment carrying tphA2IIA3IIBIIA1II on pJB866 (Fig. 2), was introduced into C. testosteroni IAM 1152, which is able to grow on PCA but not on TPA. If the IAM 1152 cells harboring pJSK63 grow on TPA as a sole source of carbon and energy, the tphA2IIA3IIA1II, and tphBII genes may code for TPADO and DCD dehydrogenase, respectively. However, this transformant was not able to grow on TPA even in the presence of 3 mM m-toluate. We found that IAM 1152 was able to grow on TPA in the absence of m-toluate when the IAM 1152 cells possessed pEJ89 containing the 8.9-kb EcoRI fragment carrying tphRII and tphCII in addition to tphA2IIA3IIBIIA1II (Fig. 4A). This indicates that either tphCII or tphRII or both genes are required for the growth of IAM 1152 on TPA.
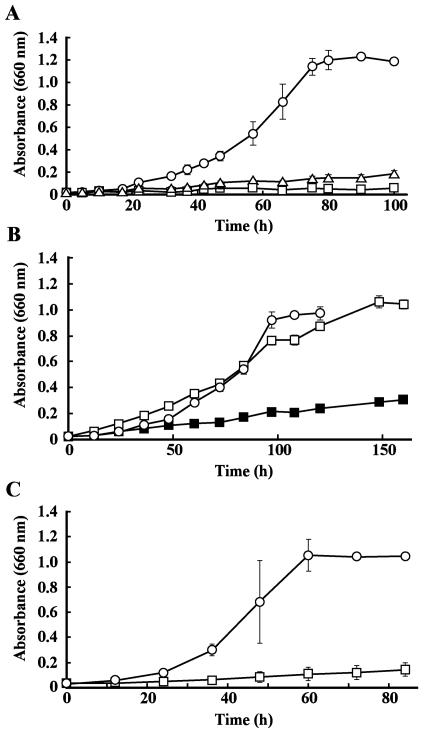
FIG. 4.
Expression of tphRIICIIA2IIA3IIBIIA1II in C. testosteroni IAM 1152. (A) Growth on TPA of IAM 1152 cells harboring pEJ89 carrying tphRIICIIA2IIA3IIBIIA1II (circles), pJSK63 carrying A2IIA3IIBIIA1II (squares), and pJB866 (vector) (triangles). (B) Disruption of tphCII in pEJ89. Growth on TPA of IAM 1152 cells harboring pEJ80 (ΔtphCII) and pKT230 (vector) at pH 7.3 (closed squares), harboring pEJ80 and pKT230 at pH 6.0 (open squares), and harboring pEJ80 and pKTC (tphCII) at pH 7.3 (circles) is shown. (C) Disruption of tphRII in pEJ89. Growth on TPA of IAM 1152 cells harboring pEJ84 (ΔtphRII) and pKT230MC (vector) (squares) and harboring pEJ84 and pKTR (tphRII) (circles) is shown.
To confirm the requirement of tphCII or tphRII, we constructed pEJ80 and pEJ84, where tphCII and tphRII, respectively, were disrupted by a deletion in each structural gene (Fig. 2). C. testosteroni IAM 1152 cells harboring each of these plasmids could not grow on TPA (Fig. 4B and C). The introductions of pKTC carrying tphCII and pKTR carrying tphRII into IAM 1152 cells harboring pEJ80 and pEJ84, respectively, conferred the TPA utilization phenotype on both transformants (Fig. 4B and C). This indicates that tphCII and tphRII are essential for IAM 1152 to grow on TPA. The fact that IAM 1152 harboring pEJ84 could not grow on TPA suggests that the transcription of the tphCIIA2IIA3IIBIIA1II genes from the Pm promoter was very weak or that m-toluate inhibited the growth of the IAM 1152 transformant on TPA. When IAM 1152 harbors pEJ89, the transcriptions of the tphRII and tphCIIA2IIA3IIBIIA1II genes appear to be initiated from their own promoters, and the transcription of the latter genes seem to be activated by the tphRII product.
The effect of pH on growth of IAM 1152 harboring pEJ80 was tested, since the lower the pH, the more TPA is in its undissociated form, which was expected to diffuse to the cells (8). This strain grew on TPA when the pH of the W medium containing TPA (pH 7.3) was adjusted to 6.0 (Fig. 4B). This result also suggests that tphCII codes for a periplasmic TPA binding receptor involved in TPA uptake.
Expression of tphA2IIA3II and tphA1II in E. coli.
The tphA2IIA3II and tphA1II genes were cloned into pETDuet-1 and pRSFDuet-1 as described in Materials and Methods to obtain pETtpa23 and pRSFtpa1, respectively. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis showed the production of 46-kDa, 17-kDa, and 36-kDa proteins in the cell extract of E. coli BL21(DE3) harboring both pETtpa23 and pRSFtpa1 (data not shown). These values were close to the molecular masses of the deduced amino acid sequences of gene products from tphA2II (46,191 Da), tphA3II (17,217 Da), and tphA1II (36,299 Da), respectively. However, a substantial amount of TphA1II was produced in an insoluble fraction.
To examine whether a cell extract containing TphA2II, TphA3II, and TphA1II shows TPA transformation activity, 100 μM TPA was incubated with the cell extract of E. coli harboring both pETtpa23 and pRSFtpa1 (100 μg of protein/ml) in the presence of 1 mM NADPH. TPA was completely degraded by the cell extract within 30 min, whereas the cell extract from E. coli harboring pETtpa23 and pRSFDuet-1 did not transform TPA. This suggests that TPADO consists of TphA2II, TphA3II, and TphA1II.
To determine the reaction product of TPADO, 1 mM of TPA was incubated with the cell extract of E. coli harboring pETtpa23 and pRSFtpa1 (1 mg of protein/ml) with 5 mM NADPH, and the reaction mixture was analyzed using HPLC. The generation of a peak with a retention time of 3.0 min (compound I) was observed (data not shown). Negative-mode electrospray ionization-mass spectrometry analysis of compound I showed a fragment at m/z 199 that corresponds to the deprotonated molecular ion of DCD. This suggests that the production of DCD from TPA is catalyzed by TPADO.
The TPA transformation rates of TPADO using NADPH and NADH were compared. When 100 μM TPA was incubated with the mixture of crude extracts of E. coli harboring pETtpa23 and E. coli harboring pRSFtpa1 (each 100 μg of protein/ml) in the presence of 1 mM NADPH, the TPA transformation activity of the crude extract measured in a 1-min reaction (560 ± 70 mU/mg protein) was 1.3 times higher than that with 1 mM NADH. Further, it required more than 5 min to degrade 100 μM TPA by TPADO with NADH, while TPA was degraded within 2 min in the presence of 1 mM NADPH. This indicates that NADPH is the preferred electron donor compared to NADH.
The TPA transformation activity of TPADO was lost when the crude enzymes were treated with 500 μM EDTA. The metal ions Fe2+, Fe3+, Mn2+, Mg2+, Cu2+, Co2+, and Zn2+ were added to the reaction mixture to a final concentration of 1 mM, and the resulting solutions were kept on ice for 1 h. The TPADO activity was partially recovered only when Fe2+ was added to the reaction mixture (39 ± 7 mU/mg). This suggests that TPADO requires Fe2+ for activity.
The substrate preference of TPADO was examined by using phthalate, isophthalate, benzoate, p-hydroxybenzoate, biphenyl, benzene, and toluene. However, the conversion of these substrates and product formation were not observed using HPLC analysis, suggesting that TPADO is specific for only TPA.
Expression of tphBII in E. coli.
The tphBII gene was cloned in pETDuet-1 to obtain pETtpb. Expression of a 33-kDa protein was observed in E. coli BL21(DE3) harboring pETtpb (data not shown); the size is close to the predicted molecular mass of TphBII (33,050 Da).
To examine whether tphBII codes for the DCD dehydrogenase, the cell extract of E. coli harboring pETtpb was incubated with the reaction product from TPA (100 μM). This reaction product (compound I) from TPA was prepared by incubating 1 mM TPA with the crude extract of E. coli harboring pETtpa23 and pRSFtpa1 (1 mg of protein/ml) and 5 mM NADPH. After a 60-min incubation of the reaction mixture containing compound I with the crude TphBII (100 μg of protein/ml) in the presence of 1 mM NADP+, compound I was completely degraded, and a new peak with a retention time of 4.4 min was generated (data not shown). By comparison of the retention times and the UV-visible spectra of the product and standard compounds, this product was identified as PCA. These results indicated that TPA was converted to PCA by two successive reactions catalyzed by TPADO and TphBII. This suggests that tphBII codes for DCD dehydrogenase.
Conclusions.
This study reports the isolation of two sets of genes involved in the transformation of TPA to PCA from Comamonas sp. strain E6, and one of the gene sets, the tphII genes, was characterized. Recently, the presence of two sets of tph genes on different plasmids in Rhodococcus was reported (7). Our preliminary experiment suggested that the E6 tph gene clusters were located on chromosome.
TPADO activity was obtained using a mixture of the crude TphA1II, TphA2II, and TphA3II enzymes. From this, it appears that E6 TPADO may be a two-component dioxygenase. To confirm this, we are now purifying TphA1II and TphA2IIA3II to reconstitute TPADO activity using the purified gene products. TphBII apparently converts the reaction product from TPA catalyzed by TPADO to PCA, and thus TphB was determined to be a DCD dehydrogenase. However, more convincing evidence that the reaction product from TPA catalyzed by TPADO is DCD is necessary. From studies of the expression of tphII genes in C. testosteroni IAM 1152, tphRII and tphCII are suggested to code for a positive regulator of the tphII genes and the TPA binding receptor, respectively. Further study is required to gain a better understanding of the regulation of tphII genes by TphRII, the TPA uptake system, and the reason for the presence of redundant tph gene sets in this strain.
Acknowledgments
We thank Y. Katayama and S. Nishikawa for helpful suggestions. We also thank T. Abe and D. Kasai for their technical assistance and T. Iwasaki for constructing pUC6C.
REFERENCES
- 1.Abe, T., E. Masai, K. Miyauchi, Y. Katayama, and M. Fukuda. 2005. A tetrahydrofolate-dependent O-demethylase, LigM, is crucial for catabolism of vanillate and syringate in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 187:2030-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine, R., F. Jacob-Dubuisson, H. Drobecq, E. Willery, S. Lesjean, and C. Locht. 2003. Overrepresentation of a gene family encoding extracytoplasmic solute receptors in Bordetella. J. Bacteriol. 185:1470-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagdasarian, M., R. Lurz, B. Ruckert, F. C. Franklin, M. M. Bagdasarian, J. Frey, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237-247. [DOI] [PubMed] [Google Scholar]
- 4.Batie, C. J., D. P. Ballou, and C. J. Correll. 1991. Phthalate dioxygenase reductase and related flavin-iron-sulfur containing electron transferases, p. 544-554. In F. Müller (ed.), Chemistry and biochemistry of flavoenzymes. CRC Press, Inc., Boca Raton, Fla.
- 5.Blatny, J. M., T. Brautaset, H. C. Winther-Larsen, P. Karunakaran, and S. Valla. 1997. Improved broad-host-range RK2 vectors useful for high and low regulated gene expression levels in gram-negative bacteria. Plasmid 38:35-51. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Choi, K. Y., D. Kim, W. J. Sul, J. C. Chae, G. J. Zylstra, Y. M. Kim, and E. Kim. 2005. Molecular and biochemical analysis of phthalate and terephthalate degradation by Rhodococcus sp. strain DK17. FEMS Microbiol. Lett. 252:207-213. [DOI] [PubMed] [Google Scholar]
- 8.Collier, L. S., N. N. Nichols, and E. L. Neidle. 1997. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J. Bacteriol. 179:5943-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford, R. L., J. W. Bromley, and P. E. Perkins-Olson. 1979. Catabolism of protocatechuate by Bacillus macerans. Appl. Environ. Microbiol. 37:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eulberg, D., S. Lakner, L. A. Golovleva, and M. Schlömann. 1998. Characterization of a protocatechuate catabolic gene cluster from Rhodococcus opacus 1CP: evidence for a merged enzyme with 4-carboxymuconolactone-decarboxylating and 3-oxoadipate enol-lactone-hydrolyzing activity. J. Bacteriol. 180:1072-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haigler, B. E., and D. T. Gibson. 1990. Purification and properties of ferredoxinNAP, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J. Bacteriol. 172:465-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haigler, B. E., and D. T. Gibson. 1990. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J. Bacteriol. 172:457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 14.Hara, H., E. Masai, Y. Katayama, and M. Fukuda. 2000. The 4-oxalomesaconate hydratase gene, involved in the protocatechuate 4,5-cleavage pathway, is essential to vanillate and syringate degradation in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 182:6950-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara, H., E. Masai, K. Miyauchi, Y. Katayama, and M. Fukuda. 2003. Characterization of the 4-carboxy-4-hydroxy-2-oxoadipate aldolase gene and operon structure of the protocatechuate 4,5-cleavage pathway genes in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 185:41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwood, C. S., and R. E. Parales. 1996. The β-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 50:553-590. [DOI] [PubMed] [Google Scholar]
- 17.Itzhaki, H., L. Naveh, M. Lindahl, M. Cook, and Z. Adam. 1998. Identification and characterization of DegP, a serine protease associated with the luminal side of the thylakoid membrane. J. Biol. Chem. 273:7094-7098. [DOI] [PubMed] [Google Scholar]
- 18.Kauppi, B., K. Lee, E. Carredano, R. E. Parales, D. T. Gibson, H. Eklund, and S. Ramaswamy. 1998. Structure of an aromatic-ring-hydroxylating dioxygenase-naphthalene 1,2-dioxygenase. Structure 6:571-586. [DOI] [PubMed] [Google Scholar]
- 19.Kersten, P. J., S. Dagley, J. W. Whittaker, D. M. Arciero, and J. D. Lipscomb. 1982. 2-Pyrone-4,6-dicarboxylic acid, a catabolite of gallic acids in Pseudomonas species. J. Bacteriol. 152:1154-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama, K. 1983. Purification and properties of 2-pyrone-4,6-dicarboxylate hydrolase. J. Biochem. (Tokyo) 93:557-565. [DOI] [PubMed] [Google Scholar]
- 21.Masai, E., K. Momose, H. Hara, S. Nishikawa, Y. Katayama, and M. Fukuda. 2000. Genetic and biochemical characterization of 4-carboxy-2-hydroxymuconate-6-semialdehyde dehydrogenase and its role in the protocatechuate 4,5-cleavage pathway in Sphingomonas paucimobilis SYK-6. J. Bacteriol. 182:6651-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masai, E., S. Shinohara, H. Hara, S. Nishikawa, Y. Katayama, and M. Fukuda. 1999. Genetic and biochemical characterization of a 2-pyrone-4,6-dicarboxylic acid hydrolase involved in the protocatechuate 4,5-cleavage pathway of Sphingomonas paucimobilis SYK-6. J. Bacteriol. 181:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishikawa, S., T. Sonoki, T. Kasahara, T. Obi, S. Kubota, S. Kawai, N. Morohoshi, and Y. Katayama. 1998. Cloning and sequencing of the Sphingomonas (Pseudomonas) paucimobilis gene essential for the O demethylation of vanillate and syringate. Appl. Environ. Microbiol. 64:836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng, X., T. Egashira, K. Hanashiro, E. Masai, S. Nishikawa, Y. Katayama, K. Kimbara, and M. Fukuda. 1998. Cloning of a Sphingomonas paucimobilis SYK-6 gene encoding a novel oxygenase that cleaves lignin-related biphenyl and characterization of the enzyme. Appl. Environ. Microbiol. 64:2520-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romine, M. F., L. C. Stillwell, K. K. Wong, S. J. Thurston, E. C. Sisk, C. Sensen, T. Gaasterland, J. K. Fredrickson, and J. D. Saffer. 1999. Complete sequence of a 184-kilobase catabolic plasmid from Sphingomonas aromaticivorans F199. J. Bacteriol. 181:1585-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito, I., and G. R. Stark. 1986. Charomids: cosmid vectors for efficient cloning and mapping of large or small restriction fragments. Proc. Natl. Acad. Sci. USA 83:8664-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 28.Schläfli, H. R., M. A. Weiss, T. Leisinger, and A. M. Cook. 1994. Terephthalate 1,2-dioxygenase system from Comamonas testosteroni T-2: purification and some properties of the oxygenase component. J. Bacteriol. 176:6644-6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigehara, K., Y. Katayama, S. Nishikawa, and Y. Hotta. January 2002. Polyamide and process for producing the same. U.S. patent 6,340,739.
- 30.Shigehara, K., Y. Katayama, S. Nishikawa, and Y. Hotta. October 2001. Polyester and process for producing the same. U.S. patent 6,303,745.
- 31.Shigematsu, T., K. Yumihara, Y. Ueda, S. Morimura, and K. Kida. 2003. Purification and gene cloning of the oxygenase component of the terephthalate 1,2-dioxygenase system from Delftia tsuruhatensis strain T7. FEMS Microbiol. Lett. 220:255-260. [DOI] [PubMed] [Google Scholar]
- 32.Short, J. M., J. M. Fernandez, J. A. Sorge, and W. D. Huse. 1988. λ ZAP: a bacteriophage λ expression vector with in vivo excision properties. Nucleic Acids Res. 16:7583-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 34.Tamaoka, J., D.-M. Ha, and K. Komagata. 1987. Reclassification of Pseudomonas acidovorans den Dooren de Jong 1926 and Pseudomonas testosteroni Marcus and Talalay 1956 as Comamonas acidovorans comb. nov. and Comamonas testosteroni comb. nov., with an emended description of the genus Comamonas. Int. J. Syst. Bacteriol. 37:52-59. [Google Scholar]
- 35.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 36.Wang, Y. Z., Y. Zhou, and G. J. Zylstra. 1995. Molecular analysis of isophthalate and terephthalate degradation by Comamonas testosteroni YZW-D. Environ. Health Perspect. 103:9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winnen, B., R. N. Hvorup, and M. H. Saier, Jr. 2003. The tripartite tricarboxylate transporter (TTT) family. Res. Microbiol. 154:457-465. [DOI] [PubMed] [Google Scholar]
- 38.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]